Abstract
Rho GTPases are regulators of signaling pathways that control actin organization and cell polarity processes in all eukaryotic cells. In Schizosaccharomyces pombe, Rho4p is involved in the regulation of septum degradation during cytokinesis. Here we show that Rho4p participates in the secretion of the glucanases Eng1p and Agn1p, which are responsible for the septum degradation. First, eng1+ or agn1+ overexpression suppressed the rho4Δ multiseptation phenotype, and simultaneous overproduction of Rho4p and Eng1p or of Rho4p and Agn1p caused a dramatic lysis. Second, Rho4p was not necessary for Eng1p-mediated glucanase activity as measured in cell extracts; however, rho4Δ cells have a lower level of (1,3)-β-d-glucanase activity in the culture medium. Additionally, Eng1- or Agn1-green fluorescent protein did not properly localize to the septum in rho4Δ cells grown at 37°C. There was a decreased amount of these enzymes in the cell wall and in the culture medium of rho4Δ cells at 37°C. These results provide evidence that Rho4p is involved in the regulation of Eng1p and Agn1p secretion during cytokinesis.
Cells of the fission yeast Schizosaccharomyces pombe are cylindrical, growing by elongation of their ends and dividing by medial septation followed by cleavage of the septum (for a review, see reference 21). Like other eukaryotic organisms, S. pombe uses an actomyosin contractile ring to provide the force necessary for cell cleavage (reviewed in references 14, 18, 21, 35, and 49). Concomitant with constriction of the actomyosin ring and addition of new membranes, synthesis of the primary septum and the new cell wall occurs. Genetic studies have identified many genes that are important for the different steps of cytokinesis, such as the positioning and assembly of the actomyosin ring, the localization of actin patches at the site of cell division, the coordination between ring contraction and nuclear cycle, and the physical assembly of the division septum (8, 14, 18, 21, 27, 35, 49). Cps1/Bgs1p is the β-1,3-glucan synthase subunit essential for the biosynthesis of the primary septum and is composed mainly of linear (1,3)-β-d-glucan (23, 28, 31). The primary septum is surrounded on both sides by two so-called secondary septa, similar to the rest of the cell wall (22). Upon septum assembly completion, the primary septum undergoes rapid autolytic degradation, accompanied by local erosion of the adjacent regions of the cell wall. This last step of cytokinesis is required to separate the two cells.
The isolation of mutants affected in cell separation has provided some insights into how septum cleavage is achieved. It has been proposed that local secretion of β-glucanases is needed, since linear (1,3)-β-d-glucan is the main component of this structure (22). In agreement with this prediction, it has been recently shown that Eng1p, a protein with endo-(1,3)-β-d-glucanase activity that transiently localizes to the septum region in a ring-like structure, is necessary for primary septum dissolution (33). Additionally, an endo-(1,3)-α-d-glucanase, Agn1p, has also been described (2, 17, 20), and this enzyme acts in concert with Eng1p to achieve efficient cell separation, hydrolyzing the cell wall material surrounding the primary septum. The exocyst complex is also required for cell separation (48), and it might be involved in the delivery of hydrolytic proteins important for cell cleavage. Other mutants with cell separation defects include, for example, those with mutations in the septins (32), the Mid2p anillin (9, 45), the calcineurin catalytic subunit Ppb1p (50), the mitogen-activated protein kinase Mpk1p (46), the Rho1GAP Rga5p (12), the putative RNA binding protein Scw1p (25, 26), or the transcription factors Sep1p and Ace2p, which regulate many septation genes (25, 33, 40, 41). It has been established that a transcriptional cascade from Sep1p to Ace2p defines a regulatory pathway at the end of the cell cycle that is required for cell separation (2, 6).
Rho GTPases are key molecules in polarity processes (reviewed in references 10 and 44). In yeasts, Rho GTPases are responsible for the coordinated regulation of cell wall biosynthesis and the actin cytoskeleton, which is required to maintain cell integrity and polarized growth (3, 11).
S. pombe has six Rho GTPases. cdc42+ and rho1+ are essential genes involved in polarity processes (1). The Rho1p GTPase localizes to sites of polarized growth, is the regulatory component of the (1,3)-β-d-glucan synthase, and is also required for maintenance of cell integrity and polarization of the actin cytoskeleton. Rho1p-GTP interacts with Pck1p and Pck2p, the two fission yeast PKC homologues (4). Rho2p-GTP also localizes to sites of polarized growth, interacts with Pck2p, and is a positive regulator of Mok1p, an essential (1,3)-α-d-glucan synthase (13). Rho3p interacts with the formin For3p and is involved in polarity processes (36). Rho3p also modulates the exocyst function (47). rho4Δ cells are defective in cell separation at high temperature. Rho4p localizes to the septum, and it has been proposed that is involved in cell wall degradation during cytokinesis (43). In this study, we have characterized the relationship between Rho4p and the glucanases Eng1p and Agn1p. eng1+ or agn1+ overexpression suppressed the rho4Δ multiseptation phenotype. Double overexpression of rho4+ and eng1+ was lethal. Eng1-green fluorescent protein (GFP) and Agn1-GFP were not properly localized in rho4Δ cells at high temperature. In fact, Eng1-GFP was not detected and Agn1-GFP was clearly reduced in the cell wall or the culture medium of rho4Δ cells grown at 37°C. Interestingly, Rho4p was not required for the (1,3)-β-d-glucanase activity of Eng1p, as measured in vitro from cell extracts; however, rho4Δ cells had reduced glucanase activity in the culture medium. From these results, we conclude that Rho4p is involved in the regulation of Eng1p or Agn1p secretion during cytokinesis.
MATERIALS AND METHODS
Strains, growth conditions, and genetic methods.
All the strains used were isogenic to wild-type strains h− 972 and h+ 975 and are described in Table 1. Standard S. pombe media and genetic manipulations were employed (34). Yeast cells were grown in YES (rich medium) or minimal medium (EMM) supplemented with the necessary requirements. For the nutritional shift experiments, cells were grown in EMM with the appropriate supplements, washed with the same medium lacking the nitrogen source (EMM−N), and resuspended in EMM−N. Growth was monitored by measurements of optical density at 600 nm.
TABLE 1.
Strains
| Strain | Genotype |
|---|---|
| PPG102 | h−leu1-32 |
| PPG371 | h−leu1-32 ura4-D18 |
| PPG1541 | h−leu1-32 ura4-D18 rho4::kanMX6 |
| YAB14 | h−ura4-D18 eng1::kanMX6 |
| PPG1568 | h−ura4-D18 eng1::kanMX6 rho4::kanMX6 |
| YAB18 | h−leu1-32 eng1-GFP |
| PPG1766 | h−leu1-32 eng1-GFP rho4::kanMX6 |
| KGY4398a | h−ura4-D18 leu1-32 ade6-M210 agn1-GFP |
| PPG3809 | h+ura4-D18 leu1-32 ade6 agn1-GFP rho4::kanMX6 |
| PPG4154 | h+bgs1-GFP rho4::kanMX6 ade6-M210 his3-D1 |
| PPG4156 | h+bgs4-GFP rho4::kanMX6 ade6-M210 his3-D1 |
Strain KGY4398 is from K. Gould's laboratory.
Escherichia coli DH5α (Gibco BRL) was used as host for propagation of plasmids. Cells were grown in LB medium supplemented with 50 μg/ml ampicillin.
All DNA and RNA manipulations were carried out by established methods (5, 42). Yeast transformations were performed by the lithium acetate method (24). The nmt1+ promoter-containing vectors pREP3X and pREP4X (19) were used for overexpression experiments. Transformed cells were grown until mid-log phase in minimal medium (EMM) containing 15 μM thiamine. Cells were then harvested, washed three times with water, and inoculated into fresh medium without thiamine, and samples were taken at the time points described for each experiment. Plasmid pND02 was used for agn1+ overexpression studies (17).
Deletion of rho4+ or eng1+ and tagging of the eng1+ or agn1+ gene have been described previously (2, 33, 43).
Microscopy techniques.
Eng1-GFP, Agn1-GFP, Bgs1-GFP, and Bgs4-GFP were observed using a Leica DMRXA microscope. Images were captured with a Photometrics Sensys charge-coupled-device camera and processed with Adobe Photoshop software.
Western blotting.
Exponentially growing cells (108) were harvested by centrifugation, washed once with lysis buffer (100 mM Tris-HCl, pH 8, 1 mM EDTA, 100 mM NaCl, 0.5% NP-40) containing protease inhibitors (Sigma), resuspended in 100 μl of lysis buffer, and broken with glass beads in a Fast-Prep (Bio 101 Savant). Lysates were centrifuged for 10 min at 14,000 × g, and protein concentration was determined with the Bio-Rad protein assay kit. Precipitated cell walls were washed once with 5% NaCl, centrifuged for 5 min at 1,000 × g, and washed twice with 1 mM EDTA. The walls were resuspended in 100 μl of lysis buffer, mixed with an equal volume of 2× concentrated Laemmli buffer, boiled for 5 min, and centrifuged. A total of 100 μg protein from the cytoplasm fraction or 20 μl from the cell wall supernatant was loaded onto polyacrylamide gels containing sodium dodecyl sulfate. After electrophoresis, proteins were blotted onto Immobilon-P (Millipore) and probed with monoclonal anti-GFP antibody (Becton Dickinson). The reactive bands were detected by using anti-mouse immunoglobulin G-horseradish peroxidase-conjugated antibodies (Bio-Rad) and the ECL detection kit (Amersham Pharmacia Biotech).
To analyze the presence of Eng1p or Agn1p in the culture medium, approximately 108 cells were centrifuged and the supernatants were concentrated 100 times using 50-ml Amicon Ultra tubes (Millipore). A volume corresponding to 107 cells was loaded into the gels.
Measurement of (1,3)-β-d-glucanase activity.
Endo-(1,3)-β-d-glucanase activity was assayed against laminarin in cell extracts or in concentrated culture supernatants as previously described (7). Determination of the reducing sugars released in the reactions was performed by the methods of Somogyi (43a) and Nelson (38). One unit of activity was defined as the amount of enzyme that catalyzed the release of reducing sugar groups equivalent to 1 μmol of glucose per hour, and specific activity was expressed as units per milligram of protein or per 106 cells.
RESULTS
Overexpression of eng1+ or agn1+ suppresses the rho4Δ multiseptation phenotype.
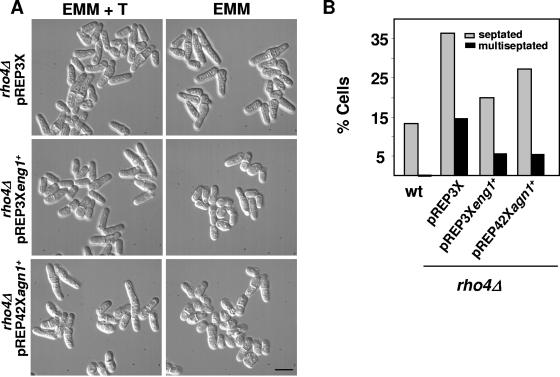
Previous work suggested that Rho4p is involved in cell wall degradation during septation (37, 43). Cells lacking Rho4p accumulate vesicles in the septation area when grown at 37°C (43). To further analyze the function of Rho4p during this process, we studied the possible relationship of this GTPase with Eng1p, the β-glucanase that degrades the primary septum, and Agn1p, the α-glucanase implicated in cell separation. First, we analyzed if overexpression of eng1+ or agn1+ was able to suppress the phenotype of rho4Δ cells. Cultures of rho4Δ mutants at 37°C contain a higher percentage of septating cells and some cells with multiple septa (43) (Fig. 1). Interestingly, eng1+ or agn1+ overexpression in rho4Δ cells decreased the percentage of septated and multiseptated cells (Fig. 1). eng1+ overexpression suppressed the separation defect better than agn1+ overexpression, most probably because different versions of nmt1 promoter were used. Overexpression of agn1+ using pREP3X is lethal (20).
FIG. 1.
Overexpression of eng1+ or agn1+ suppresses the rho4Δ multiseptation phenotype. (A) Differential interference contrast images of rho4Δ cells transformed with pREP3X, pREP3Xeng1+, or pREP42Xagn1+ (pND02) and grown at 37°C for 22 h in the presence (EMM + T) or absence (EMM) of thiamine. Bar, 10 μm. (B) Quantification of the septation phenotype (n = 300). Calcofluor white was used to stain the septa. wt, wild type.
Simultaneous overexpression of rho4+ and eng1+ or agn1+ causes severe lysis.
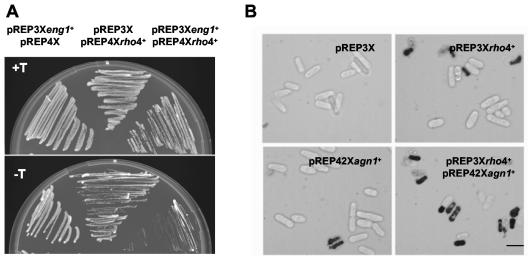
Rho4p overproduction causes 20 to 30% lysis to cells growing in liquid culture. However, the remaining cells survive and are able to grow as rounded cells (43). In solid medium, Rho4p-overproducing cells are able to grow. Overexpression of eng1+ does not cause any morphological or lytic phenotype (Fig. 2) (33). Interestingly, when Eng1p and Rho4p were simultaneously overproduced, the cells were unable to grow in solid medium (Fig. 2A), and the fraction of lysed cells increased to 50 to 60% in liquid medium (data not shown). Lysis also increased to similar levels (45 to 50%) when Agn1p and Rho4p were simultaneously overproduced (Fig. 2B). A possible explanation for this additive effect would be that the glucanases are Rho4p targets; therefore, Rho4p might either act as an activator of the glucanase activities or be necessary for their polarized secretion during septum degradation. In such cases, overexpression of rho4+ would cause, respectively, either excessive activation or ectopic glucanase secretion, and both of these situations would result in cell lysis. Alternatively, the additive effects could be caused by the activation of two independent pathways.
FIG. 2.
Simultaneous overexpression of rho4+ and eng1+ or agn1+ causes dramatic cell lysis. (A) Wild-type cells transformed with plasmids pREP3Xeng1+ and pREP4X, pREP3X and pREP4Xrho4+, or pREP4Xrho4+ and pREP3Xeng1+ were grown at 32°C in EMM with 15 μM thiamine (nmt promoter off, top panel) or in EMM without thiamine (nmt promoter on, bottom panel) to induce rho4+ and eng1+ overexpression. (B) Images of wild-type cells transformed with plasmids pREP3X and pREP4X, pREP3Xrho4+ and pREP4X, pREP3X and pREP42Xagn1+, and pREP3Xrho4+ and pREP42Xagn1+. Cells were grown at 32°C in EMM without thiamine for 22 h and stained with methylene blue. Lysed cells appear dark. Bar, 10 μm.
The eng1Δ rho4Δ double mutant is similar to the rho4Δ mutant at high temperature.
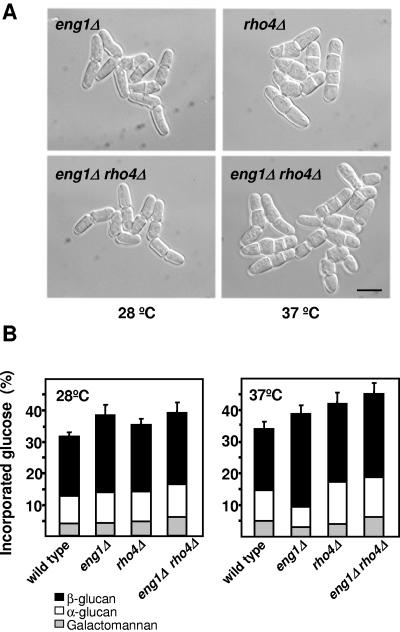
To check if Eng1p and Rho4p participate in the same or different pathways, we constructed the eng1Δ rho4Δ double mutant. Eng1p and Rho4p are both involved in cell separation, but the phenotypes of the eng1Δ and rho4Δ cells are quite different. Most of the eng1Δ cells are clustered in groups of four cells (Fig. 3A) (33), while rho4Δ cells contain multiple septa only when grown at 37°C (Fig. 3A) (43). As shown in Fig. 3A, the eng1Δ rho4Δ double mutant, grown at 28°C, is similar to the eng1Δ mutant, showing clusters of four cells. However, at 37°C, eng1Δ rho4Δ cells are similar to rho4Δ cells, suggesting that rho4+ is epistatic over eng1+. To corroborate further the results described above, we directly analyzed the incorporation of radioactive glucose into the walls of eng1Δ, rho4Δ, and eng1Δ rho4Δ mutants in comparison with wild-type cells. eng1Δ cells contained more cell wall material, mainly due to an increase of the β-glucan (Fig. 3B). rho4Δ cells also showed an increase in the cell wall content, which was more evident during growth at high temperature (Fig. 3B). The eng1Δ rho4Δ cell wall composition is similar to that of eng1Δ cells at 28°C, but at high temperature is closer to that of rho4Δ cells (Fig. 3B). These results suggest that Eng1p and Rho4p are in the same pathway, and they are consistent with the hypothesis that Rho4p participates in the secretion of lytic enzymes, including Eng1p and Agn1p, during cell separation at high temperature.
FIG. 3.
Phenotype of the eng1Δ rho4Δ double mutant. (A) Differential interference contrast images of eng1Δ, rho4Δ, and eng1Δ rho4Δ cells grown at 28°C or 37°C in rich medium. Bar, 10 μm. (B) Cell wall composition of wild-type, eng1Δ, rho4Δ, and eng1Δ rho4Δ cells grown in rich medium at 28°C or 37°C. [14C]glucose was added 4 h before harvesting the cells. The [14C]glucose radioactivity incorporated into each cell wall polysaccharide is shown. Values are the means from three independent experiments; the standard deviations for total carbohydrate values are shown.
Eng1p glucanase activity is not dependent on Rho4p levels.
To further study the relationship between Rho4p and Eng1p, we first analyzed if Rho4p is an activator of the Eng1-mediated (1,3)-β-d-glucanase activity. β-Glucanase activity levels were measured in extracts of cells without Rho4p or in cells overexpressing this GTPase. Eng1p glucanase activity did not depend on Rho4p, since the activity of extracts of either rho4Δ or rho4+-overexpressing cells grown at 37°C was similar to or slightly higher than that of wild-type extracts (Fig. 4A). We next analyzed the effect of altering Rho4p levels in cells overexpressing eng1+. When eng1+ was overexpressed using the plasmid pREP3X-eng1+, the β-glucanase activity in cell extracts from the wild-type strain increased by around five times, and this increase was slightly higher in cells lacking Rho4p grown at 37°C (Fig. 4B). Simultaneous overproduction of both Eng1p and Rho4p decreased the activity detected in the cell extracts (Fig. 4B); however, this decrease could be partly due to the induced cell lysis (see Fig. 2A). Thus, these results suggest that Rho4p is neither an activator of the Eng1p glucanase nor required for its activity in cell extracts.
FIG. 4.
Eng1p glucanase activity is not dependent on rho4+ levels. β-1,3-Glucanase activity was assayed in extracts from wild-type (wt), eng1Δ, rho4Δ, and rho4+-overexpressing cells (A); in extracts from wild-type, rho4Δ, and rho4+-overexpressing cells containing pREP3Xeng1+ in the presence (hatched bars) or absence (black bars) of thiamine (B); and in culture supernatants of wild-type, eng1Δ, and rho4Δ cells grown at 37°C (C). Values are the means from at least three independent experiments. Error bars represent the standard deviations.
Rho4p is required for proper secretion of Eng1p at 37°C.
We then considered the possibility that Rho4p was necessary for the localized secretion of Eng1p at 37°C, since rho4Δ cells accumulate vesicles in the septum area (43). When the secreted (1,3)-β-d-glucanase activities of wild-type cells and cells lacking Rho4p grown at 37°C were measured, we found that the activity was around 50% lower in the culture medium of rho4Δ cells (Fig. 4C). Similarly, when eng1+ was overexpressed using the plasmid pREP3X-eng1+, the β-glucanase activity in the culture medium of rho4Δ cells was 50% of the activity measured in cultures of wild-type cells (data not shown).
Eng1p or Agn1p localization is defective in cells lacking Rho4p when grown at high temperature.
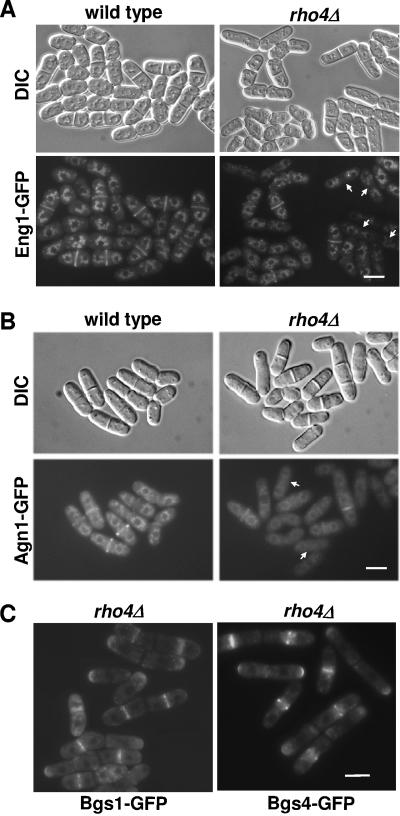
Eng1p is a secreted protein that is expressed only during cytokinesis and can be visualized in the septum area (Fig. 5A) (33). To further confirm the possible role of Rho4p in Eng1p polarized secretion, the Rho4p GTPase requirement for proper localization of Eng1-GFP to the septum was analyzed. At high temperature (36°C), Eng1-GFP was visualized in most of the septating wild-type cells (80%), whereas only 26% of the septating rho4Δ cells displayed Eng1-GFP at the septa (Fig. 5A). Agn1p is a cell cycle-regulated protein that localizes to the septum (2, 20). Agn1-GFP is also partially delocalized in rho4Δ cells grown at 37°C. At high temperature, Agn1-GFP was visualized in most of the septating wild-type cells (82%), whereas only 56% of the septating rho4Δ cells displayed Agn1-GFP at the septa (Fig. 5B). Other proteins involved in cell wall organization and localized to the septum, such as the β-glucan synthases Bgs1p and Bgs4p (15, 16, 30), were analyzed, and no differences were observed between wild-type and rho4Δ cells even at high temperature (Fig. 5C). Therefore, these results indicate that Rho4p specifically affects the localization of the glucanases involved in cell separation but not other septum proteins.
FIG. 5.
Eng1p and Agn1p localizations are defective in rho4Δ cells grown at high temperature. (A and B) Wild-type and rho4Δ cells expressing eng1-GFP (A) or agn1-GFP (B) from their own promoter were grown at 36°C and observed by fluorescence microscopy. Arrows point to rho4Δ cells lacking Eng1-GFP or Agn1-GFP at the septa. DIC, differential interference contrast. (C) Localization of Bgs1-GFP and Bgs4-GFP in rho4Δ cells grown at 37°C. Bar, 10 μm.
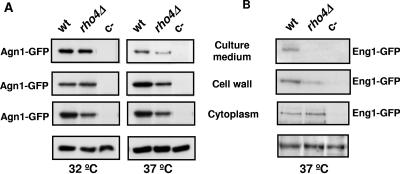
These observations were corroborated using Western blot analysis to measure Agn1-GFP and Eng1-GFP levels in cells grown at 37°C. Specific bands of both proteins were detected in cell extracts, cell walls, and concentrated culture medium. Comparison of Agn1-GFP protein levels in cytoplasm, cell walls, and concentrated culture media of wild-type and rho4Δ cells grown at 32°C or 37°C indicated that Agn1-GFP was slightly reduced in the cytoplasm at both temperatures. However, Agn1-GFP levels were reduced in the cell wall and the medium in rho4Δ cells grown at high temperature (Fig. 6A). Eng1-GFP was also reduced in the walls of rho4Δ cells and could not be detected in the culture medium, but no significant changes were found in the cytoplasmic fraction (Fig. 6B).
FIG. 6.
Agn1p and Eng1p levels are reduced in the cell walls and culture medium of rho4Δ cells grown at 37°C. Wild-type (wt) and rho4Δ cells containing agn1-GFP (A) or eng1-GFP (B) were analyzed by Western blotting. Proteins from cytoplasm, cell walls, and concentrated culture medium were separated in polyacrylamide gels containing sodium dodecyl sulfate. Gels were blotted and probed with monoclonal anti-GFP antibodies. Cells lacking the GFP tag were included as a negative control (c−). A 80-kDa specific band corresponding to Agn1-GFP and a 130-kDa specific band corresponding to Eng1-GFP are shown. Lower panels represent the loading control of the cytoplasm fraction, using tubulin in panel A or a nonspecific band in panel B.
Since Eng1p is expressed only during cytokinesis, we synchronized the cultures by nitrogen starvation in order to have most of the cells septating. Cells growing logarithmically were kept at 28°C or 37°C in medium without nitrogen for 16 h and then transferred to medium containing nitrogen for 4 h. When the first round of separation took place, we analyzed the presence of Eng1-GFP in the cytoplasm and the cell walls by Western blotting. Similar levels of Eng1-GFP were present in the cell walls of both wild-type and rho4Δ cells grown at 28°C; in contrast, Eng1p could not be detected in the walls of rho4Δ cells grown at 37°C (data not shown). A faint Eng1p-GFP band was also detected in cell extracts of the wild-type and rho4Δ synchronized cultures grown at 28°C and only in the cell extracts of rho4Δ cells grown at 37°C (data not shown). Thus, Rho4p is necessary for proper Eng1p and Agn1p localized secretion to the septum area during cytokinesis at 37°C.
DISCUSSION
Septum formation needs cell wall synthesis, and cell separation needs cell wall degradation. Enzymatic hydrolysis of the lateral cell wall and the primary septum is required to produce two independent daughter cells. The recent characterization of the endo-(1,3)-β-d-glucanase encoded by eng1+ indicates that it is responsible for the primary septum degradation (33). Another protein, named Eng2p, also exhibits endo-(1,3)-β-d-glucanase activity, but it does not seem to be involved in cell separation (33). There are also two genes coding for putative α-glucanases in the S. pombe genome, and at least one of them (agn1+) has recently been shown to be involved in cell separation, being required for degradation of the lateral cell wall around the primary septum (2, 17, 20). The proper secretion of those hydrolases during cell division is necessary for the process to be achieved successfully. In previous work, we demonstrated that Rho4p regulates wall degradation during cell division (43). The results shown in this work indicate that Rho4p participates in the proper secretion of Eng1p and Agn1p. First, overexpression of eng1+ or agn1+ was able to suppress the rho4Δ multiseptation phenotype, suggesting that rho4Δ cells have a defect in lytic enzymes that results in cell separation defects. Second, overexpression of eng1+ did not cause any lysis unless rho4+ was overexpressed simultaneously, suggesting that Eng1p requires an excess of Rho4p to cause lysis. Overexpression of agn1+ and rho4+ also causes drastic lysis, but in this case the interpretation is less clear because both display a lysis phenotype on their own. Interestingly, rho4+ overexpression in eng1Δ agn1Δ cells still produces 20% cell lysis in liquid medium, but the remaining surviving cells are not rounded (our unpublished results). This could be explained if other lytic enzymes, besides Eng1p and Agn1p, were regulated by Rho4p. Another possible explanation could be that the lytic effect could be due to possible interference with Rho1p regulators caused by the excess of Rho4p. Alternatively, the glucanases and Rho4p could act in two different additive pathways leading to cell lysis. However, results from subsequent experiments and detailed studies with the eng1Δ rho4Δ double mutant suggested a relationship between Rho4p and Eng1p. Third, in rho4Δ cells the intracellular β-glucanase activity was similar to that of wild-type cells, but the extracellular activity and the Eng1p and Agn1p protein levels were clearly reduced in the cell wall or the culture medium when cells were grown at 37°C. Together, these results therefore support the idea that Rho4p is not an activator of the glucanases but is specifically required for the secretion of Eng1p and Agn1p at high temperature. The effect of Rho4p on glucanases secretion is most probably specific, since other Rho proteins do not cause lysis when overproduced. Moreover, other Rho proteins, except Rho3p, do not have phenotypes especially related to septation. rho3+ deletion causes a general secretion defect, and, in that sense, it most probably affect the Eng1 secretion.
Eng1p and Agn1p are secreted proteins. It has been proposed that the exocyst is essential in S. pombe for the delivery of proteins that are important for cell cleavage, including hydrolytic enzymes. Mutants with mutations in the exocyst accumulate 100-nm vesicles, and the glucanases could be one of the components transported in such vesicles.
The polarized localization of the exocyst in budding yeast is controlled by some members of the Rho family, such as Cdc42p, Rho1p, Rho3p, or Rho4p (29). In S. pombe, only Rho3p has been described to modulate the exocyst function, controlling the fusion of secretory vesicles to specific sites on the cell surface (39). rho4Δ cells, similar to cells having a defective exocyst complex, accumulate vesicles in the septum area (43, 48). One possibility is that besides Rho3p, Rho4p is also required for the exocyst function, especially at high temperature, when the cells grow faster and regulation of secretion seems to be more crucial. Preliminary results indicate that the Rho4p GTPase is required for the proper function of the exocyst at high temperature (our unpublished results). Interestingly, Rho4p is not required for the secretion of a well-known secreted protein, the acid phosphatase (43), in contrast to the case for exocyst mutants (48); it is possible that Rho4p will be more related to a precise polarized secretion during cell separation. That could explain why Eng1p secretion it is clearly more affected than that of Agn1p, since Eng1p participates specifically in the digestion of the primary septum, whereas Agn1p is involved in the digestion of the α-glucan present not only in the septum but also in the lateral cell wall.
Besides Eng1p and Agn1p, Rho4p might participate in the secretion of other lytic enzymes. Further studies will address this issue.
Acknowledgments
J. C. Ribas, Henar Valdivieso, and Pedro San Segundo provided critical comments on the manuscript. We thank D. Posner for reviewing the English. We thank Elvira Portales for technical help.
A.B.M.-C. was supported by a fellowship from the Spanish Ministerio de Educación. This work was supported by grants BIO-2001-1531 and BIO-2001-2041 from the Comisión Interministerial de Ciencia y Tecnología, Spain, and SA026/02 from Junta de Castilla y León.
REFERENCES
- 1.Adamo, J. E., J. J. Moskow, A. S. Gladfelter, D. Viterbo, D. J. Lew, and P. J. Brennwald. 2001. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155:581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Nuñez, M. L., H. An, A. B. Martin-Cuadrado, S. Mehta, C. Petit, M. Sipiczki, F. del Rey, K. L. Gould, and C. R. Vázquez de Aldana. 2005. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 16:2003-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arellano, M., P. M. Coll, and P. Pérez. 1999. Rho GTPases in the control of cell morphology, cell polarity, and actin localization in fission yeast. Microsc. Res. Technol. 47:51-60. [DOI] [PubMed] [Google Scholar]
- 4.Arellano, M., M. H. Valdivieso, T. M. Calonge, P. M. Coll, A. Durán, and P. Pérez. 1999. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 112:3569-3578. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. Jonh Wiley and Sons, New York, N.Y.
- 6.Bahler, J. 2005. A transcriptional pathway for cell separation in fission yeast. Cell Cycle 4:39-41. [DOI] [PubMed] [Google Scholar]
- 7.Baladrón, V., S. Ufano, E. Dueñas, A. B. Martín-Cuadrado, F. del Rey, and C. R. Vázquez de Aldana. 2002. Eng1p, an endo-1,3-beta-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot. Cell 1:774-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasubramanian, M. K., E. Bi, and M. Glotzer. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14:R806-R818. [DOI] [PubMed] [Google Scholar]
- 9.Berlin, A., A. Paoletti, and F. Chang. 2003. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burridge, K., and K. Wennerberg. 2004. Rho and Rac take center stage. Cell 116:167-179. [DOI] [PubMed] [Google Scholar]
- 11.Cabib, E., J. Drgonova, and T. Drgon. 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67:307-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calonge, T. M., M. Arellano, P. M. Coll, and P. Pérez. 2003. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity in Schizosaccharomyces pombe. Mol. Microbiol. 47:507-518. [DOI] [PubMed] [Google Scholar]
- 13.Calonge, T. M., K. Nakano, M. Arellano, R. Arai, S. Katayama, T. Toda, I. Mabuchi, and P. Pérez. 2000. Schizosaccharomyces pombe Rho2 GTPase regulates the cell wall α-glucan biosynthesis, through the protein kinase Pck2p. Mol. Biol. Cell. 11:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, F. 2001. Studies in fission yeast on mechanisms of cell division site placement. Cell Struct. Funct. 26:539-544. [DOI] [PubMed] [Google Scholar]
- 15.Cortés, J. C., E. Carnero, J. Ishiguro, Y. Sánchez, A. Durán, and J. C. Ribas. 2005. The novel fission yeast (1,3)beta-d-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 118:157-174. [DOI] [PubMed] [Google Scholar]
- 16.Cortés, J. C., J. Ishiguro, A. Duran, and J. C. Ribas. 2002. Localization of the (1,3)beta-d-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115:4081-4096. [DOI] [PubMed] [Google Scholar]
- 17.Dekker, N., D. Speijer, C. H. Grun, M. van den Berg, A. de Haan, and F. Hochstenbach. 2004. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell. 15:3903-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feierbach, B., and F. Chang. 2001. Cytokinesis and the contractile ring in fission yeast. Curr. Opin. Microbiol. 4:713-719. [DOI] [PubMed] [Google Scholar]
- 19.Forsburg, S. L. 1993. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21:2955-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García, I., D. Jiménez, V. Martín, A. Durán, and Y. Sánchez. 2005. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell 97:569-576. [DOI] [PubMed] [Google Scholar]
- 21.Guertin, D. A., S. Trautmann, and D. McCollum. 2002. Cytokinesis in eukaryotes. Microbiol. Mol. Biol Rev. 66:155-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbel, B. M., M. Konomi, T. Takagi, N. Kamasawa, S. A. Ishijima, and M. Osumi. 2001. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18:433-444. [DOI] [PubMed] [Google Scholar]
- 23.Ishiguro, J., A. Saitou, A. Duran, and J. C. Ribas. 1997. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J. Bacteriol. 179:7653-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, Q. W., and D. McCollum. 2003. Scw1p antagonizes the septation initiation network to regulate septum formation and cell separation in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 2:510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannis, J., R. Oulton, and P. G. Young. 2002. The Scw1 RNA-binding domain protein regulates septation and cell-wall structure in fission yeast. Genetics 162:45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krapp, A., M. P. Gulli, and V. Simanis. 2004. SIN and the art of splitting the fission yeast cell. Curr. Biol. 14:R722-R730. [DOI] [PubMed] [Google Scholar]
- 28.Le Goff, X., A. Woollard, and V. Simanis. 1999. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 262:163-172. [DOI] [PubMed] [Google Scholar]
- 29.Lipschutz, J. H., and K. E. Mostov. 2002. Exocytosis: the many masters of the exocyst. Curr. Biol. 12:R212-R214. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., X. Tang, H. Wang, S. Oliferenko, and M. K. Balasubramanian. 2002. The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell 13:989-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., H. Wang, D. McCollum, and M. K. Balasubramanian. 1999. Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 153:1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longtine, M. S., D. J. DeMarini, M. L. Valencik, O. S. Al-Awar, H. Fares, C. De Virgilio, and J. R. Pringle. 1996. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8:106-119. [DOI] [PubMed] [Google Scholar]
- 33.Martín-Cuadrado, A. B., E. Dueñas, M. Sipiczki, C. R. Vázquez de Aldana, and F. del Rey. 2003. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 116:1689-1698. [DOI] [PubMed] [Google Scholar]
- 34.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizasaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 35.Mulvihill, D. P., and J. S. Hyams. 2003. Myosin-cell wall interactions during cytokinesis in fission yeast: a framework for understanding plant cytokinesis? Cell Biol. Int. 27:239-240. [DOI] [PubMed] [Google Scholar]
- 36.Nakano, K., J. Imai, R. Arai, E. A. Toh, Y. Matsui, and I. Mabuchi. 2002. The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 115:4629-4639. [DOI] [PubMed] [Google Scholar]
- 37.Nakano, K., T. Mutoh, R. Arai, and I. Mabuchi. 2003. The small GTPase Rho4 is involved in controlling cell morphology and septation in fission yeast. Genes Cells 8:357-370. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, M. J. 1957. Colorimetric analysis of sugars. Methods Enzymol. 3:85-86. [Google Scholar]
- 39.Reinhard, M., K. Giehl, K. bel, C. Haffner, T. Jarchau, V. Hoppe, B. M. Jockusch, and U. Walter. 1995. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 14:1583-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribar, B., A. Grallert, E. Olah, and Z. Szallasi. 1999. Deletion of the sep1(+) forkhead transcription factor homologue is not lethal but causes hyphal growth in Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 263:465-474. [DOI] [PubMed] [Google Scholar]
- 41.Rustici, G., J. Mata, K. Kivinen, P. Lio, C. J. Penkett, G. Burns, J. Hayles, A. Brazma, P. Nurse, and J. Bahler. 2004. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 36:809-817. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Santos, B., J. Gutiérrez, T. M. Calonge, and P. Pérez. 2003. Novel Rho GTPase involved in cytokinesis and cell wall integrity in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 2:521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Somogyi, H. 1952. Notes on sugar determination. J. Biol. Chem. 195:19-23. [PubMed] [Google Scholar]
- 44.Takai, Y., T. Sasaki, Tanaka, and T. Matozaki. 2001. Small GTP-binding proteins. Phys. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 45.Tasto, J. J., J. L. Morrell, and K. L. Gould. 2003. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toda, T., S. Dhut, G. Superti-Furga, Y. Gotoh, E. Nishida, R. Sugiura, and T. Kuno. 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16: 6752-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, H., X. Tang, and M. K. Balasubramanian. 2003. Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 164:1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, H., X. Tang, J. Liu, S. Trautmann, D. Balasundaram, D. McCollum, and M. K. Balasubramanian. 2002. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13:515-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfe, B. A., and K. L. Gould. 2005. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 15:10-18. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body. J. Cell Sci. 107:1725-1735. [DOI] [PubMed] [Google Scholar]