Abstract
The sigB gene of Bacillus cereus ATCC 14579 encodes the alternative sigma factor σB. Deletion of sigB in B. cereus leads to hyperresistance to hydrogen peroxide. The expression of katA, which encodes one of the catalases of B. cereus, is upregulated in the sigB deletion mutant, and this may contribute to the hydrogen peroxide-resistant phenotype.
Bacillus cereus is a gram-positive bacterium that is a frequent cause of foodborne illnesses, with diarrheal and emetic symptoms (18, 22). It is also an important spoilage organism, especially in the dairy industry, as B. cereus spores that are present in raw milk can survive pasteurization. During storage, spores can germinate and cell growth can occur in milk or milk-based products (9).
Recently, the close genetic relationship between bacteria in the B. cereus group has received considerable attention. The B. cereus group includes B. cereus itself but also Bacillus anthracis, which is the causative agent of anthrax, and Bacillus thuringiensis, which is used as a biopesticide (17). Recent complete-genome sequence studies confirmed the close genetic relationships between the organisms in the B. cereus group (21), and this supports the view that the identification of genes that are crucial for important cellular processes in B. cereus can have implications for understanding the biology of B. anthracis (16). This may be especially true for the alternative sigma factor σB, because the genes coding for σB and its regulators are highly conserved throughout the B. cereus group (24).
The alternative sigma factor σB, which is encoded by the sigB gene, has a central role in the stress responses of several gram-positive bacteria, including Bacillus subtilis, Listeria monocytogenes, Staphylococcus aureus, and B. cereus (26). In B. cereus, σB is activated upon stress exposure (including exposure to ethanol and osmotic upshock but most profoundly heat shock) and entry into stationary phase (6, 24). The phenotypic characterization of the B. cereus sigB deletion mutant revealed that σB plays a role in the adaptive heat stress response (24).
In other gram-positive bacteria, the deletion of sigB generally leads to a decreased resistance to reactive oxygen species (7, 8, 10, 13). The sole exception to this rule is provided in a report in which the increased resistance of a sigB deletion mutant of a L. monocytogenes strain towards the oxidative agent cumene hydroperoxide is described (19). However, neither quantitative nor mechanistic details were provided in this study.
In this study, we set out to assess the role of σB in B. cereus ATCC 14579 in resistance to the oxidizing agent hydrogen peroxide (H2O2).
The B. cereus sigB deletion mutant is hyperresistant to H2O2.
B. cereus ATCC 14579 and its isogenic sigB deletion mutant were grown aerobically as described earlier (24). For experiments under anaerobic conditions, B. cereus was precultured overnight (final optical density at 600 nm [OD600] of 1.0 to 1.2) and grown until mid-exponential phase (OD600 of 0.2) in brain heart infusion (BHI) medium in tightly stoppered flasks, which were purged with N2 gas for 45 min before inoculation. The indicator resazurin was added to the medium at a final concentration of 0.0002% (wt/vol). Upon inoculation of freshly purged BHI medium with an anaerobic overnight preculture, there were still minimal amounts of oxygen present in the medium, as indicated by the incomplete disappearance of the pink color of resazurin. After 2 h of culturing, however, the indicator dye had become completely colorless, indicating anaerobic conditions. The cultures were then grown for an additional 4-h period until the culture reached the mid-exponential growth phase, after which cells were either exposed to H2O2 or harvested for the catalase activity assay (see below). The H2O2 resistance of aerobically grown vegetative B. cereus cells was assayed by adding H2O2 from a 30% stock solution directly to a culture in the mid-exponential growth phase to a final concentration of 10 mM H2O2. In a parallel experiment, aerobic cultures were heat shocked from 30°C to 42°C for 30 min to activate σB in B. cereus ATCC 14579, prior to the addition of H2O2. When anaerobically grown, wild-type cells in the mid-exponential growth phase were exposed to 10 mM H2O2, no survivors could be detected, but when 2 mM H2O2 was added to the culture, a killing efficiency that was comparable to that of the exposure of aerobically grown wild-type cells to 10 mM H2O2 was observed. Survival was determined by plating appropriate dilutions on BHI plates, followed by overnight incubation at 30°C. For each time point, samples were plated in duplicate. All survival experiments were performed with three independent cultures.
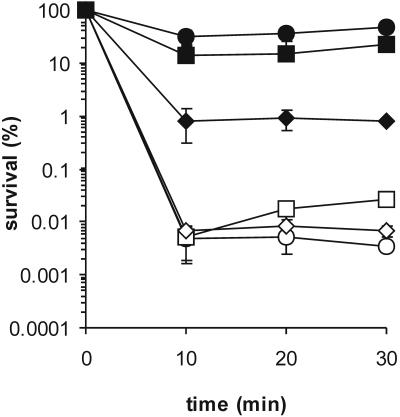
The sigB deletion mutant survived between 6,000- and 10,000-fold better than the parent strain (Fig. 1). In wild-type cells, a heat shock from 30°C to 42°C has a slight cross-protecting effect on H2O2 resistance. Interestingly, the survival rate of heat-shocked B. cereus FM1400 cells after exposure to H2O2 is twofold lower than that of cells of the mid-exponential growth phase. Under anaerobic conditions, the sigB deletion mutant is also more resistant to H2O2 than the parent strain, but the difference in survival rate is now approximately 100-fold, which is markedly less than that under aerobic conditions.
FIG. 1.
Survival rates of B. cereus ATCC 14579 and B. cereus FM1400 during exposure to H2O2. The survival rates of B. cereus ATCC 14579 cells (open symbols) and the cells of sigB deletion mutant B. cereus FM1400 (closed symbols) were determined upon exposure to H2O2. Cells were grown until the mid-exponential growth phase either aerobically or anaerobically. Aerobically grown cultures were exposed to 10 mM H2O2 in the mid-exponential growth phase (circles) or after a heat shock from 30°C to 42°C for 30 min (squares). Anaerobically grown cultures were exposed to 2 mM H2O2 (diamonds). The averages of three independent experiments are shown. The error bars indicate standard deviations.
Increased catalase activity in the sigB deletion mutant.
An important line of defense of bacteria against H2O2 is the enzyme catalase, which breaks down two molecules of H2O2 to water and oxygen. Most bacteria produce one or more catalases to cope with the generation of H2O2, which is a fortuitous by-product of aerobic growth of bacteria (4, 12, 15). Under conditions for which H2O2 concentrations are high, catalase appears to be the most important cellular H2O2-scavenging enzyme (23).
The determination of the catalase activity of whole cells of B. cereus was performed based on previously described methodology (2, 5) with slight modifications. Two-milliliter (for aerobically grown cultures) or 10-milliliter (for anaerobically grown cultures) aliquots of cultures in the mid-exponential growth phase and cultures that were heat shocked from 30°C to 42°C for 30 min were pelleted by centrifugation (13,000 × g, 1 min), washed once in ice-cold phosphate-buffered saline (PBS) (138 mM NaCl, 2.7 mM KCl, 140 mM Na2HPO4, 1.8 mM KH2PO4, adjusted to pH 7.4 with HCl), and resuspended in 1 ml ice-cold PBS. One hundred microliters of the cell suspension was then added to a quartz cuvette containing 900 μl PBS with 44.4 mM H2O2 (the final concentration of H2O2 in the assay was 40 mM). After a mixing, the cuvette was placed in a Shimadzu UV-1601 spectrophotometer and the decrease of the absorbance at 240 nm (A240) was measured over time at a constant temperature of 30°C. One unit of catalase activity was defined as a decrease of 1 in A240 per minute. Catalase activity was corrected for the amount of cells added to the assay buffer as indicated by the value of the OD600 of the cell suspension. This assay was performed in triplicate for all tested conditions.
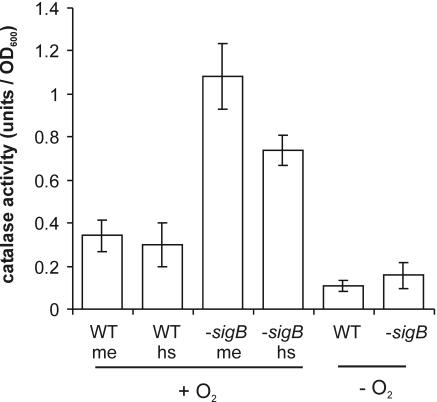
The assay of the catalase activity of whole cells revealed that during the mid-exponential growth phase, catalase activity in the sigB deletion mutant is threefold higher than that in the parent strain (Fig. 2). A heat shock from 30°C to 42°C has no effect on the cellular catalase activity of wild-type cells. This suggests that the catalase KatE, of which the structural gene is expressed only upon heat shock in a σB-dependent fashion (25), has a minor role in H2O2 resistance of B. cereus. In the sigB deletion mutant, a significant decrease is observed in catalase activity upon a heat shock, and this may explain the somewhat lower survival rate of heat-shocked B. cereus FM1400 cells. The catalase activity of anaerobically grown cells is clearly lower than that of cells growing in an aerobic culture, which may explain the increased H2O2 sensitivity of the anaerobically grown cells. Furthermore, under anaerobic conditions, there is no significant difference between the catalase activity of B. cereus ATCC 14579 and the sigB deletion mutant. This suggests that there are other, as yet unidentified, mechanisms than catalase that are operating in the sigB deletion mutant which are responsible for the increased resistance to H2O2 under anaerobic conditions.
FIG. 2.
Catalase activity in B. cereus ATCC 14579 and B. cereus FM1400. Levels of catalase activity of whole cells of B. cereus ATCC 14579 (WT) and B. cereus FM1400 (−sigB) were determined. Levels of catalase activity of aerobically (+O2) grown cells in the mid-exponential growth phase in BHI (me) and after a 30-min heat shock from 30°C to 42°C (hs) were determined. Catalase activity of cells grown in anaerobic (−O2) culture was also determined. Cells were pelleted by centrifugation, washed once in PBS, and resuspended in PBS containing 40 mM H2O2. One unit of catalase activity was defined as a decrease in the absorbance at 240 nm of 1 per minute and adjusted for the OD600 of the cultures. The averages of three independent experiments are shown. The error bars indicate standard deviations.
Expression of katA, which encodes the main vegetative-cell catalase, is upregulated in the sigB deletion mutant.
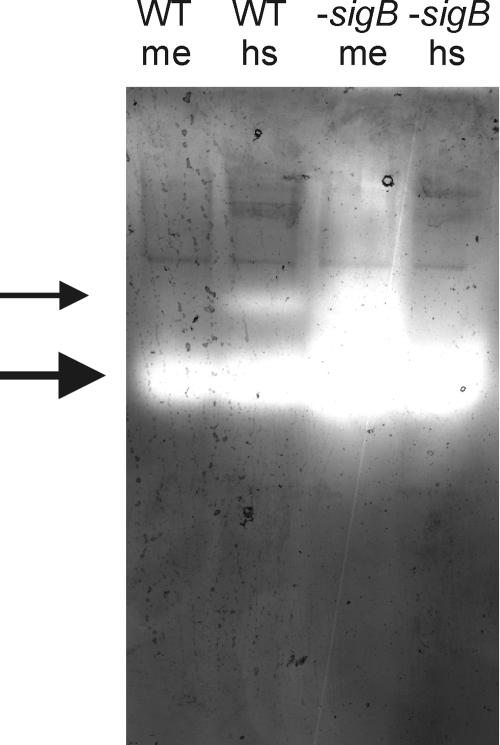
In the B. cereus ATCC 14579 genome sequence (16), three open reading frames encoding catalases can be identified. We termed these catalase genes katA, katB, and katE. The original B. cereus genome sequence codes for these genes are rzc06308, rzc07268, and rzc01424, respectively. katE was previously found to be expressed upon a heat shock in a σB-dependent fashion (25). Because there is such a dramatic increase in catalase activity in the sigB deletion mutant under aerobic conditions, the expression of catalases under these conditions was studied in more detail. First, the catalases that are present in B. cereus during mid-exponential aerobic growth and upon a heat shock from 30°C to 42°C for 30 min were visualized by activity staining of catalases on a native polyacrylamide gel (Fig. 3). Total proteins were isolated from B. cereus cultures as described previously (20), and crude cell-free protein extracts of B. cereus were obtained by pelleting insoluble material by centrifugation at 13,000 × g for 30 min. One hundred micrograms of cell-free protein extracts were separated on a native 10% polyacrylamide gel, and catalases were stained by the method described by Woodbury et al. (27), which results in yellow catalase bands against a dark-green background.
FIG. 3.
Visualization of catalase activity on a native polyacrylamide gel. Cell-free protein extracts were isolated from B. cereus ATCC 14579 (WT) and B. cereus FM1400 (−sigB) cells in the mid-exponential growth phase in BHI (me) and after a 30-min exposure to 42°C (hs). Proteins (100 μg) were separated on a nondenaturing 10% polyacrylamide gel and stained for catalases as described by Woodbury et al. (27), resulting in light catalase bands against a dark background. The large arrow points to the main vegetative-cell catalase. The small arrow points to the σB-dependent catalase KatE.
The highest level of activity staining is observed in the cell extract of the B. cereus sigB deletion mutant, while a heat shock leads to a decrease in catalase activity staining. A single catalase seems to be responsible for the staining, with the exception of the heat-shocked wild-type sample, in which a relatively weak second catalase band appears. This band is probably the σB-dependent catalase KatE, as no such band is present in the protein samples isolated during mid-exponential growth and from the sigB deletion mutant. This again indicates that KatE does not contribute significantly to the total catalase activity of the cell under these conditions.
As the expression of katE was determined previously (25), here the expression of katA and katB in B. cereus ATCC 14579 and its sigB deletion mutant during exponential growth and upon a heat shock from 30°C to 42°C was studied. The techniques used (Northern and primer extension analysis) are described in reference 24. katB is not expressed under the tested conditions, which makes katA the only catalase gene that is expressed during aerobic exponential growth. Indeed, katA is clearly more expressed during exponential growth in the sigB deletion mutant than in the parent strain (Fig. 4A). katA is mainly transcribed as a monocistronic transcript of approximately 1.6 kb. A second, very weak transcript can be a read-through mRNA from the upstream gene rzc05783. Upon a heat shock, the expression of katA is upregulated in both B. cereus ATCC 14579 and the sigB deletion mutant. This is seemingly at odds with the enzyme activity data in Fig. 2, but it is not unlikely that a heat shock leads to the partial inactivation of the catalase, as this enzyme has been previously reported to be thermolabile (1). Furthermore, σB-dependent proteins that are upregulated after a heat shock may act as chaperones, which can repair misfolded proteins in B. cereus ATCC 14579 (25). The absence of these σB-dependent rescue processes may explain the relatively large decrease in cellular catalase activity after a heat shock in the sigB deletion mutant.
FIG. 4.
Transcriptional analysis of katA of B. cereus. (A) Northern blot analysis of the transcription of katA. Total RNA was extracted from B. cereus ATCC 14579 and B. cereus FM1400 cells during mid-exponential growth in BHI (lane 1 and 3, respectively) and after a 10-min exposure to 42°C (lane 2 and 4, respectively). A 32P-labeled internal PCR product of katA was used as a probe. Hybridization of the probe with target RNA was visualized by exposure to a Phosphoscreen and scanning with a Storm scanner. Marker sizes (in kb) are indicated. (B) Primer extension analysis of the promoters 5′ of katA. Total RNA was extracted from B. cereus ATCC 14579 and B. cereus FM1400 cells during mid-exponential growth in BHI (lane 1 and 3, respectively) and after a 10-min exposure to 42°C (lane 2 and 4, respectively). The mapped transcriptional start site is indicated with an arrow. The lanes labeled A, C, G, and T are the corresponding sequencing ladder for the localization of the transcripts. (C) Sequence of the promoter 5′ of katA. Identified −35 and −10 regions are underlined. Bold type indicates the mapped transcriptional start site. The putative PerR-binding site is indicated in italics. The spacing to the ATG start codon of katA is also indicated.
Subsequently, the transcriptional start site of the katA mRNA was mapped by primer extension analysis to determine which promoter sites are responsible for the upregulated katA expression in the sigB deletion mutant (Fig. 4B and C). A single transcriptional start site upstream of katA could be determined. The promoter site that is predicted to be responsible for the transcription of katA is practically identical to the consensus B. subtilis σA-dependent promoter (11). No other promoter sites could be identified upstream from katA, which shows that the σA-dependent promoter is responsible for the transcription of katA. Overlapping the transcriptional start site of the katA mRNA is a sequence that is essentially identical to the recognition site of the regulator PerR in B. subtilis, which has a consensus sequence of TTATAATnATTATAA (12). A practically identical consensus PerR-binding sequence in S. aureus has been described (14). PerR is a metal-binding protein which functions as the central regulator of the peroxide stress response in gram-positive bacteria. In B. subtilis and S. aureus, the deletion of perR leads to the upregulation of catalase activity and increased H2O2 resistance by the abolishment of the repression of katA transcription (3, 14). If, in B. cereus, expression of perR would be dependent on σB, this could serve as an explanation for the H2O2-hyperresistant phenotype of the sigB deletion mutant. However, Northern analysis showed that transcription of perR is not dependent on σB. In fact, perR is transcribed at higher levels in the sigB mutant than in the parent strain (data not shown), which indicates that an abolished repression of katA expression by PerR is not a likely explanation of the H2O2-resistant phenotype.
Concluding remarks.
This study illustrates that mutations in regulatory genes can have unexpected phenotypic consequences. Such pleiotropic effects should be taken into account when industrial processes or antibiotic therapies are developed which target the σB response of gram-positive bacteria. The inactivation of σB may have important consequences, as it may actually lead to an unexpected increased resistance to a subset of stress conditions. This study underlines the importance of thorough phenotypic investigations of sigB deletion mutants, as these can provide crucial knowledge on the precise role of σB in the parental strains.
REFERENCES
- 1.Baillie, A., and J. R. Norris. 1963. Studies of enzyme changes during sporulation in Bacillus cereus, using starch gel electrophoresis. J. Appl. Bacteriol. 26:102-106. [Google Scholar]
- 2.Beers, R. F., and I. W. Sizer. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133-140. [PubMed] [Google Scholar]
- 3.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Chelikani, P., I. Fita, and P. C. Loewen. 2004. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61:192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, Y. P., L. M. Hornstra, W. M. de Vos, and T. Abee. 2004. Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl. Environ. Microbiol. 70:2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelmann, S., and M. Hecker. 1996. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol. Lett. 145:63-69. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira, A., C. P. O'Byrne, and K. J. Boor. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank, J. F. 2001. Milk and dairy products, p. 111-126. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, D.C.
- 10.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmann, J. D., and C. P. Moran, Jr. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 12.Herbig, A. F., and J. D. Helmann. 2002. Metal ion uptake and oxidative stress, p. 405-414. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 13.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, E. S. Dusko, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, G. B., B. M. Hansen, J. Eilenberg, and J. Mahillon. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5:631-640. [DOI] [PubMed] [Google Scholar]
- 18.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 19.Moorhead, S. M., and G. A. Dykes. 2003. The role of the sigB gene in the general stress response of Listeria monocytogenes varies between a strain of serotype 1/2a and a strain of serotype 4c. Curr. Microbiol. 46:461-466. [DOI] [PubMed] [Google Scholar]
- 20.Periago, P. M., W. van Schaik, T. Abee, and J. A. Wouters. 2002. Identification of proteins involved in the heat stress response of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 68:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasko, D. A., M. R. Altherr, C. S. Han, and J. Ravel. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303-329. [DOI] [PubMed] [Google Scholar]
- 22.Schoeni, J. L., and A. C. Wong. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636-648. [DOI] [PubMed] [Google Scholar]
- 23.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Schaik, W., M. H. Tempelaars, J. A. Wouters, W. M. De Vos, and T. Abee. 2004. The alternative sigma factor σB of Bacillus cereus: response to stress and role in heat adaptation. J. Bacteriol. 186:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schaik, W., M. H. Zwietering, W. M. de Vos, and T. Abee. 2004. Identification of σB-dependent genes in Bacillus cereus by proteome and in vitro transcription analysis. J. Bacteriol. 186:4100-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Schaik, W., and T. Abee. 2005. The role of σB in the stress response of gram-positive bacteria—targets for food preservation and safety. Curr. Opin. Biotechnol. 16:218-224. [DOI] [PubMed] [Google Scholar]
- 27.Woodbury, W., A. K. Spencer, and M. A. Stahman. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 44:301-305. [DOI] [PubMed] [Google Scholar]