Abstract
Formins are downstream effector proteins of Rho-type GTPases and are involved in the organization of the actin cytoskeleton and actin cable assembly at sites of polarized cell growth. Here we show using in vivo time-lapse microscopy that deletion of the Candida albicans formin homolog BNI1 results in polarity defects during yeast growth and hyphal stages. Deletion of the second C. albicans formin, BNR1, resulted in elongated yeast cells with cell separation defects but did not interfere with the ability of bnr1 cells to initiate and maintain polarized hyphal growth. Yeast bni1 cells were swollen, showed an increased random budding pattern, and had a severe defect in cytokinesis, with enlarged bud necks. Induction of hyphal development in bni1 cells resulted in germ tube formation but was halted at the step of polarity maintenance. Bni1-green fluorescent protein is found persistently at the hyphal tip and colocalizes with a structure resembling the Spitzenkörper of true filamentous fungi. Introduction of constitutively active ras1G13V in the bni1 strain or addition of cyclic AMP to the growth medium did not bypass bni1 hyphal growth defects. Similarly, these agents were not able to suppress hyphal growth defects in the wal1 mutant which is lacking the Wiskott-Aldrich syndrome protein (WASP) homolog. These results suggest that the maintenance of polarized hyphal growth in C. albicans requires coordinated regulation of two actin cytoskeletal pathways, including formin-mediated secretion and WASP-dependent endocytosis.
Cell polarity establishment and maintenance of polarized secretion are essential for morphogenesis and development (10). Cell polarization is required in neuronal cells to establish a growth cone that maintains a polarized growth direction in response to extracellular stimuli (9, 20). Cell polarization is also required for epithelium formation and in migrating cells. Similarly, in plant cells, establishment and maintenance of cell polarity are used during root hair or pollen tube growth (31). Both actin and microtubule cytoskeletons play important roles in maintaining cell polarization and in providing cellular tracks for vesicle delivery. This requires complex processes of spatial and temporal coordination of protein localization and activation at sites of polarized growth (32).
The actin cytoskeleton is involved in three basic structures: actin patches, actin cables, and the cytokinetic ring at sites of cell cleavage in animal and fungal cells. Actin patches are found at sites of endocytosis, actin cables provide tracks for vesicle delivery, and dynamic constriction of the actin ring is required for cytokinesis (32).
Rho-type GTPases, such as Cdc42, are known regulators of the actin cytoskeleton in that they activate downstream effector proteins (11). Two major classes of conserved effector protein families are the Wiskott-Aldrich syndrome proteins (WASPs) and the formins. WASP-like proteins are involved in endocytosis and play a role in Arp2/3-dependent actin assembly (7, 26, 43). The C. albicans WASP homolog, Wal1, was shown to be required for endocytosis and vacuolar morphology as well as polarized hyphal growth (40). Formins represent the conserved family of Diaphanous-related proteins that control the assembly of actin cables (33, 34). Formins assemble linear actin cables in an Arp2/3 complex-independent manner (12, 13, 33, 34).
The sole Aspergillus nidulans formin, SEPA, is so far the only formin analyzed in filamentous fungi. SEPA localizes to sites of polarized growth both at the hyphal tip and at septal sites. The tip localization resembles the position of a structure termed the Spitzenkörper (17). In Saccharomyces cerevisiae the formin Bni1 colocalizes with Spa2, Bud6, and Pea2 at sites of polarized growth and forms a complex termed the polarisome (15, 36). In C. albicans, SPA2 has been analyzed recently and was found to localize to the tips of growing hyphae. Consistently, deletion of SPA2 resulted in polarity and hyphal growth defects (44).
In this study, we exploit the human fungal pathogen Candida albicans as a model to understand the role and contribution of formins in the regulation of polarized morphogenesis. In C. albicans, the yeast-to-hyphal transition contributes to its virulence and allows the penetration of epithelia and the evasion of the host cellular immune response (6). The genetic basis of morphogenetic switching in C. albicans relies on the activation of the Ras1-GTPase by extracellular signals which induce two downstream signal cascades: a mitogen-activated protein (MAP) kinase pathway and the cyclic AMP (cAMP) pathway. Both activate transcriptional regulators, Cph1 and Efg1, respectively, that induce hypha-specific gene expression (4). Deletions of CPH1 and EFG1 or a single deletion of both RAS1 alleles yields viable mutant strains that are nonfilamentous under most conditions and avirulent in animal models (14, 25). On the other hand, constitutive activation of RAS1, using a ras1G13V allele, enhances filamentous growth (15). In this study, we investigate the role of the C. albicans formins as part of a potential Spitzenkörper complex and present a model including the actin cytoskeleton machinery in the regulatory network that establishes hyphal morphogenesis in C. albicans.
MATERIALS AND METHODS
Strains and media.
C. albicans strains used in this study are listed in Table 1. Media and the lithium acetate transformation procedure were used as described previously (39, 40).
TABLE 1.
Strains used in this study
| Straina | Genotype | Reference |
|---|---|---|
| SC5314 | Candida albicans wild type | 16 |
| BWP17 | ura3::limm34/ura3::llimm34 arg4::hisG/arg4::hisG his1::hisG/his1::hisG | 42 |
| CAT4 | WAL1/wal1::HIS1 | 40 |
| CAT6 | wal1::HIS1/wal1::URA3 | 40 |
| CAT37 | wal1::HIS1/wal1::SAT1 | This study |
| CAT40 | wal1::HIS1/wal1::SAT1ADE2/ade2::MAL2p-ras1G13V::URA3 | This study |
| GC11 | BNI1/bni1::URA3 | This study |
| GC13 | BNR1/bnr1::URA3 | This study |
| GC14 | bni1::URA3/bni1::HIS1 | This study |
| GC19 | bnr1::URA3/bnr1::HIS1 | This study |
| GC33 | MAL2p-BNI1::HIS1/bni1::URA3 | This study |
| GC40 | bni1::HIS1/bni1::ARG4 | This study |
| GC42 | ADE2/ade2::MAL2p-ras1G13V::URA3 | This study |
| GC46 | bni1::HIS1/bni1::ARG4 ADE2/ade2::MAL2p-ras1G13V::URA3 | This study |
| GC59 | BNI1/BNI1-GFP::URA3 | This study |
All strains whose names begin with GC and CAT are derivates of BWP17 but have the indicated genotypic alterations.
Targeting of C. albicans genes.
The C. albicans homologs of the Saccharomyces cerevisiae formins BNI1 and BNR1 were identified in the C. albicans genomic sequence (http://www-sequence.stanford.edu/group/candida). Deletions of the complete open reading frames (ORFs) of both alleles of C. albicans BNI1 (CaBNI1) or BNR1 were performed by PCR-generated URA3 and HIS1 disruption cassettes containing 100 bp of a target homology region at both ends of the cassettes as described previously (18). Similarly, regulatable expression of BNI1 under the control of the CaMAL2 promoter was achieved by using amplified HIS1-MAL2p cassettes in the transformation of a BNI1/bni1 strain so that the endogenous promoter of the only remaining copy of BNI1 is replaced with the MAL2 promoter. To reconstruct strains generating new marker combinations in the absence of a sexual cycle, a homozygous strain (in this case the bni1::HIS1/bni1::URA3 strain) was used and transformed with PCR-amplified cassettes derived from the pFA-ARG4 plasmid. Upon double selection for histidine and arginine prototrophy, transformants whose URA3 marker was replaced with the ARG4 marker gene were selected. Either this method or use of a suitable heterozygous strain can result in generating the desired marker combinations. To increase the arsenal of available marker genes, we generated a pFA-SAT1 plasmid which contains the SAT1 gene (kindly provided by Joachim Morschhäuser). Its use will be described in detail in an upcoming update on pFA modules.
In order to fuse BNI1 with green fluorescent protein (GFP), a fusion cassette was cloned. To this end, a PCR fragment was amplified from genomic DNA which contains the 3′ end of BNI1. The in vivo recombination machinery of Saccharomyces cerevisiae was used to fuse the end of the BNI1 ORF with GFP. To this end, a GFP-URA3 PCR cassette was amplified from the standard set of modules. This cassette contains C. albicans URA3, which is also functional in S. cerevisiae. Gene targeting in S. cerevisiae requires shorter homology regions that flank the target locus (45 bp were used in our case). This detour via S. cerevisiae was used to generate a cassette and to verify the correct in-frame fusion after plasmid recovery from yeast and amplification in Escherichia coli by sequencing. This cassette was excised from the plasmid backbone and used for the transformation of BWP17, yielding strain GC59.
The constitutively active ras1G13V allele was used as described previously, which resulted in targeted integration of the CaMAL2 promoter-driven alleles at the C. albicans ADE2 locus (14).
Primers used for the construction of cassettes and the verification of deletions are listed in Table 2 or were described previously (40). Disruptions were verified by PCR of whole yeast cells.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea |
|---|---|
| 392 XFP | GAGTGCCATGCCCGAAGGTTATG |
| 599 U3 | GGAGTTGGATTAGATGATAAAGGTGATGG |
| 600 U2 | GTGTTACGAATCAATGGCACTACAGC |
| 601 H2 | CAACGAAATGGCCTCCCCTACCACAG |
| 602 H3 | GGACGAATTGAAGAAAGCTGGTGCAACCG |
| 797 S1-BNI1 | ATCACAAACATCTTTTCATCATCCACTACTATCACCAGTGCTAGTGCAGTTTCAATTAATTACCATATTCTTCAACCTCATCCTCCCTTACCTCCTCCCTgaagcttcgtacgctgcaggtc |
| 798 S2-BNI1 | CTTAACTTGTAAACTCACATGGTATATAATATGTAAATACAACTTTGTACAGATATAAAAATTAATAGCTATATTTCATTTAAATACATATACAAAAAAAAtctgatatcatcgatgaattcgag |
| 799 G1-BNI1 | CTCTCTGAGGGAGACAACGC |
| 800 G4-BNI1 | CAGGTTTCCAAATTGAATCGTCC |
| 801 G4-BNI1-MAL2p | GCACCCTTGTCGTATGGAC |
| 802 G1-BNI1-FP | CCGCCTGAAGGATGCTG |
| 804 S2-BNI1-MAL2p | GAGTTGAGTTCTGACTTGCTGATGATAATATACTAGCTGAGTCATTGGAAGACATGTCGGACACACTGTCGGTGTTGTGCTTGTCT TTATGTCGTCTCCTcattgtagttgattattagttaaaccac |
| 805 S1-BNR1 | GTTGTTTTTTTTTTTCAAACGCGACTTCTAAATCTCTAGCCAT ACCCGATCCAGAAACACTTGTTTTAAATTTTTTTGGTTACCACACACACAAAAATATTCgaagcttcgtacgctgcaggtc |
| 806 S2-BNR1 | AGAAAGTGAAAAAAAAGAAAAAAGAAAAAAAAAAAATAGTTGTTCTTTTTTAAGGAAGAGCATCACAAAATTTTTTAGACGTGTAT ATGCAGTATCGGTGTAGtctgatatcatcgatgaattcgag |
| 811 G1-BNR1 | GTAAGCACCGAGTCTTGTCGC |
| 812 G4-BNR1 | GGAAATTTCTACTCAACGAGCG |
| 1088 I1-BNI1 | GGAAATCAAGAACCAGAGCCTTG |
| 1089 I2-BNI1 | CTCTTGGCAAAGCCGGCAACAC |
| 1090 I1-BNR1 | GAGATAGATTCCAGGAACACGAG |
| 1091 I2-BNR1 | CACCAATGCCTTGACGACGTACAC |
| 1203 CaBNI1 | CGCGGATCCGCGGGCTCACCAACTAATGTCTCACC |
| 1204 CaBNI1 | TGCTCTAGAGCACGACTCTATTTATGATGACGAAGATGAAG |
| 1242 ADE2-down | GGTCGTATGATTGTTGAAGCAGCAC |
| 1243 ADE2-up | CCAGAGTTGTGAGGTCTTGGTGC |
| 1244 RAS1-down | GGAAAGACAAGTTAGTTATCAAGATGG |
| 1268 S1-BNI1-GFP | GTTCAAATAGATCTTGATGAAGTGGCTAAGAATAACAATAGTGAGggtgctggcgcaggtgcttc |
| 1269 S2-BNI1-GFP | CTCGAATTCATCGATGATATCAGAGGCCTGCTAAGGAGAAGCACTtttttttgtatatgtatttaaatg |
Uppercase sequences correspond to C. albicans genomic DNA. Lowercase sequences correspond to 3′-terminal annealing regions for the amplification of transformation cassettes. Bold letters indicate restriction sites used for cloning. All sequences are written from 5′ to 3′.
Hyphal induction of C. albicans.
Different protocols were used to induce hyphal formation in C. albicans strains at 37°C. On solid plates, hyphal induction was done either on Spider medium (24) or on medium containing 10% serum (calf serum; Sigma). Plates were incubated for 4 to 7 days prior to photography. In liquid culture, the addition of 10 mM cAMP was used besides serum to induce filament formation.
Staining procedures.
Chitin staining was done by directly adding 1 μl calcofluor (1 mg/ml) to a 100-μl cell suspension, followed by an incubation of 15 min at room temperature and a subsequent washing step to optimize the signal-to-noise ratio. Vacuolar staining of overnight cultures was done using the lipophilic dye FM4-64 (0.2 μg/ml). Cells were stained for 30 min at 30°C prior to photography. To monitor the uptake of FM4-64 by in vivo time-lapse microscopy, cells in the exponential growth phase were transferred to deep-well microscopy slides containing the dye in the culture medium. Imaging of the Spitzenkörper-like structure was done by staining cells induced for germ tube formation with FM4-64 immediately prior to microscopy. Staining of vacuoles in germ tubes was done by adding FM4-64 to the culture during hyphal induction, which was carried out for 3 h at 37°C in the dark in minimal medium containing 10% serum. Rhodamine-phalloidin staining of the actin cytoskeleton was done as described previously (29).
Time-lapse microscopy.
Strains were grown to exponential phase, harvested, washed, and resuspended in sterile water. Small aliquots of cells (1.5 μl) were applied to microscopy slides with deep wells as described previously (38). Temperature control was achieved via a heat stage mounted on the microscope table. Microscopy was performed on an automated Zeiss Axioplan II imaging microscope. Image acquisition using Metamorph software (Universal Imaging Corporation) and movie processing were done as described previously (40). Movies are posted at the corresponding author's homepage at http://pinguin.biologie.uni-jena.de/phytopathologie/pathogenepilze/index.html.
Sequence data for Candida albicans was obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida.
RESULTS
Functional analysis of C. albicans formins.
Candida formins were identified in the C. albicans genome sequence using the Saccharomyces cerevisiae Bni1 protein. Two formins were found: CaBNI1 and CaBNR1 of 1,732 and 1,485 amino acids in length, respectively. CaBNI1 is annotated as orf19.4927 and CaBNR1 as orf19.7537. Both formins correspond in their protein structure to A. nidulans SEPA and other formins in that they possess an N-terminal G protein binding domain, followed by a formin homology 3 (FH3) domain and a C-terminal FH1-FH2-Diaphanous autoinhibitory domain (DAD) involved in actin filament assembly and regulation of formin activity by autoinhibition (30). The highest sequence identity of more than 50% between C. albicans Bni1 and the S. cerevisiae Bni1 is found in the C termini, including the FH2 domains, while the N termini are less well conserved and show less than 30% identity on the amino acids level. The presence of conserved residues of the G protein binding domain and DAD indicates that regulatory mechanisms similar to those that have been described for other systems may apply in C. albicans, particularly the direct binding to a Rho-type GTPase.
To investigate the role of both C. albicans formins during yeast and hyphal growth, we constructed mutant strains in which the complete ORFs of both alleles of BNI1 or BNR1 were sequentially deleted using PCR-based gene targeting methods (18). Homozygous mutant strains were generated from independent heterozygous strains for each formin and were phenotypically identical, indicating that correct gene targeting had occurred, as was verified by colony PCR. The absence of the formin ORFs in the homozygous but not in the heterozygous mutant strains was shown using internal primers. Additionally, to provide further proof that the observed phenotypes described below were solely due to the disruption of BNI1, a heterozygous BNI1/bni1 mutant strain was used to place the only remaining copy of the target gene under the control of the regulatable CaMAL2 promoter. This generated strains with wild-type-like phenotypes, including the ability to filament under permissive conditions during growth on maltose, while a shutdown of the MAL2 promoter during growth on glucose restored mutant phenotypes (not shown).
Polarized growth defects of formin mutants during the yeast growth phase.
Cell cycle lengths of the wild type and the bni1 and bnr1 strains were determined using growth curves generated with cultures grown in liquid rich medium at 30°C and using in vivo time-lapse microscopy recordings calculating as the average cell cycle duration the time required between two consecutive bud emergence events. This revealed prolonged cell cycle durations of about 20 min for both formin mutants compared to that for the wild type (Table 3). Wild-type cells are characteristically ellipsoidal and show a length-versus-width ratio of approximately 1.3. During growth on solid media, cytokinesis in the wild type is most obvious by a rotational movement of mother and daughter cells out of the mother-bud axis (Fig. 1A). Heterozygous mutants with either BNI1/bni1 or BNR1/bnr1 were phenotypically like the wild type, suggesting the absence of gene dosage effects.
TABLE 3.
Yeast phase growth characteristicsa
| Parameter | Wild-type SC5314 | GC14 (bni1/bni1) | GC19 (bnr1/bnr1) |
|---|---|---|---|
| Cell cycle duration (min) | 71 ± 7 | 92 ± 15 | 99 ± 14 |
| Cell length (μm) (n = 500) | 6.0 ± 0.5 | 6.4 ± 0.6 | 6.7 ± 0.5 |
| Cell width (μm) (n = 500) | 4.6 ± 0.7 | 5.7 ± 0.4 | 3.9 ± 0.4 |
| Bud scar diameter (μm) (n = 200) | 1.5 ± 0.3 | 2.4 ± 0.9 | 1.5 ± 0.4 |
Values are means ± standard deviations.
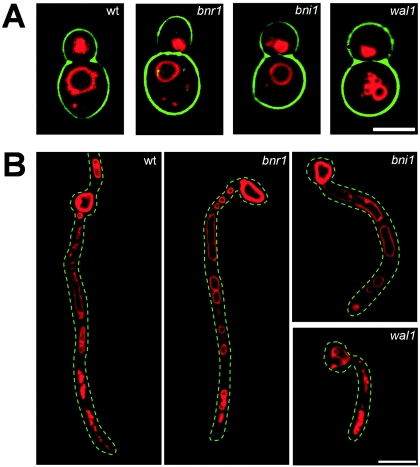
FIG. 1.
Growth defects of C. albicans formin mutants during yeast growth. Growth of the wild type and formin mutant strains was monitored using time-lapse microscopy over several hours (timescale is in hours:minutes) (A-C). In the movie of the wild type at http://pinguin.biologie.uni-jena.de/phytopathologie/pathogenepilze/index.html, a characteristic change of cell axes after cytokinesis can be observed (previous mother-bud axes are indicated as white, dotted lines and newly established axes as black, dashed lines in panels A to C). This results in lateral movement of cells such that wild-type colonies form a single cell layer (A). In the bni1 mutant, growth was irregular and cells were dispatched in three dimensions. A shift of mother-bud axes occurs but often only due to mechanical forces generated by new buds. The arrows denote enlarged septal sites (B). In the bnr1 mutant, growth axes are kept over several cell cycles, resulting in a linear array of mother and daughter cells (C). Cell shape and budding pattern of the indicated strains were analyzed after staining the cells with calcofluor (D). wt, wild type. Scale bar, 5 μm.
Bud emergence in bni1 cells was not inhibited. However, newly formed buds showed drastic defects in polarized growth. This resulted in the formation of swollen and rounded yeast cells with a length/width index of 1.1, resembling mutant wal1 cells bearing a deletion of the WASP homolog WAL1. Strong defects were observed at the bud neck, which was broadened and revealed enlarged septa (Table 3). Separation of mother and daughter cells did not take place as readily as in the wild type. Instead mother and daughter cells were separated by pushing forces generated by newly formed buds during growth on solid media as observed during in vivo time-lapse recordings (Fig. 1B).
Characteristically, bnr1 cells were more elongated than the wild type, which resulted in a length/width ratio of 1.7. Cell separation defects of bnr1 cells were not as drastic as those seen in bni1 cells but still resulted in mother and daughter cells that were moved apart by colonial growth rather than by the cytokinesis of mother and daughter cells (Fig. 1C).
To determine if the swollen morphology of bni1 cells resulted also in an altered bud site selection pattern, cells were stained with calcofluor to visualize chitin-rich septal rings (Fig. 1D). In the wild type and in bnr1 cells grown at 30°C, a bipolar budding pattern was observed. In contrast, bni1 cells showed increased random budding resembling that of spa2 and wal1 cells described in previous studies (Fig. 1D) (40, 44).
C. albicans formin mutants do not show defects in endocytosis.
Since we found defects in endocytosis in wal1 mutant cells in a previous study, we wanted to determine any deficiencies in vacuolar morphology and transport to the vacuole in formin mutant strains. To this end, we analyzed the uptake of the lipophilic dye FM4-64 by in vivo fluorescence time-lapse microscopy. This revealed no vacuolar phenotypes in bni1 and bnr1 yeast cells compared to the drastic defects in the wal1 mutant, suggesting that formins are not involved in endocytosis or in determining the vacuolar morphology of yeast cells (Fig. 2A; movies M4 to M6 at http://pinguin.biologie.uni-jena.de/phytopathologie/pathogenepilze/index.html). For comparison of FM4-64 uptake under inducing conditions, we induced yeast cells of the wild type and the formin and wal1 mutant strains to form germ tubes in the presence of serum at 37°C and simultaneously stained these cells with FM4-64. In the wild type, dye uptake resulted in staining of the apical compartment. Smaller vesicles in apical regions—sometimes at the hyphal apex—were followed by larger vacuoles in subapical compartments. A similar distribution of endosomes and vacuoles was found in the bni1 and bnr1 mutant strains (Fig. 2B). In contrast, defects in endocytosis were observed in induced wal1 cells. Here, we found that dye uptake revealed an accumulation of vesicles at the tip of the germ tubes, indicating defects in the transport or fusion of vesicles.
FIG. 2.
Analysis of endocytosis. Endocytosis and the vacuolar morphology of the indicated strains were analyzed by monitoring the uptake of FM4-64 during the yeast stage (A) and hyphal stage (B). In panel A, images of yeast cells that were grown overnight and subsequently stained with FM4-64 are shown. The wild type (wt) and formin mutants generate a single large vacuole. In contrast, the wal1 cells exhibit fragmented vacuoles. Movies 4 to 6 at the website http://pinguin.biologie.uni-jena.de/phytopathologie/pathogenepilze/index.html show the time course of uptake of FM4-64. In panel B, images of FM4-64-stained cells induced to form germ tubes (3 h at 37°C in the presence of serum) are shown. Wild-type and formin mutant germ tubes show the characteristic larger vacuoles in their terminal regions, while the wal1 mutant displays fragmented vacuoles throughout the initial germ tube. Scale bars, 5 μm (A) and 10 μm (B).
The C. albicans formin Bni1 is required for the maintenance of polarized hyphal growth.
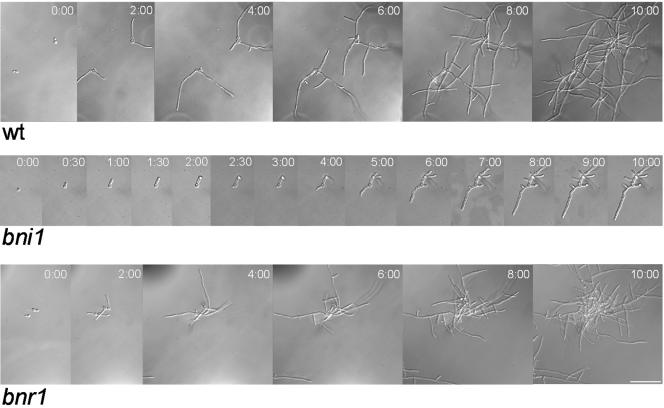
To analyze growth defects during the hyphal growth phase in formin mutants, germ tube formation was initiated under different inducing conditions. First, we used in vivo time-lapse microscopy and followed germ tube induction and polarized hyphal growth of the wild type and the formin mutant strains (Fig. 3; movies M7 to M9). The wild type and also the bnr1 strain responded to serum as a hypha-inducing cue, with vigorous filamentation and the development of mycelia after several hours of growth. In contrast, bni1 cells initiated germ tube formation but generated swollen germ tubes with widened diameters. These hyphal tubes were not able to maintain polarized cell growth, did not generate fast-growing hyphal filaments and lateral branches, and were thus not able to develop into mycelia. Frequently, constrictions were observed at septal sites in otherwise enlarged and swollen cells.
FIG. 3.
Time-lapse analysis of the hyphal induction of formin mutants. In vivo time-lapse microscopy was used to monitor the initial phases of germ tube induction in the wild-type (wt), the bni1, and the bnr1 strains on solid media containing serum at 37°C (for complete movies, see movies M7 to -9 at http://pinguin.biologie.uni-jena.de/phytopathologie/pathogenepilze/index.html). Images from these movies acquired at the indicated time points are shown.
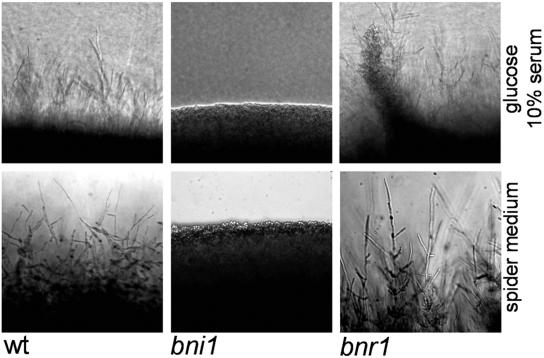
Growth on solid-medium plates containing serum or on Spider plates revealed abundant filamentation at the edges of wild-type and bnr1 colonies, while bni1 colonies showed smooth edges generated by yeast cell growth, supporting our time-lapse data and indicating that even after prolonged incubation, filamentation is crippled in bni1 strains (Fig. 4).
FIG. 4.
Mycelial growth defects of the bni1 mutant. The indicated strains were grown for 4 days at 37°C on plates containing either 10% serum or Spider medium prior to photography. Representative images of the edges of colony sectors which demonstrate the mycelial growth defects of the bni1 mutant are shown. wt, wild type.
Analysis of the actin cytoskeleton in formin mutants.
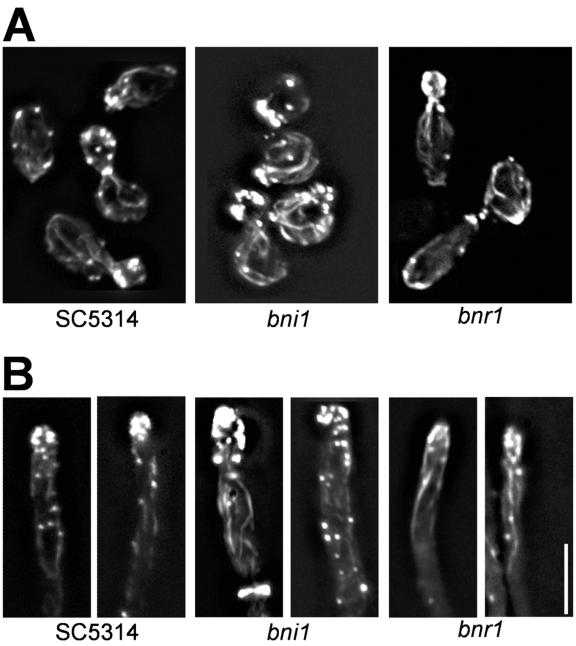
Distribution of actin patches and cables was analyzed to determine if the morphological defects observed can be attributed to a disorganization of the actin cytoskeleton (Fig. 5). During wild-type yeast growth, cortical actin patches accumulate in the growing bud and actin cables are found oriented at the mother-bud axis. This was also observed in the formin mutant strains, although some partial delocalization of actin patches in bni1 cells was found, resulting in patch positioning in the mother instead of a total accumulation of patches in the bud. This partial delocalization besides the prolonged cell cycle and the cytokinesis defect may explain the cell shape defects in bni1 cells. In bnr1 mutant cells, elongated buds showed clustering of cortical actin patches at the broadened tip region (Fig. 5A). During germ tube induction, the actin cytoskeleton in the wild type is strongly polarized towards the hyphal tip as in true filamentous fungi. Such an organization of the actin cytoskeleton was also found in the formin mutants (Fig. 5B). This was expected in bnr1 hyphae since hyphal development is not blocked in this mutant. However, in the bni1 germ tubes, given their morphological defects, it was rather unexpected to find such a clearly polarized arrangement of the actin cytoskeleton. Since cortical actin patches are sites of endocytosis, Bni1 may not be involved in the correct positioning of patches.
FIG. 5.
Analysis of the actin cytoskeleton. Images of rhodamine-phalloidin-stained cells of the indicated strains are shown. Cells were grown overnight in yeast extract-peptone-dextrose (YPD) at 30°C, inoculated into fresh YPD (A) or YPD plus 10% serum (B), and grown for 3 hours at 30°C (A) or 37°C (B) prior to fixation and staining. Bar, 10 μm.
Subcellular localization of Bni1-GFP during hyphal growth.
In S. cerevisiae, Bni1 forms a polarisome complex with Spa2 and Bud6 (36). Since the Spa2 homolog in C. albicans was localized to the tips of growing hyphae, we wanted to determine the subcellular localization of Bni1. To this end, a C-terminal fusion of GFP to Bni1 was employed in a heterozygous background (Fig. 6A). Throughout polarized hyphal growth, Bni1-GFP was found to localize to the hyphal tip as a punctate spot. However, the label was too weak to generate prolonged time-lapse series. Interestingly, during our studies on the uptake of FM4-64, we found that very short incubation periods of germ tubes or hyphae with the dye resulted in a spot-like staining at the hyphal tip. Staining of Bni1-GFP-expressing hyphae with FM4-64 showed that both spots colocalize (Fig. 6A). This suggests that in C. albicans hyphae, a structure is present that resembles the Spitzenkörper of true filamentous fungi, which on the molecular level can be described by the localization of Bni1 (17, 19). Such a Spitzenkörper was not found in yeast-like cells and thus represents a cellular marker that distinguishes the different cell types in C. albicans. Bni1-GFP was found to simultaneously localize to the hyphal tip as well as to a site of future septation in C. albicans (Fig. 6B).
FIG. 6.
Localization of Bni1-GFP and the Spitzenkörper. (A) A BNI1-GFP-expressing strain (BNI1/BNI1-GFP) was induced with serum and stained with FM4-64. GFP fluorescence and differential inference contrast (DIC) images were used in an overlay showing the colocalization of Bni1-GFP with the FM4-64-stained Spitzenkörper. (B) Simultaneous localization of Bni1-GFP to the hyphal tip and to a future septal site. Note that in the DIC image no septum is apparent. (C) Comparison of Spitzenkörper morphologies in the wild-type (wt) and bni1 strains using FM4-64. Images of FM4-64-stained germ tubes that were induced by serum at 37°C for 3 hours are shown. Bars, 5 μm.
Next, we determined if the Spitzenkörper is also present in bni1 germ tubes. In contrast to the wild type, which possesses a focused, point-like Spitzenkörper, in the bni1 germ tubes, the Spitzenkörper was much broader and enlarged, which indicates that the subcellular structure of the Spitzenkörper correlates with the hyphal diameter (Fig. 6C).
ras1G13V does not suppress the bni1 and wal1 phenotypes.
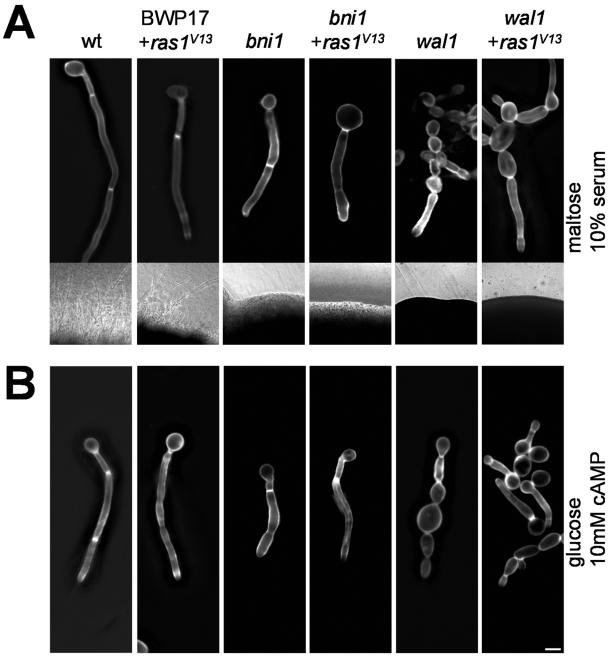
Maintenance of polarized hyphal growth, but not the initiation of germ tube formation, was found to be blocked in both the bni1 and wal1 germ tubes. Since morphogenetic switching depends on the activation of the Ras1-GTPase, we wanted to determine if constitutive activation of Ras1 could bypass the bni1 or wal1 defects in polarized hyphal growth (Fig. 7A). To this end, the homozygous bni1::HIS1/bni1::URA3 strain was used and its URA3 marker was exchanged for the ARG4 marker using a PCR-based approach. We then used an integrative cassette containing the URA3 selectable marker gene, which targets the ras1G13V allele under the control of the MAL2 promoter to the ADE2 locus and placed this cassette in the BWP17, bni1, and wal1 backgrounds (14). Under inducing conditions (37°C and 10% serum) in the presence of maltose as the carbon source, wild-type and BWP17 strains (with or without the activated ras1G13V allele) showed profuse filaments both in liquid culture and on solid media (Fig. 7A). In contrast, bni1 germ tubes were phenotypically identical to those of strains harboring the activated ras1G13V allele. Moreover, the constitutive ras1G13V allele in the bni1 mutant background did not result in filamentation on solid media (insets in Fig. 7A). This indicates that ras1G13V, which in the wild-type background induces filamentation even at 30°C in complete medium (not shown), is not able to bypass the bni1 defect. Similarly, the phenotype of the wal1 mutant, which was previously shown to be defective in maintaining filamentous growth, was also not suppressed by the constitutive ras1G13V allele. This suggests that both pathways, including polarisome function and secretion, as well as endocytosis, are required for a stable hyphal growth phase in C. albicans.
FIG. 7.
Germ tube induction in C. albicans strains harboring the ras1G13V allele. Overnight cultures of the indicated strains were grown in liquid medium containing maltose as the sole carbon source to allow for MAL2 promoter-driven expression of the ras1G13V allele when applicable. Strains were then induced for 3 h with either 10% serum (A) or 10 mM cAMP (B) at 37°C and stained with calcofluor prior to photography. The insets of panel A depict microscopic images of colony edges of the strains grown on solid-medium plates containing serum. Expression of the constitutive ras1G13V allele did not enable mycelial growth in the bni1 or wal1 mutant strains. wt, wild type.
Furthermore, as with the expression of the ras1G13V allele, addition of cAMP to the culture medium did not generate mycelial growth in either bni1 or wal1 cells but readily resulted in filamentation in the wild-type strain (Fig. 7B).
DISCUSSION
C. albicans can adopt a variety of morphologies; however, there are growth conditions ensuring stable yeast and filamentous-growth phases. The ability to induce hyphal growth in Candida allows its use as a model system to understand the underlying molecular mechanisms. One of the interesting questions is how a conserved basic protein network can be regulated differentially to achieve dimorphic growth in contrast to the continuous polarized growth of true filamentous fungi, for example, Neurospora crassa, Aspergillus nidulans, and Ashbya gossypii (5, 27, 41).
In true filamentous fungi, an apical body can be distinguished, that is, it is phase dark and forms at growing hyphal tips, and was found to be responsible for the growth direction of hyphal tips (1, 17, 19). This apical body, or Spitzenkörper, serves as the vesicle supply center in support of hyphal growth (2, 17). This Spitzenkörper can be stained by the lipophilic dye FM4-64, which stains endocytic vesicles (19). We have previously used this dye in the analysis of endocytosis in C. albicans. Deletion of the Wiskott-Aldrich syndrome protein C. albicans homolog WAL1 resulted in mutant strains that showed defects in the maintenance of polarized hyphal growth and were unable to form mycelia (40). Staining of yeast cells with FM4-64 did not reveal a prominent accumulation of vesicles but rather resulted in the delivery of the dye to and the visualization of vacuolar membranes (see also movies M4 to -6). Staining of germ tubes and hyphae resulted in almost immediate accumulation of the dye at the hyphal tip. This shows that C. albicans hyphae resemble in this respect hyphae of other filamentous ascomycetes. In a study by Crampin et al., a distinction based on molecular markers was drawn between polarisome components, for example, Cdc42, Spa2, and Bud6, and Spitzenkörper components, for example, Bni1 (8). In A. nidulans, the formin SEPA was shown to localize to the hyphal tip either as a crescent at the tip or as a spot near the tip, suggesting that SEPA is part of the Spitzenkörper (35). While in S. cerevisiae and in A. nidulans C-terminal tagging of formins appeared to result in functional fusion proteins, it appeared to be difficult to generate Bni1-GFP in C. albicans as the sole source of Bni1 (22). We as well as others found that Bni1-GFP colocalizes with the FM4-64-stained Spitzenkörper and in some cases simultaneously at the latest septal site as was also observed for SEPA in A. nidulans, suggesting mechanistic similarities (8, 22, 35). Very recently, Li et al. presented an analysis of BNI1 in C. albicans, which confirms our central findings of the role of Bni1 in the regulation of cell polarity. Their report shows that Bni1 is required for correct alignment and positioning of the mitotic spindle and that bni1 defects correlate with the mislocalization of the C. albicans Kar9 homolog (22).
In S. cerevisiae, Bni1 was shown to form a polarisome complex with Spa2, Bud6, and Pea2 (36). In C. albicans, Spa2 was localized in a manner similar to that of Bni1-GFP (44). This provides some further evidence for a conserved role of the polarisome in polarized hyphal growth. Interestingly, the C. albicans genome encodes a BUD6 homolog (orf19.5087) but not a PEA2 homolog (at least according to assembly 19), which suggests that species-specific regulation of the central morphogenetic protein network exists in fungal species.
Function of formins.
The function of formins in the assembly of linear actin filaments is conserved in nature and has elegantly been analyzed in several recent studies (12, 13, 33, 34). Our analysis of the single formin deletion mutants did not reveal any drastic phenotypes in the assembly of the cortical actin cytoskeleton in growing yeast cells or germ tubes. Double deletion of both formin genes, BNI1 and BNR1, in S. cerevisiae results in synthetic lethality (22, 37), suggesting overlapping functions of both formins. This may also suggest a surrogate mechanism in which Bnr1 partially takes over Bni1 functions in C. albicans (22). Since bnr1 strains are able to form mycelia, either Bnr1 does not contribute to polarized morphogenesis or its function can be taken over by Bni1. We found that in a bnr1 deletion strain, cells are more elongated than in the wild type. Therefore, Bnr1 may have some function in the redirection of polarized secretion during septation which is promoted at a time when Bni1 may not localize to the bud neck and thus cannot take over Bnr1 function. Bni1, however, is required for the maintenance of polarized hyphal growth. Both C. albicans formins possess G protein binding and FH2 and FH3 domains corresponding to other Diaphanous-related formins. At the very C terminus, both C. albicans formins also contain a small stretch that is conserved with other DADs, suggesting similar regulations of formins in C. albicans by activation via G protein binding. The C-terminal localization of the DAD may explain the problems of generating C-terminal GFP fusions to BNI1 as the sole source of BNI1 in C. albicans, which may require a longer linker region between the DAD and GFP [three copies of (Gly-Ala) repeats in our construct].
The bni1 mutant phenotypes resemble those of the SPA2 and WAL1 deletions. In bni1 and wal1 cells, polarized morphogenesis could be induced, but after germ tube formation, hyphal growth ceased and mycelial development was abolished. This is of biological significance, indicating that upstream events of signal perception and signal transduction are executed in these mutant strains but run into a morphogenetic block at the level of secretion and/or endocytosis (Fig. 8). As a response, polarized delivery of vesicles can be initiated, but subsequently defects in the organization of the actin cytoskeleton generate decreased polar growth rates and swelling of the germ tubes. The less drastic effects in bni1 cells than in spa2 and wal1 cells may in fact be the result of the BNR1 background and at least partial suppression (22). However, Bnr1 is almost 250 amino acids shorter than Bni1 and thus may lack some of the interaction potential of Bni1, which needs to be determined in future analyses.
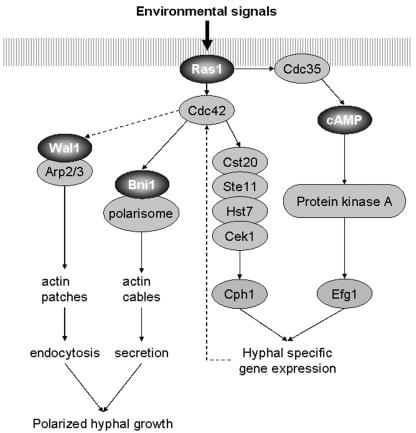
FIG. 8.
The actin cytoskeleton as a downstream part of signal transduction in regulating polarized morphogenesis. A model is presented in which the central pathways to the actin cytoskeleton regulating endocytosis and secretion are incorporated via Cdc42 into the morphogenetic cascade of C. albicans. Ras1-induced signaling activates the cAMP pathway and a MAP kinase cascade that activates hypha-specific gene expression and may also lead to activation of the Cdc42 module. Defects in the actin cytoskeleton machinery affecting either endocytosis (e.g., via Wal1) or polarisome function (e.g., via Bni1 or Spa2) inhibit mycelial development, indicating the relevance of membranous vesicle transport and cycling for polarized growth. The dashed arrow connecting Cdc42 and Wal1 is to indicate the fact that Wal1 lacks a G protein binding domain and can thus be activated only indirectly by Cdc42. Some other pathways regulating gene expression upon hyphal induction, e.g., the Tup1/Nrg1/Rbf1 pathway and the pH-Rim101 pathway, have been omitted for clarity.
Role of the actin cytoskeleton for polarized hyphal growth.
Morphogenetic switching in C. albicans was shown to be dependent on Ras1 signaling and the activation of the transcriptional regulators Cph1 and Efg1 by a MAP kinase cascade and the cAMP pathway, respectively (4, 21, 23). In a previous study, we showed that defects in endocytosis due to the deletion of WAL1, which encodes the C. albicans homolog of the human WASP crippled mycelial development (40). In this study, we demonstrate a defect in the maintenance of hyphal growth due to the deletion of BNI1. Bni1 is a direct effector protein of Rho protein signaling, whereas Wal1 lacks a G protein binding domain (in contrast to its human homolog) and is activated by an as-yet-unknown mechanism. Tip localization of both Bni1-GFP (this study and reference 22) and Spa2-GFP (44) suggests the presence of a polarisome complex in C. albicans hyphae. The role of Bni1 as part of the C. albicans polarisome is, therefore, the targeted delivery of secretory vesicles. Another conserved function of Bni1 is the formation of the actin ring at septal sites (potentially overlapping with Bnr1), and consistent with that Bni1-GFP was also found to localize to septal sites. The lack of a strong defect in mislocalization of cortical actin patches is consistent with a role of patches in endocytosis as a parallel pathway of the actin cytoskeleton. In our bni1 strains, cortical actin patches were polarized, although larger cells showed some mislocalization of patches to the mother cells, which was also observed by Li et al. (22).
In a recent study, an explanation of how transcription via Efg1 may influence growth decisions and activate actin cytoskeleton dynamics was provided. Under serum-inducing conditions, transcript levels of CDC24, encoding the guanine nucleotide exchange factor of Cdc42, transiently increase, which may result in an increase in Cdc42 activity that may trigger germ tube formation (3). Other genes involved in the organization of the actin cytoskeleton, e.g., RHO3 and BEM2, were found to be regulated in a similar manner (28).
Taken together, our results on the characterization of WASP and formins in C. albicans suggest a model of how regulatory networks signal to the actin cytoskeleton to promote growth decisions in C. albicans (Fig. 8). Our data also indicate that there are two largely separate pathways leading to secretion via formins and to endocytosis via WASP. Deletion of genes affecting either pathway will likely cripple the maintenance of polarized hyphal growth and may provide new means and targets for antifungal drug therapy to specifically perturb the hyphal growth phase in C. albicans.
Future research will be directed to elucidate how the interplay between secretion and endocytosis is regulated at the molecular level to achieve a balanced state of polarized cell growth.
Acknowledgments
We thank Gerry Fink for providing the ras1 allele-containing plasmids and Wang Yue for comments on the manuscript and communicating results prior to publication.
This research was supported by the Deutsche Forschungsgemeinschaft, the Friedrich-Schiller University, and the Hans-Knöll Institute.
REFERENCES
- 1.Bartnicki-Garcia, S., D. D. Bartnicki, G. Gierz, R. Lopez-Franco, and C. E. Bracker. 1995. Evidence that Spitzenkörper behavior determines the shape of a fungal hypha: a test of the hyphoid model. Exp. Mycol. 19:153-159. [DOI] [PubMed] [Google Scholar]
- 2.Bartnicki-Garcia, S., F. Hergert, and G. Gierz. 1989. Computer simulation of fungal morphogenesis and the mathematical basis for hyphal tip growth. Protoplasma 153:46-57. [Google Scholar]
- 3.Bassilana, M., J. Hopkins, and R. A. Arkowitz. 2005. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot. Cell 4:588-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 5.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68:1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 7.Cory, G. O., R. Cramer, L. Blanchoin, and A. J. Ridley. 2003. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell 11:1229-1239. [DOI] [PubMed] [Google Scholar]
- 8.Crampin, H., K. Finley, M. Gerami-Nejad, H. Court, C. Gale, J. Berman, and P. Sudbery. 2005. Candida albicans hyphae have a Spitzenkörper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 118:2935-2947. [DOI] [PubMed] [Google Scholar]
- 9.Dent, E. W., and F. B. Gertler. 2003. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40:209-227. [DOI] [PubMed] [Google Scholar]
- 10.Drubin, D. G., and W. J. Nelson. 1996. Origins of cell polarity. Cell 84:335-344. [DOI] [PubMed] [Google Scholar]
- 11.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista, M., D. Pruyne, D. C. Amberg, C. Boone, and A. Bretscher. 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4:32-41. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista, M., S. Zigmond, and C. Boone. 2003. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116:2603-2611. [DOI] [PubMed] [Google Scholar]
- 14.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara, T., K. Tanaka, A. Mino, M. Kikyo, K. Takahashi, K. Shimizu, and Y. Takai. 1998. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9:1221-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 17.Girbardt, M. 1957. Der Spitzenkörper von Polystictus versicolor. Planta 50:47-59. [Google Scholar]
- 18.Gola, S., R. Martin, A. Walther, A. Dunkler, and J. Wendland. 2003. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20:1339-1347. [DOI] [PubMed] [Google Scholar]
- 19.Harris, S. D., N. D. Read, R. W. Roberson, B. Shaw, S. Seiler, M. Plamann, and M. Momany. 2005. Polarisome meets Spitzenkörper: microscopy, genetics, and genomics converge. Eukaryot. Cell 4:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiryushko, D., V. Berezin, and E. Bock. 2004. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann. N. Y. Acad. Sci. 1014:140-154. [DOI] [PubMed] [Google Scholar]
- 21.Leberer, E., D. Harcus, D. Dignard, L. Johnson, S. Ushinsky, D. Y. Thomas, and K. Schroppel. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42:673-687. [DOI] [PubMed] [Google Scholar]
- 22.Li, C. R., Y. M. Wang, X. De Zheng, H. Y. Liang, J. C. Tang, and Y. Wang. 2005. The formin family protein CaBni1p has a role in cell polarity control during both yeast and hyphal growth in Candida albicans. J. Cell Sci. 118:2637-2648. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H., J. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 25.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 26.Machesky, L. M., and K. L. Gould. 1999. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11:117-121. [DOI] [PubMed] [Google Scholar]
- 27.Momany, M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5:580-585. [DOI] [PubMed] [Google Scholar]
- 28.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A. P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberholzer, U., A. Marcil, E. Leberer, D. Y. Thomas, and M. Whiteway. 2002. Myosin I is required for hypha formation in Candida albicans. Eukaryot. Cell 1:213-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otomo, T., C. Otomo, D. R. Tomchick, M. Machius, and M. K. Rosen. 2005. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell 18:273-281. [DOI] [PubMed] [Google Scholar]
- 31.Parton, R. M., S. Fischer-Parton, M. K. Watahiki, and A. J. Trewavas. 2001. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J. Cell Sci. 114:2685-2695. [DOI] [PubMed] [Google Scholar]
- 32.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 33.Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond, A. Bretscher, and C. Boone. 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297:612-615. [DOI] [PubMed] [Google Scholar]
- 34.Sagot, I., A. A. Rodal, J. Moseley, B. L. Goode, and D. Pellman. 2002. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4:626-631. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless, K. E., and S. D. Harris. 2002. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol. Biol. Cell 13:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheu, Y. J., B. Santos, N. Fortin, C. Costigan, and M. Snyder. 1998. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18:4053-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong, A. H., M. Evangelista, A. B. Parsons, H. Xu, G. D. Bader, N. Page, M. Robinson, S. Raghibizadeh, C. W. Hogue, H. Bussey, B. Andrews, M. Tyers, and C. Boone. 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294:2364-2368. [DOI] [PubMed] [Google Scholar]
- 38.Walther, A., and J. Wendland. 2004. Apical localization of actin patches and vacuolar dynamics in Ashbya gossypii depend on the WASP homolog Wal1p. J. Cell Sci. 117:4947-4958. [DOI] [PubMed] [Google Scholar]
- 39.Walther, A., and J. Wendland. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42:339-343. [DOI] [PubMed] [Google Scholar]
- 40.Walther, A., and J. Wendland. 2004. Polarized hyphal growth in Candida albicans requires the Wiskott-Aldrich syndrome protein homolog Wal1p. Eukaryot. Cell 3:471-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendland, J., and A. Walther. 2005. Ashbya gossypii: a model for fungal developmental biology. Nat. Rev. Microbiol. 3:421-429. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter, D. C., E. Y. Choe, and R. Li. 1999. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA 96:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng, X. D., Y. M. Wang, and Y. Wang. 2003. CaSPA2 is important for polarity establishment and maintenance in Candida albicans. Mol. Microbiol. 49:1391-1405. [DOI] [PubMed] [Google Scholar]