Abstract
The Japanese common plataspid stinkbug, Megacopta punctatissima, deposits small brown particles, or symbiont capsules, on the underside of the egg mass for the purpose of transmission of symbiotic bacteria to the offspring. We investigated the microbiological aspects of the bacteria contained in the capsule, such as microbial diversity, phylogenetic placement, localization in vivo, and fitness effects on the host insect. Restriction fragment length polymorphism analysis of 16S ribosomal DNA clones revealed that a single bacterial species dominates the microbiota in the capsule. The bacterium was not detected in the eggs but in the capsules, which unequivocally demonstrated that the bacterium is transmitted to the offspring of the insect orally rather than transovarially, through probing of the capsule content. Molecular phylogenetic analysis showed that the bacterium belongs to the γ-subdivision of the Proteobacteria. In adult insects the bacterium was localized in the posterior section of the midgut. Deprivation of the bacterium from the nymphs resulted in retarded development, arrested growth, abnormal body coloration, and other symptoms, suggesting that the bacterium is essential for normal development and growth of the host insect.
The Heteroptera, known as true bugs, is one of the most diverse groups of insects among those with incomplete metamorphosis. The group includes some 30,000 species of relatively large insects which differ from the Homoptera (cicadas, plant hoppers, aphids, coccids, etc.) primarily in the structure of the mouthpart and in having half-membranous forewings. Symbiotic bacteria of the Heteroptera are most often extracellular in the lumina of midgut caeca, although some are exceptionally located in the midgut epithelia, the abdominal mycetomes, or elsewhere (4, 7, 12, 21, 22). In many groups of the Heteroptera, the posterior end of the midgut is characterized by the presence of sac-like appendages opening into it. These evaginations, called caeca or crypts, vary considerably in number and arrangement in different taxonomic groups and almost always contain specific bacteria (12, 13, 18). The symbiotic bacteria harbored in the midgut caeca of the Heteroptera are, in general, maternally inherited by superficial contamination of the egg shell with excretion containing the bacteria. Upon hatching the nymphs exhibit a marked resting behavior in which they remain associated with their egg shells and constantly probe the surface with their proboscis. The bacteria ingested during this period become associated with the alimentary tract and proliferate in the lumina of the sterile midgut caeca. Owing to the external carriage and transmission of the symbionts, sterile aposymbiotic nymphs can be experimentally obtained through surface sterilization of the eggs. Aposymbiotic nymphs of several heteropterans were reported to exhibit retarded growth and/or nymphal mortality (1, 2, 5, 14, 19, 23), suggesting that the symbionts play some important roles for the host insects. The microbiological nature of symbiotic bacteria has been poorly understood. Although several bacteria have been isolated from the gut of some heteropterans (7), it has scarcely been confirmed whether the isolates are identical to the predominant bacteria harbored in the midgut caeca.
Insects dependent on restricted diets, such as plant sap, vertebrate blood, or woody material, commonly carry symbiotic microorganisms that are thought to provide nutritional supplements for their hosts (4, 7). In this respect the Heteroptera are an interesting research subject for understanding the diversity and evolution of insect-microbe symbiotic associations, because a number of plant sap feeders, vertebrate blood feeders, and predators of other arthropods are found in this well-defined insect group. Certainly, symbiotic relationships in the Heteroptera correlate reasonably well with diet: symbiotic bacteria tend to be found in plant sap feeders and vertebrate blood feeders but are absent from predators of other arthropods (4, 7). Because of their medical importance, endosymbionts of several vertebrate blood feeders, such as Triatoma infestans and Cimex lectularius, have been relatively well characterized (16, 17, 20). On the other hand, no endosymbionts of plant sap feeders have been identified by a molecular phylogenetic approach.
Among the plant-sucking groups of the Heteroptera, the family Plataspidae shows the most remarkable behavioral and anatomical arrangement for transmission of the symbiont, in which eggs are provided with symbiont-filled particles, so-called symbiont capsules, instead of direct surface contamination with the symbiont (4). Coptosoma scutellatum is the only representative of the Plataspidae found in central and southern Europe as well as the only plataspid species whose endosymbiotic system was investigated in detail (19, 22). In adult insects of this species, the anterior section of the midgut ended in a blind sac and the separated posterior section of the midgut was transformed into a voluminous mycetome-like symbiotic organ with numerous crypts whose lumina were filled with bacteria. When female adults laid eggs on the host plant, small brownish symbiont capsules were deposited on the underside of the egg mass. Upon hatching, the nymphs immediately probed the capsule with their proboscis and ingested the symbionts. Deprivation of the capsules resulted in retarded growth of the nymphs. The bacteria in the capsules could not be cultured (19, 22). Since these early reports, although interesting, the microbiota in the capsule of C. scutellatum and any other plataspids have not been microbiologically characterized.
In the Japanese common plataspid stinkbug, Megacopta punctatissima (Insecta: Heteroptera: Plataspidae), we found similar capsules associated with egg masses. In the present study microbiological aspects, such as microbial diversity, phylogenetic placement, localization in vivo, and fitness effects on the host insect, of the bacteria contained in the capsule were investigated.
MATERIALS AND METHODS
Materials.
Samples of M. punctatissima used in this study are listed in Table 1. Adult insects and egg masses were collected from Pueraria lobata in the field. Some of the samples were preserved in acetone for DNA analysis (9).
TABLE 1.
Samples of M. punctatissima used in this study
| Sample code | Localitya | Collection date | Collector |
|---|---|---|---|
| KOBE00 | Kobe University, Hyogo | 22 June 2000 | T. Hosokawa |
| TCUR01 | Tsuchiura, Ibaraki | 23 May 2001 | T. Fukatsu |
| MITO01 | Mito, Ibaraki | 20 May 2001 | Y. Kikuchi |
| ONMC01 | Onomichi, Hiroshima | 4 May 2001 | T. Fukatsu |
| KOCH01 | Kochi University, Kochi | 23 April 2001 | R. Arakawa |
| FKOK01 | Kashii, Fukuoka | 13 May 2001 | T. Hosokawa |
| KGSM01 | Kagoshima University, Kagoshima | 16 May 2001 | N. Ijichi |
| YKSM01 | Yakushima Island, Kagoshima | 13 May 2001 | N. Ijichi |
All locations listed are in Japan.
Dissection of insects.
Some of the adult insects were subjected to dissection to obtain isolated tissues and organs. An adult insect was carefully dissected by using pins, fine forceps, and razors under a dissection microscope in a petri dish covered with silicon rubber and filled with sterile phosphate-buffered saline. Isolated tissues and organs were individually washed several times with fresh phosphate-buffered saline to minimize possible microbial contamination.
Molecular biological procedures.
Extraction of DNA from whole insects or dissected tissues and/or organs was conducted with the QIAamp DNA Mini kit (QIAGEN). PCR amplification of eubacterial 16S ribosomal DNA (rDNA) with universal primers 16SA1 and 16SB1, cloning of the products, typing of the clones by restriction fragment length polymorphism (RFLP), and sequencing of the clones were conducted as previously described (10).
Molecular phylogenetic analysis.
A multiple alignment of 16S rDNA sequences was conducted with the program package Clustal W (25). The final alignment was inspected and corrected manually. Ambiguously aligned regions were excluded from the phylogenetic analysis. Nucleotide sites that included an alignment gap(s) were also omitted from the aligned data set. Neighbor-joining trees were constructed with Kimura’s two-parameter distance by using Clustal W. Maximum parsimony trees were constructed with the program package PAUP 3.0.1 (24). A bootstrap test was conducted with 100 resamplings.
Diagnostic PCR.
Specific PCR detection of 16S rDNA from the symbiont of M. punctatissima was conducted with the specific primer MRKM16SF (5′-TATAGAGATATATAAGTGCCTTTCG-3′) and the universal eubacterial primer 16SB1 under a temperature profile of 94°C for 2 min followed by 30 cycles of 94°C for 1 min, 50°C for 1 min, and 70°C for 1 min. Specific detection of the mitochondrial rDNA (MtrDNA) of the host insect was conducted using the primers MtrA1 and MtrB1 under a temperature profile of 94°C for 2 min followed by 30 cycles of 94°C for 1 min, 48°C for 1 min, and 65°C for 3 min (11).
Measurement of the fitness effects.
Eggs and capsules of M. punctatissima were carefully isolated from egg masses, which were collected at Kobe University on 22 June 2000, by using forceps under a dissection microscope. Eggs and capsules from many egg masses were combined and mixed, respectively, to average the effects of their genetic background. Some of the capsules were heat-treated in a petri dish at 100°C for 15 min in a laboratory oven. To obtain symbiotic insects, 160 eggs and 120 normal capsules were placed on filter paper in a humidified petri dish at 20°C. To obtain aposymbiotic insects, 160 eggs and 120 heat-treated capsules were combined in the same way. After a week of incubation the number of eggs hatched was counted and hatchlings were transferred to potted soybean (Glycine max) seedlings. The insects, reared on the soybean plants at room temperature, were transferred to new plants every 8 to 13 days, at which time their developmental stage was determined. Finally, all the surviving insects were fixed in acetone, air-dried to measure dry body weight, and subjected to DNA extraction for diagnostic PCR detection of the endosymbiont.
Nucleotide sequence accession number.
The 16S rDNA sequence of the capsule symbiotic bacterium from M. punctatissima was deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under the accession number AB067723.
RESULTS
General observations.
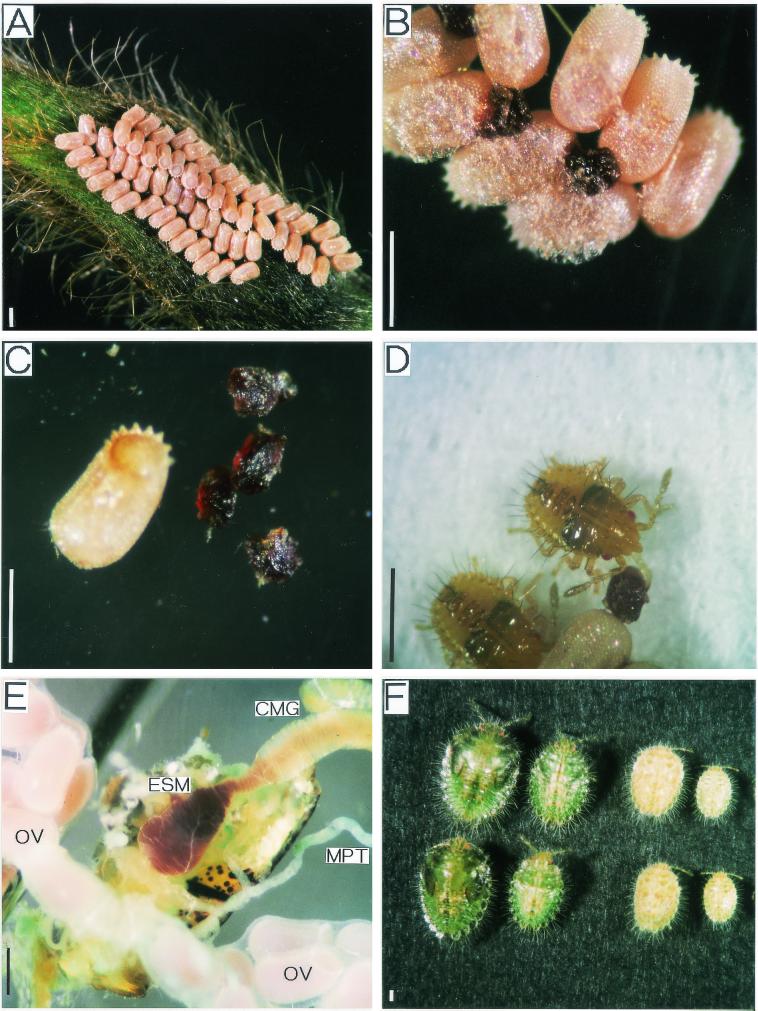
In the field, M. punctatissima females were observed to deposit egg masses on buds of P. lobata. An egg mass was composed of 6 to 30 eggs arranged in two lines in parallel (Fig. 1A). On the underside of the egg masses, small objects, 0.2 to 0.3 mm in diameter and dark brown in color, were always attached (Fig. 1B). Morphologically the objects were quite reminiscent of the symbiont capsules reported for C. scutellatum (19, 22). The aubergine-shaped eggs and the capsules were easily separated with forceps under a dissection microscope (Fig. 1C). Within a week after oviposition, first-instar nymphs hatched from the eggs kept in petri dishes. Soon after hatching the newborn nymphs always approached a capsule and probed it with their proboscis for a considerable period (Fig. 1D). After sucking the capsule they entered a stationary period for several days. In contrast, when deprived of the capsules the newborn nymphs restlessly crawled around in petri dishes and did not enter a rest period.
FIG. 1.
General observation of M. punctatissima. (A) Egg masses laid on a bud of P. lobata. An egg mass produced by a female is composed of two lines of eggs in parallel. Thus, two egg masses are seen on the bud. (B) Brown capsules placed on the underside of the egg mass. (C) An isolated egg and capsules. (D) A newly hatched nymph probing a symbiont capsule. (E) Internal organs of a female adult. CMG, crypt-bearing midgut; ESM, enlarged end section of midgut; MPT, Malpighian tubule; OV, ovariole. Also see Fig. 4 and 5. (F) Comparison of symbiotic and aposymbiotic insects. Note that these insects are of almost the same age, 7 to 8 weeks after hatching. From left to right, fifth-instar nymphs fed with normal capsule; fourth-instar nymphs fed with normal capsule; fourth-instar nymphs fed with heat-treated capsule; third-instar nymphs fed with heat-treated capsule. Also see Fig. 6. Bars, 0.5 mm.
Detection of eubacterial 16S rDNA from the capsule.
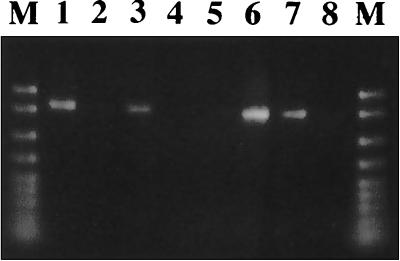
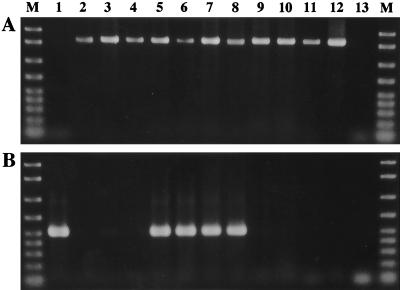
DNA samples separately extracted from the eggs and the capsules, respectively, were subjected to a PCR assay (Fig. 2). Using the primers for MtrDNA of insects, the PCR product was detected from the eggs (lane 1) but not from the capsules (lane 2). In contrast, when primers for 16S rDNA of eubacteria were used a very thick band was detected from the capsules (lane 6), whereas no band was amplified from the eggs (lane 5).
FIG. 2.
PCR detection of insect and bacterial genes from eggs and capsules of M. punctatissima. Lanes 1 to 4, detection of insect mitochondrial rDNA by using primers MtrA1 and MtrB1; lanes 5 to 8, detection of bacterial 16S rDNA by using universal eubacterial primers 16SA1 and 16SB1. Lanes 1 and 5, DNA extracted from eggs; lanes 2 and 6, DNA extracted from capsules; lanes 3 and 7, positive control, DNA from the pea aphid (Acyrthosiphon pisum) containing an intracellular symbiont, Buchnera sp.; lanes 4 and 8, negative control, no template; lane M, DNA size markers (2,000; 1,500; 1,000; 700; 500; 400; 300; 200; and 100 bp from top to bottom). About 1 ng of DNA was subjected to the PCR for each sample.
RFLP analysis of 16S rDNA clones from the capsule.
From the DNA sample extracted from the capsules, a 1.5-kb segment of eubacterial 16S rDNA was amplified by PCR, cloned, and subjected to RFLP analysis. Notably, all 30 clones examined showed completely identical RFLP patterns (data not shown), which indicated that a single bacterial species dominates the microbiota in the capsules.
Molecular phylogenetic analysis of the capsule bacterium.
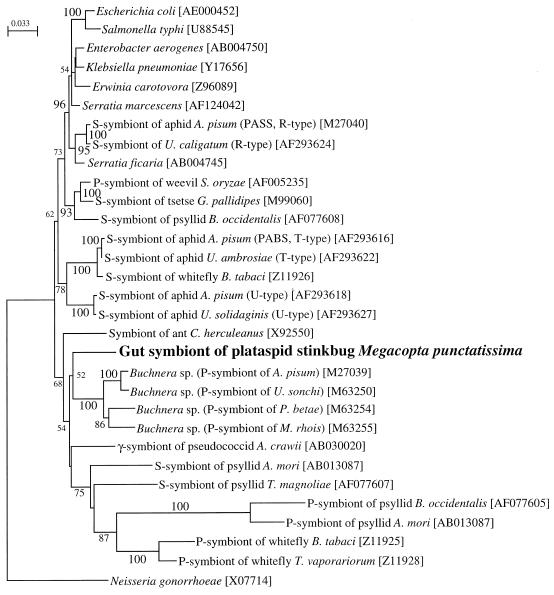
To characterize the bacterium in the capsule, the 16S rDNA clones were sequenced. The four partial (1,468-bp) sequences determined were completely identical, except for two single-nucleotide substitutions that might have been the result of PCR errors. The consensus sequence was subjected to molecular phylogenetic analysis (Fig. 3). The sequence, placed in the γ-subdivision of the Proteobacteria, had no closely related bacterial sequences in the DNA databases. In the neighbor-joining tree the sequence showed a phylogenetic affinity to those of Buchnera spp., the primary intracellular symbiont of aphids, although the bootstrap support for the clade was extremely low (52%).
FIG. 3.
Molecular phylogenetic analysis of bacterial 16S rDNA sequences identified from the capsule of M. punctatissima. A total of 1,264 unambiguously aligned nucleotide sites were subjected to analysis. A neighbor-joining phylogeny is shown, whereas maximum parsimony analysis gave essentially the same result. The bootstrap values obtained with 100 resamplings are shown at the nodes in percentages, although values of less than 50% were omitted. The numbers in brackets are accession numbers.
Localization of the capsule bacterium in adult insect.
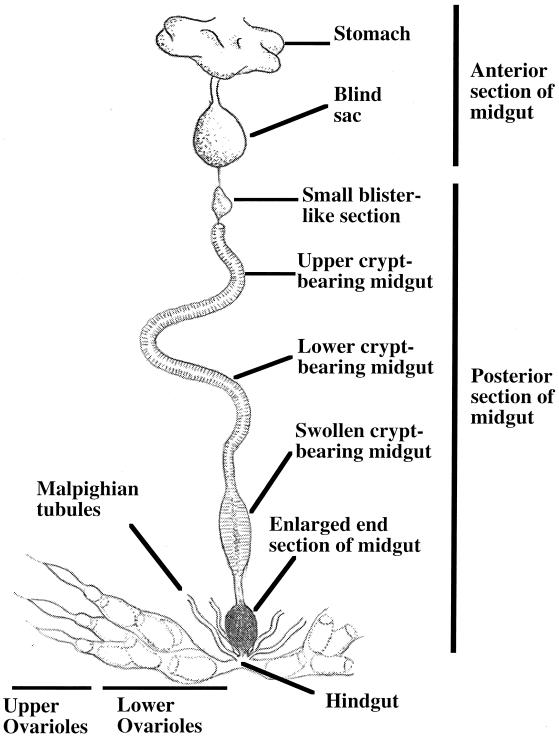
Figure 4 shows the organization of the internal tissues and organs of an M. punctatissima female adult. To identify the localization of the capsule bacterium in vivo, we conducted diagnostic PCR analysis of dissected tissues and organs of M. punctatissima. Based on the 16S rDNA sequence we determined, a specific PCR primer, MRKM16SF, was designed to amplify the 16S rDNA of the capsule bacterium in combination with a general reverse primer, 16SB1. Of the various tissues and organs of the female insect, the capsule bacterium was specifically detected in the posterior section of the midgut (Fig. 5). Microscopic examination confirmed that the lumen of the midgut section was densely populated by bacterial cells (data not shown). Notably, the enlarged end section of the midgut of the female was filled with brown material (Fig. 1E), indicating that the capsule is produced in this section. In males the bacterium was also detected in the posterior section of the midgut, although the enlarged end section was lacking (data not shown).
FIG. 4.
Organization of midgut sections and ovarioles of M. punctatissima female adults.
FIG. 5.
Localization of the bacterial symbiont in M. punctatissima. (A) PCR detection of insect mitochondrial rDNA by using primers MtrA1 and MtrB1. (B) Specific PCR detection of the capsule symbiont by using primers MRKM16SF and 16SB1. Lane 1, capsule; lane 2, egg; lane 3, stomach; lane 4, blind sac; lane 5, upper crypt-bearing midgut; lane 6, lower crypt-bearing midgut; lane 7, swollen crypt-bearing midgut; lane 8, enlarged end section of midgut; lane 9, upper ovariole containing nurse tissues and young oocytes; lane 10, lower ovariole containing mature oocytes; lane 11, Malpighian tubule; lane 12, thoracic muscle; lane 13, no template control; lane M, DNA size markers (2,000; 1,500; 1,000; 700; 500; 400; 300; 200; and 100 bp from top to bottom). For the organization of midgut sections and ovarioles, see Fig. 4.
Prevalence of the capsule bacterium in natural populations.
Adult insects collected from eight Japanese populations of M. punctatissima were subjected to diagnostic PCR detection of the capsule bacterium. In total 130 individuals were examined: 10 males and 10 females from KOBE00; 10 males and 10 females from TCUR01; 5 males and 5 females from MITO01; 10 males and 10 females from ONMC01; 5 males and 10 females from KOCH01; 10 males and 10 females from FKOK01; 5 males and 10 females from KGSM01; and 5 males and 5 females from YKSM01 (see Table 1). Infection with the capsule bacterium was detected in all the insects.
Comparison of the fitness of symbiotic and aposymbiotic insects.
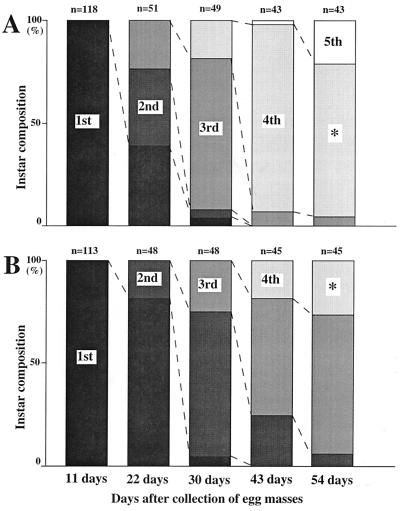
We attempted to estimate the contribution of the capsule bacterium to the fitness of M. punctatissima. By providing the hatchlings with normal capsules and heat-treated capsules, respectively, we prepared two groups of insects: the normal capsule group and the heat-treated capsule group. These two groups were reared on the soybean plants in the laboratory, and their survival, growth, and development were monitored. The survival of the insects showed a similar pattern between the two groups: high mortality in the first instar and lower mortality among older nymphs (Fig. 6). The initial high mortality was probably due to the failure to settle on the host plant and/or to desiccation. Notably, development of the nymphs exhibited a striking difference between these groups: the normal capsule group grew and molted faster than the heat-treated capsule group. As a result, on the 54th day after collection of egg masses, 21% of individuals were at the fifth instar and 74% were at the fourth instar in the normal capsule group, whereas in the heat-treated capsule group none was at the fifth instar, 24% were at the fourth instar, and 68% were still at the third instar (Fig. 6). In addition, the body sizes of the heat-treated capsule group were smaller than those of the normal capsule group. For example, on the 54th day after collection of egg masses, dry body weight of fourth-instar nymphs of the heat-treated capsule group was 588 ± 187 mg (mean ± standard deviation), whereas that of the normal capsule group was 1,516 ± 566 mg. The difference was statistically highly significant (P < 0.0001; Mann-Whitney U test). Notably, insects of the heat-treated capsule group showed morphological symptoms. While normal nymphs were green in color, nymphs of the heat-treated capsule group were an abnormal pale yellow (Fig. 1F). Not only the body coloration but body shape and other characteristics were abnormal (data not shown). Finally, we checked for the presence of the capsule bacterium in these insects by diagnostic PCR analysis. It was clearly demonstrated that the insects of the normal capsule group possessed the bacterium, whereas the insects of the heat-treated capsule group were essentially free of it (data not shown). However, after 35 cycles of amplification, some individuals of the heat-treated capsule group exhibited a faint band, indicating the presence of a small amount of the bacterium.
FIG. 6.
Growth and development of M. punctatissima nymphs reared on soybean plants (G. max). (A) Nymphs fed with normal capsules. (B) Nymphs fed with heat-treated capsules. 1st, 2nd, 3rd, 4th, and 5th indicate the instars of the nymphs. The numbers on the top of each column indicate the number of individuals that survived. The groups marked with asterisks are those from which the fourth-instar insects shown in Fig. 1F were selected. These groups were also the ones subjected to the dry weight comparison.
DISCUSSION
In the present study we identified a novel bacterial species, which belongs to the γ-subdivision of the Proteobacteria, in the symbiont capsule of M. punctatissima (Fig. 3). Based on RFLP analysis of 16S rDNA clones from the capsule, the bacterium was essentially the only bacterial species in the capsule, although the possible presence of minor microbial components cannot be excluded. In adult insects the bacterium showed a specific localization to the posterior section of the midgut (Fig. 5). Disrupted transmission of the bacterium to newborn nymphs by heat treatment of the capsules resulted in retarded development, arrested growth, abnormal body coloration, and other symptoms (Fig. 1 and 6). From these results we concluded that the bacterium is a mutualistic gut symbiont of M. punctatissima which is vertically transmitted through the capsule and is essential for normal development and growth of the host insect.
The characteristics of the endosymbiotic system of the Japanese common plataspid bug, M. punctatissima, such as deposition of the capsules in egg masses, vertical transmission of bacterial symbiont in the capsules to newborn nymphs by oral ingestion, localization of the symbiont in the posterior section of the midgut, and possible biological roles of the symbiont for growth and development of the nymphs, were essentially consistent with those described in previous reports on the European plataspid species C. scutellatum (19, 22). Therefore, these remarkable endosymbiotic arrangements are likely to be commonly found in the bugs of the family Plataspidae.
Although the morphology of the symbiotic bacteria in the midgut caeca of a number of plant-sucking species has been described (4, 12, 21, 22), none of these bacteria have been characterized by a molecular phylogenetic approach. Therefore, this study is the first report of phylogenetic position of a gut symbiont from plant-sucking bugs. On the other hand, endosymbiotic bacteria of several vertebrate blood feeders have been characterized phylogenetically, probably due to their medical importance. The assassin bugs, which belong to the genera Triatoma and Rhodnius of the family Reduviidae, feed on the blood of mammals, including humans, and are known as carriers of Trypanosoma cruzi (the etiological agent of Chagas’ disease), Yersinia pestis, and Leptospira spp. From the midgut lumina of T. infestans and Rhodnius prolixus a nocardioform actinomycete, Rhodococcus rhodnii, was identified which provides B vitamins and is essential for normal development and growth of the hosts (2, 20). From T. infestans, in addition to the gut symbiont a gram-negative intracellular symbiotic bacterium, “Candidatus Arsenophonus triatominarum,” was identified (15, 17). The bedbugs, which belong to the genus Cimex and others of the family Cimicidae, also feed on the blood of humans. From C. lectularius two morphotypes of rickettsia-like intracellular symbiotic bacteria were identified in the cytoplasm of abdominal mycetomes (6). Molecular phylogenetic analysis demonstrated that one belongs to the genus Wolbachia in the α-subdivision of the Proteobacteria, and the other is closely related to a bacterial associate of a leafhopper, Euscelidius variegatus, in the γ-Proteobacteria (16). Notably, these symbiotic bacteria from blood-sucking heteropterans together with the gut symbiont of M. punctatissima did not show phylogenetic affinity to each other. The diversity of symbiotic bacteria as well as that of endosymbiotic mechanisms in the Heteroptera suggests that the endosymbiotic associations have evolved multiple times independently in a dynamic manner, although the number of species so far examined is quite limited. Examination of more species from diverse taxa of the Heteroptera is needed.
As for the evolutionary origin of intracellular symbiotic bacteria harbored by mycetocytes of various insects, it has been hypothesized that they originated from gut bacteria which were intimately associated with the alimentary tract and played beneficial biological roles for the ancestors of the insects (4). In this context, it appears meaningful that the gut symbiont of M. punctatissima was phylogenetically related to intracellular symbiotic bacteria of other insects, such as aphids and ants in the γ-Proteobacteria, although statistical support for the grouping was not significant (Fig. 3). The gut symbiont of M. punctatissima, harbored in a special structure like the crypt-bearing midgut and vertically transmitted by a special mechanism like the capsules, might represent a specialized intermediate status of endosymbiosis between gut bacteria and mycetocyte symbionts.
The newborn nymphs of M. punctatissima orally acquire the symbiont by probing the capsule (Fig. 1D). In adult insects, on the other hand, the symbiont shows a specific localization to the posterior section of the midgut (Fig. 5). Therefore, the symbiont must colonize the specific part of the midgut in the developmental course of M. punctatissima. In adult insects, notably, the alimentary tract is segmented and differentiated into a series of peculiarly compartmentalized structures (Fig. 4). It will be of great interest to investigate the development of the alimentary canal of M. punctatissima in connection with the localization of the symbiont.
When transmission of the symbiont to newborn nymphs was disrupted by heat treatment of the capsules, the aposymbiotic nymphs showed deficient symptoms, such as retarded development, arrested growth, and abnormal body coloration (Fig. 1 and 6). Similar symptoms were observed by tetracycline treatment of the capsules as well as by simple deprivation of the capsules (T. Fukatsu, unpublished data). Therefore, these negative effects are certainly attributed to the disrupted endosymbiotic association. In this study, however, the disruption did not necessarily mean complete elimination of the symbiont. From some of the insects fed with heat-treated capsules a very small amount of the symbiont was detected by PCR. When eggs and capsules were separated with forceps a trace amount of the symbiont probably occasionally remained on the surface of several eggs, and the nymphs managed to take it. To remove the symbiont completely surface sterilization of the eggs will be necessary, although such treatment may cause negative effects on the viability of the eggs.
Notably, the insects with a trace amount of the symbiont also exhibited the deficient symptoms, suggesting the possibility that a threshold titer of the symbiont must be ingested by newborn nymphs to establish normal endosymbiotic association. In M. punctatissima, the number of eggs in an egg mass is around twice that of capsules (T. Fukatsu, unpublished data). Therefore, it is conceivable that newborn nymphs of a clutch compete with each other for the capsules, which might lead to ecological and evolutionary consequences, such as size of capsules, number of capsules in an egg mass, timing of hatching, and so forth.
The nature of the positive fitness effects caused by the symbiont is unknown. The symbionts probably provide the host insect with nutritional supplements, such as essential amino acids and vitamins, as has been reported for other plant-sucking insects (3, 8). To investigate the physiological aspects of endosymbiosis, development of a nutritionally defined artificial diet for M. punctatissima is needed.
In general it is extremely difficult to experimentally manipulate mutualistic symbiotic microorganisms of insects for any of the following reasons: (i) they are structurally and developmentally integrated in the host to such an extent that the host and symbiont are often no longer separable; (ii) due to specialization for endosymbiotic life, they are physically and biologically too fragile to be manipulated without fatal damage; and (iii) they are generally difficult to culture in vitro. In plataspid bugs such as M. punctatissima and C. scutellatum, the symbiotic bacterium is uniquely packaged in the capsules and is easily separable from the host eggs by using forceps. The endosymbiotic system would provide us with unprecedented experimental approaches to host-symbiont interaction, coevolution, and coadaptation. For example, as conducted in this study, biological roles of the symbiont can be assessed by experimental disruption of the symbiotic association by using heat treatment, antibiotic treatment, or simple deprivation of the capsules. By exchanging the capsules between populations, species, and genera of the plataspid bugs and by measuring the effects of artificially generated symbiotic associations, we would be able to experimentally and quantitatively assess the degree of coevolution and coadaptation between the symbionts and the hosts. By using artificial capsule-like bacterial droplets, bacteria (including genetically engineered ones) might be able to be introduced into newborn nymphs of the plataspid bugs. If the gut symbiont of M. punctatissima is cultured in vitro and genetically engineered in future, we expect that the endosymbiotic system would potentially be an excellent experimental model to investigate the physiology, molecular genetics, and evolutionary biology of insect-microbe endosymbiosis.
Acknowledgments
We thank R. Arakawa, Y. Kikuchi, and N. Ijichi for insect samples and A. Sugimura, S. Kumagai, and K. Sato for technical and secretarial assistance.
This research was supported by the Industrial Science and Technology Frontier Program “Technological Development of Biological Resources in Bioconsortia” of the Ministry of International Trade and Industry of Japan.
REFERENCES
- 1.Abe, Y., K. Mishiro, and M. Takanashi. 1995. Symbiont of brown-winged green bug. Plautia stali Scott. Jpn. J. Appl. Entomol. Zool. 39:109–115. [Google Scholar]
- 2.Bains, S. 1956. The role of the symbiotic bacteria in the nutrition of Rhodnius prolixus (Hemiptera). J. Exp. Biol. 33:533–541. [Google Scholar]
- 3.Baumann, P., L. Baumann, C.-Y. Lai, D. Rouhbakhsh, N. A. Moran, and M. A. Clark. 1995. Genetics, physiology and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu. Rev. Microbiol. 49:55–94. [DOI] [PubMed] [Google Scholar]
- 4.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, N.Y.
- 5.Chang, K. P. 1974. Effects of elevated temperature on the mycetome and symbiotes of the bed bug Cimex lectularis (Heteroptera). J. Invertebr. Pathol. 23:333–340. [DOI] [PubMed] [Google Scholar]
- 6.Chang, K. P., and A. J. Musgrave. 1973. Morphology, histochemistry, and ultrastructure of mycetome and its rickettsial symbiotes in Cimex lectularis L. Can. J. Microbiol. 19:1075–1081. [DOI] [PubMed] [Google Scholar]
- 7.Dasch, G. A., E. Weiss, and K. P. Chang. 1984. Endosymbionts of insects, p.811–833. In N. R. Krieg and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 8.Douglas, A. E. 1989. Mycetocyte symbiosis in insects. Biol. Rev. 64:409–434. [DOI] [PubMed] [Google Scholar]
- 9.Fukatsu, T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935–1945. [DOI] [PubMed] [Google Scholar]
- 10.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukatsu, T., T. Tsuchida, N. Nikoh, and R. Koga. 2001. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 67:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glasgow, H. 1914. The gastric caeca and the caecal bacteria of the Heteroptera. Biol. Bull. 3:101–171. [Google Scholar]
- 13.Goodchild, A. J. P. 1966. Evolution of the alimentary canal in the Hemiptera. Biol. Rev. 41:97–140. [Google Scholar]
- 14.Huber-Schneider, L. 1957. Morphologische und physiologische Untersuchungen an der Wanze Mesocerus marginatus L. und ihren Symbionten (Heteroptera). Z. Morphol. Ökol. Tiere 46:433–480. [Google Scholar]
- 15.Hypsa, V. 1993. Endocytobionts of Triatoma infestans: distribution and transmission. J. Invertebr. Pathol. 61:32–38. [Google Scholar]
- 16.Hypsa, V., and S. Aksoy. 1997. Phylogenetic characterization of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera: Cimicidae). Insect Mol. Biol. 6:301–304. [DOI] [PubMed] [Google Scholar]
- 17.Hypsa, V., and C. Dale. 1997. In vitro culture and phylogenetic analysis of “Candidatus Arsenophonus triatominarum,” an intracellular bacterium from the triatomine bug Triatoma infestans. Int. J. Syst. Bacteriol. 47:1140–1144. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto, S. 1961. Comparative morphology of alimentary organs of Heteroptera, with the phylogenetic consideration. Sieboldia 2:197–259. [Google Scholar]
- 19.Müller, H. J. 1956. Experimentelle Studien an der Symbiose von Coptosoma scutellatum Geoffr. (Hem. Heteropt.). Z. Morphol. Ökol. Tiere 44:459–482. [Google Scholar]
- 20.Rainey, F. A., J. Burghardt, R. M. Kroppenstedt, S. Klatte, and E. Stackebrandt. 1995. Phylogenetic analysis of the genera Rhodococcus and Norcardia and evidence for the evolutionary origin of the genus Nocardia from within the radiation of Rhodococcus species. Microbiology 141:523–528. [Google Scholar]
- 21.Rosenkranz, W. 1939. Die Symbiose der Pentatomiden (Hemiptera, Heteroptera). Z. Morphol. Ökol. Tiere 36:279–309. [Google Scholar]
- 22.Schneider, G. 1940. Beiträge zur Kenntnis der symbiontischen Einrichtungen der Heteropteren. Z. Morphol. Ökol. Tiere 36:565–644. [Google Scholar]
- 23.Schorr, H. 1957. Zür Verhaltensbiologie und Symbiose von Brachypelta aterrima Först (Cydnidae, Heteroptera). Z. Morphol. Ökol. Tiere 45:561–602. [Google Scholar]
- 24.Swofford, D. L. 1993. PAUP: phylogenetic analysis using parsimony version 3.1.1. Smithsonian Institution, Washington, D.C.
- 25.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]