Abstract
In Chlamydomonas reinhardtii cells, H2 photoproduction can be induced in conditions of sulfur deprivation in the presence of acetate. The decrease in photosystem II (PSII) activity induced by sulfur deprivation leads to anoxia, respiration becoming higher than photosynthesis, thereby allowing H2 production. Two different electron transfer pathways, one PSII dependent and the other PSII independent, have been proposed to account for H2 photoproduction. In this study, we investigated the contribution of both pathways as well as the acetate requirement for H2 production in conditions of sulfur deficiency. By using 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), a PSII inhibitor, which was added at different times after the beginning of sulfur deprivation, we show that PSII-independent H2 photoproduction depends on previously accumulated starch resulting from previous photosynthetic activity. Starch accumulation was observed in response to sulfur deprivation in mixotrophic conditions (presence of acetate) but also in photoautotrophic conditions. However, no H2 production was measured in photoautotrophy if PSII was not inhibited by DCMU, due to the fact that anoxia was not reached. When DCMU was added at optimal starch accumulation, significant H2 production was measured. H2 production was enhanced in autotrophic conditions by removing O2 using N2 bubbling, thereby showing that substantial H2 production can be achieved in the absence of acetate by using the PSII-independent pathway. Based on these data, we discuss the possibilities of designing autotrophic protocols for algal H2 photoproduction.
Hydrogen is often considered a promising energy vector, provided that economically and environmentally relevant ways of production can be developed. Present large-scale methods for H2 production are based on fossil fuels cracking and therefore parallel CO2 emissions. In the long term, clean H2 production should be ideally based on renewable energy sources. Several unicellular green algae have the capacity to produce H2 by using water and sunlight as an energy source. The discovery of H2 photoproduction by photosynthetic eukaryotic algae is rather ancient (7), but the productivity of algae-based systems is still limited and needs to be improved. As a result, investigations are being conducted worldwide to optimize the ability of microalgae to produce H2 (10, 18).
Chlamydomonas reinhardtii is one of the unicellular green algae able to produce H2 under anoxic conditions. During photosynthetic growth, light energy is harvested by chlorophyll antennae, resulting in charge separation at photosystem II (PSII) and O2 release by water photolysis. Electrons are transported through the photosynthetic chain to plastoquinones (PQs), the cytochrome b6/f complex, plastocyanin, PSI, and ferredoxin (Fd). Reduced ferredoxin is used to convert NADP+ to NADPH, thanks to the Fd-NADP+-reductase, and NADPH is then used in reactions of the photosynthetic CO2 reduction cycle (Calvin cycle) to form carbohydrate compounds. Under anoxic conditions, C. reinhardtii cells synthesize an Fe-hydrogenase (14), catalyzing the reversible reduction of protons into molecular hydrogen. In green algae, the Fe-hydrogenase is localized in the chloroplast (13) and accepts electrons directly from reduced ferredoxin to generate H2 (6). Because the Fe-hydrogenase is strongly inhibited by O2, H2 production is sustained only in anoxic conditions (2, 9).
H2 photoproduction can result from two different electron transfer pathways. The first is PSII dependent and involves water photolysis as the source of electrons for PSI, Fd, and the Fe-hydrogenase. The second is PSII independent and uses the catabolism of endogenous organic compounds as a source of reducing power (16). This catabolism provides electrons to the photosynthetic chain at the PQ level, probably through the chlororespiratory pathway (17). An NAD(P)H-plastoquinone oxidoreductase activity is likely involved in the supply of electrons from stromal donors to the PQ pool (11). For both PSII-dependent and PSII-independent pathways, the release of H2 gas would contribute to maintain the photosynthetic chain partially oxidized under anoxia and therefore sustain a basal level of chloroplast and mitochondrial electron transport activity for the generation of ATP needed for survival (16).
One method to induce anoxic conditions is to place C. reinhardtii cells in a sulfur-deprived medium. This triggers a progressive reduction of photosynthetic capacity due to the inactivation of the PSII while respiration is maintained, resulting in a decrease in O2 concentration (22). After 1 to 2 days in these conditions, anoxia is reached, the Fe-hydrogenase is induced, and H2 starts to be released. By this way, C. reinhardtii cells produce H2 for a few days under continuous illumination (17). In these conditions, even if PSII is deeply inhibited, H2 production has been reported to depend essentially on PSII activity (1). In the experiments reported so far in C. reinhardtii cells, significant H2 production has required the addition of acetate to the sulfur-deprived culture medium. The acetate requirement for H2 production implies several limitations for biotechnological applications. Acetate is a relatively expensive compound which is not readily available in nature and must be synthesized. When added to a culture medium, acetate allows rapid development of heterotrophic microorganisms like bacteria, thereby requiring rigorous axenic conditions that could be difficult to apply to large-scale cultures. The role of acetate in the stimulation of H2 production is not clearly understood. By stimulating respiration (17), acetate may help to establish and maintain anoxia when the photosynthetic activity is reduced under sulfur-deprived conditions. C. reinhardtii is capable of efficient heterotrophic growth in the presence of acetate (3, 4), which could be seen as a carbon source for intracellular carbohydrate accumulation, the degradation of which could participate in H2 production. Furthermore, recent data have shown that under sulfur deprivation, algal cells survive by developing a photofermentative pathway (21). Posewitz et al. (19), by studying starch-deficient mutants severely impaired in H2 production, recently illustrated the importance of starch for reaching maximal rates of H2 production.
The aim of this study is to investigate the metabolic pathways involved in H2 production when C. reinhardtii cells are placed under sulfur-deprived conditions. We first investigated the nature of the electron pathway (PSII dependent versus PSII independent) involved in H2 production and then investigated the requirement for acetate. We show that substantial, although not maximal, H2 photoproduction can be obtained in fully photoautotrophic conditions, provided that PSII inhibition is achieved when deprivation-induced carbohydrate accumulation is maximal.
MATERIALS AND METHODS
Culture conditions.
Wile-type Chlamydomonas reinhardtii strain 137c (Chlamydomonas Genetic Center, Duke University, Durham, NC) was used in all experiments. A protocol with two phases was defined for H2 production by microalgae. During the first phase, photosynthetic growth conditions were applied, which allowed cells to duplicate. In the second phase, cells were placed under sulfur-deprived conditions to induce H2 production.
For the first phase, cultures were prepared at 25°C in Erlenmeyer flasks, under constant agitation (130 rpm) and continuous illumination (110 μmol photons · m−2 · s−1) supplied with fluorescent tubes (a mixed array of Cool White and Grolux tubes Sylvania, Germany). Cells density was measured using a Malassez hemacytometer. Cells were cultivated during the first phase until a density of about 5 × 106 cells/ml was reached, corresponding to a late logarithmic growth state. At this stage, 250 ml of algal culture was centrifuged at 1,000 × g for 5 min, washed, resuspended in a sulfur-deprived medium, and placed in a 250-ml Schott bottle closed by a tight septum. Identical temperature, light, and agitation conditions were applied as in the growth phase. Liquid and gas samples were taken daily with a sterile syringe through the septum. Culture liquid samples were used for cell counting and starch reserve quantification. Gas samples were analyzed by mass spectrometry to determine gas phase composition.
Analysis of collected gases.
Gas samples (0.5 ml) were taken from the bottle through the septum with a tight syringe and introduced through a vacuum line into a mass spectrometer (model MM 8-80; VG Instruments, Cheshire, United Kingdom) in order to measure the gas phase composition of the bottles (H2, O2, and CO2).
Description of media used in experiments.
Several experimental conditions were tested for both growth and sulfur-deprived phases. Two different media were tested in the growth phase. The first was Tris-acetate-phosphate (TAP) medium at pH 7.2, and the second was a minimum medium similar to the TAP medium but supplemented with 20 mM NaHCO3 (pH 7.3) instead of acetate. Two methods were employed to transfer cultures to anoxic conditions. The first one consisted of incubating the algae in a sulfur-deprived medium (supplemented either with acetate or with bicarbonate), as sulfur deprivation leads to the inhibition of O2 production by PSII (22). In the second method, PSII was blocked by supplying it with 20 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). In some experiments, nitrogen gas was bubbled to eliminate dissolved oxygen in the medium and to reach anoxic conditions more rapidly.
Starch measurements.
Starch determination was performed using a method slightly modified from that of Klein and Betz (15). Two-ml aliquots of cell suspension were taken from the bottle through the septum with a syringe, centrifuged at 18,000 × g for 2 min, suspended in 1 ml of methyl alcohol for chlorophyll extraction, and centrifuged again. The pellets were rinsed with 1 ml of Na-acetate buffer (100 mM, pH 4.5), resuspended in 350 μl of Na-acetate buffer, and autoclaved for 15 min at 120°C for starch solubilization. Starch assays were then performed with a commercial kit (starch assay kit SA-20; Sigma-Aldrich) based on an enzymatic method following the supplier's recommendations.
RESULTS
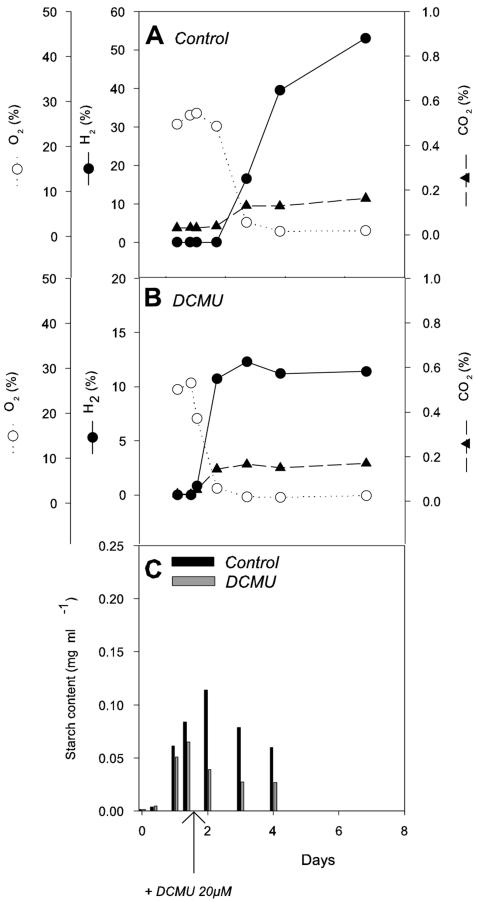
Sulfur deprivation has been reported to trigger H2 production due to a reversible and pronounced decline in PSII-mediated O2 production, resulting in anoxia and hydrogenase induction (17, 22). Under our experimental conditions, anoxia was reached 3 days after sulfur deprivation and a rapid H2 production was observed (Fig. 1A). In order to better understand the effect of sulfur deficiency on H2 production, PSII inhibition was achieved alternatively by using DCMU, a PSII inhibitor. C. reinhardtii cultures were transferred to a sulfur-deprived medium, treated with DCMU, and bubbled with N2 to reach anoxia. In these conditions, a very low H2 production rate was detected (Fig. 1B), clearly showing that the effect of sulfur deprivation is not restricted to the establishment of anoxia and to the inhibition of PSII. Carbohydrate reserves and, more precisely, starch have been recently reported to be an important parameter for H2 production (19). Starch content measurements were performed on the culture (Fig. 1C), showing that the initial starch content (about 0.02 mg · ml−1) rapidly increased in response to sulfur deficiency and reached about 0.22 mg · ml−1 after 1 day. The starch level then quickly decreased until the fourth day. When cells were incubated with DCMU, the initial starch content was low and remained mostly unchanged throughout the experiment, which probably explains why H2 production was scarce in these conditions.
FIG. 1.
Effects of DCMU on H2 production, O2, CO2 exchange, and starch accumulation in sulfur-deprived Chlamydomonas cells. (A) Control in the absence of DCMU. (B) DCMU (20 μM final concentration) was added at t0. Sulfur deprivation was realized by resuspending cells in sulfur-deprived TAP medium at t0. Relative quantities of gases contained in closed flasks were measured by mass spectrometry and are expressed as the percentage of the gas phase volume. (C) Intracellular starch amounts measured at different times in the culture conditions described for panels A and B.
When DCMU was added 24 h after the beginning of sulfur deprivation, significant H2 production was observed (Fig. 2B). Starch accumulation occurred before DCMU addition but stopped once DCMU was added (Fig. 2C). In these conditions, H2 production was lower than in the absence of DCMU (about 20% when compared to standard conditions) (Fig. 2A) but much higher than when DCMU was added at time zero (Fig. 1B). When DCMU was added before optimal starch accumulation, subsequent H2 production was observed but at a lower rate (intermediate between those observed in Fig. 1B and 2B and data not shown).
FIG. 2.
Effects of retarded DCMU addition on H2 production, O2, CO2 exchange, and starch accumulation in sulfur-deprived Chlamydomonas cells. (A) Control in the absence of DCMU. (B) DCMU (20 μM final concentration) was added 24 h after the beginning of sulfur deprivation. (C) Intracellular starch amounts measured at different times in the culture conditions described for panels A and B. Other experimental conditions are similar to those described for Fig. 1.
In the following experiments, the role of acetate in H2 production was investigated by placing algae in an acetate-free medium. Bicarbonate was provided to the culture to optimize photosynthetic performance in photoautotrophic conditions. In the first experiment, following an initial phase of photoautotrophic growth, algae were transferred to sulfur-deprived TAP medium for H2 production. In these conditions, H2 production and starch variations were comparable to previously observed ones (Fig. 1 and 3). In a second experiment, acetate was omitted from the sulfur-deprived minimal medium, which was instead supplemented with bicarbonate. In these conditions, initial starch accumulation was of the same magnitude as that in the acetate-containing medium but it stopped after 1 day, whereas in the presence of acetate, it continued until the second day (Fig. 3C). In the absence of acetate, anoxia was not reached. As a consequence, no H2 production could be detected, although starch was consumed through oxic metabolism. Indeed, an important O2 release occurred after the transition into a sulfur-deprived medium (Fig. 3B), preventing cells from reaching anoxia and producing H2. After 24 h of sulfur deprivation, nitrogen bubbling was performed, allowing the culture to reach anoxia, but anoxia was not maintained. Sulfur deprivation in itself, then, is not sufficient to decrease PSII activity enough in order to maintain anoxia in minimal medium. It should be noted that if acetate was added at this stage instead of bubbling N2, anoxia could be reached and maintained, resulting in H2 production (data not shown).
FIG. 3.
Effects of acetate on H2 production, O2, CO2 exchange, and starch accumulation in sulfur-deprived Chlamydomonas cells. (A) Control in the presence of acetate (TAP medium). (B) Acetate was omitted from the culture medium (minimal medium supplemented with 20 mM bicarbonate); in this experiment, N2 bubbling was achieved 24 h after the beginning of sulfur deprivation. For both experiments, sulfur deprivation was achieved at t0. (C) Intracellular starch amounts measured at different times in the culture conditions described for panels A and B.
Therefore, we decided to test whether H2 production could be obtained in autotrophic conditions under a stronger PSII inhibition, i.e., by DCMU. When DCMU was added at 0 h, no starch accumulation was observed and no H2 was released (Fig. 4A). When DCMU was added after 24 h, the cells had accumulated starch (around 0.06 mg · ml−1), probably from HCO3− fixation by photosynthesis. After DCMU addition, a consumption of the accumulated starch was observed simultaneously with O2 uptake. Once anoxia was reached, some H2 production was observed (Fig. 4B). It appears then that most of the starch accumulated in these conditions was mobilized for O2 uptake rather than for H2 production; therefore, we decided to test whether H2 production could be improved by externally scavenging O2 at the time of DCMU injection. The protocol chosen was to bubble N2 in the vessels for 5 min, after DCMU injection. With DCMU addition and N2 bubbling performed 24 h after the start of sulfur deprivation, the O2 concentration decreased immediately in the medium and H2 production appeared as follows: H2 concentration reached 0.3% of the gas phase after 4 h, 6.9% after 22 h, and 11.6% after 28 h following inhibitor addition (Fig. 4). In this case, H2 was then released in the same amount as when DCMU was added in the presence of acetate (Fig. 2).
FIG. 4.
Effects of DCMU addition and N2 flushing on H2 production, O2, CO2 exchange, and starch accumulation by Chlamydomonas cells in sulfur-deprived minimal medium supplemented with 20 mM bicarbonate. (A) DCMU was added at t0, (B) DCMU was added at 24 h, and (C) DCMU was injected and N2 bubbling was performed at 24 h. For the three experiments, sulfur deprivation was achieved at t0. (D) Intracellular starch amounts measured at different times in the culture conditions described for panels A, B, and C.
In order to test whether starch accumulation and H2 production were indeed due to HCO3− mobilization in the previous experiments, another set of experiments was conducted where cells were cultivated in sulfur-deprived medium with no carbon sources (no acetate and no bicarbonate were added). DCMU was injected into the medium at 0 h, 24 h, and 48 h after the sulfur deprivation was applied. In these three cases, no starch was synthesized and no H2 production was observed, even if anoxic conditions were reached after DCMU addition (data not shown). In order to test the potential for HCO3− utilization for H2 production, we checked whether HCO3− fixation into starch could be used to feed H2 production in the presence of acetate. Then we added 20 mM HCO3− to a sulfur-deprived TAP medium and put C. reinhardtii cells into this medium in H2-producing conditions. Interestingly, this HCO3− addition induced around 20% stimulation in starch accumulation and 20% stimulation in subsequent H2 production when compared to the standard sulfur-deprived TAP control medium (data not shown).
DISCUSSION
H2 production, which occurs in C. reinhardtii in response to sulfur deprivation, relies on the succession of two phases: a growth phase in a standard medium under aerobic conditions, followed by transfer to a sulfur-deprived medium leading to anoxia, thereby allowing H2 production. We have shown here that the aerobic growth phase could be conducted either mixotrophically or autotrophically without a major impact on subsequent H2 production. On the other hand, the presence or absence of acetate during the H2 production stage had dramatic effects on H2 production. During this period, two important phenomena, starch accumulation and the establishment of anoxia (due to PSII decline), are required to obtain optimal H2 production. Starch accumulation is a general and fast response to nutrient deprivation in Chlamydomonas (12). Starch accumulation observed during the transition to a sulfur-deprived medium was an order of magnitude higher than during normal growth. Starch accumulation started during the initial period of sulfur deprivation, in conditions of active photosynthesis. When PSII activity was blocked at the beginning of the sulfur deprivation stage, starch did not accumulate, showing that photosynthetic activity is necessary for starch accumulation. Both PSII activity and sulfur deprivation, then, are necessary to obtain optimal starch formation. In mixotrophic conditions, maximum starch accumulation occurred between 24 and 48 h. In autotrophic conditions, it was observed after 24 h and was immediately followed by a sharp decline (stronger than in mixotrophic conditions). Therefore, the period during which starch content was high (i.e., optimal for transition toward H2-producing conditions) appeared shorter in this case than in the presence of acetate.
Indeed, our experiments have shown a clear correlation between the starch content decrease and the kinetics of H2 production, indicating a central role of starch in H2 production. When PSII was inhibited by DCMU at the beginning of sulfur deprivation, no starch accumulated, resulting in a very small H2 release. When PSII was inhibited after starch started to accumulate, significant H2 production was measured. This clearly shows that PSII-independent H2 production does not operate in the absence of starch. Melis and Happe (16) previously reported the existence of an important catabolism of endogenous substrates simultaneous to H2 production. The nature of substrates being used as an electron source for H2 production was not fully identified, but starch was assumed to be involved, at least as an initial electron donor (6). Indeed, starch is often considered the main endogenous carbohydrate reserve in C. reinhardtii (12). The involvement of starch was recently confirmed by the study of starch-deficient mutants, which were reported to have an 80% reduced H2 production rate compared to the wild type (19). PSII-independent H2 production, measured in the presence of DCMU, did not account for more than about 20% of H2 production measured in the absence of DCMU. Therefore, as stated by Posewitz et al. (19), the contribution of starch is probably not restricted to the alimentation of the PSII-independent pathway; otherwise, the 80% reduction in H2 production observed in starch-deficient mutants would be difficult to explain. Other contributions of starch to H2 production in the standard sulfur deprivation protocol could be (i) acting as a fermentative substrate which would maintain the acting reduced chloroplast pools, (ii) acting as a respiratory substrate which would favor O2 scavenging during H2 production by the PSII-dependent pathway, and (iii) contributing to hydrogenase synthesis and activity, as proposed by Posewitz et al. (19).
The establishment of anoxia is also an important parameter for H2 production. Anoxia is required for induction (20) and activation of the Fe-hydrogenase, and it must be maintained throughout the H2 production phase. In mixotrophic conditions (in the presence of acetate), PSII decrease induced by sulfur deprivation leads to anoxia and H2 production. In photoautotrophic conditions, although photosynthesis activity was reduced by sulfur deprivation, respiration was not sufficiently active to maintain anoxia. As a consequence, the Fe-hydrogenase was not induced and/or active and no H2 was produced. Acetate intervenes during anoxia establishment by both stimulating respiration (16) and accelerating PSII activity decline in the absence of sulfur (8). Once anoxia is reached and H2 production has started, acetate may contribute to the maintenance of a respiration rate sufficient for sustaining anoxic conditions by consuming O2 produced by the remaining PSII activity. However, Ghirardi et al. (10) observed that in anoxia, acetate was not (or was slowly) consumed, showing the importance of another metabolic pool for sustaining O2 scavenging in microoxic conditions. Our experiments and those of Posewitz et al. (19) show that this pool is likely to be constituted by starch. The conjunct presence of acetate and starch is probably critical for sustaining anoxia and optimal H2 production. Alternatively, anoxia can be reached in photoautotrophic conditions by inhibiting PSII (using DCMU). In this case, reaching anoxia consumed a large amount of previously accumulated starch, resulting in a decrease in the H2 production potential.
H2 production is initiated once anoxia is reached and can be achieved using two main pathways, PSII-dependent and PSII-independent pathways. As discussed above, the PSII-dependent pathway can be sustained only if acetate is provided, but it is by far the most efficient. Indeed, although it is always hazardous to infer partitions from observations made in the presence of inhibitors, we can estimate, based on the best rates obtained for H2 production in the presence of DCMU, that about 70 to 80% of this production is PSII dependent (versus 20 to 30% PSII independent) in the standard sulfur deprivation protocol. This is in line with previous reports concluding that photosynthetic water oxidation was the main source of electrons for H2 production (1, 8). Note, however, that the H2 production rate under DCMU is only an approximation and could be an underestimation of the contribution of the PSII-independent pathway. Indeed, the Fe-hydrogenase amount or activity could represent a limitation in DCMU experiments since the long-term effects of such a treatment on hydrogenase synthesis and activation have not been tested. Anyway, the potential for PSII-dependent H2 production depends on how much PSII is still active and how much respiration is able to consume O2. It therefore relies on a subtle equilibrium which might be difficult to maintain. Improving such a process would require the concerted tuning of both PSII activity and respiration. Also, the PSII-dependent pathway has a higher light requirement (four photons/H2) than the PSII-independent one (two photons/H2).
Although PSII-independent H2 production capacity was lower, its initial rate in optimal conditions (i.e., when DCMU was added in the second day in sulfur-deprived TAP, for instance) was near that of the initial H2 production measured in standard sulfur-deprived TAP medium. But the duration of PSII-independent H2 production barely exceeded 24 h, and production stopped before the starch was fully consumed. Improving the capacity of this pathway will require clarifying which enzymes and electron carriers are involved and where the limitations in starch utilization reside. During PSII-independent H2 photoproduction, reduced equivalents from the fermentative catabolism of the endogenous substrates (starch pool) are driven to the photosynthetic chain at the level of PQ (16). In C. reinhardtii, different activities have been proposed to be involved in nonphotochemical PQ reduction, using NADPH or NADH as an electron donor. The existence of a rotenone-sensitive multisubunit complex I was initially suggested by Godde and Trebst (11). However, based on recent sequencing data on the C. reinhardtii nuclear and chloroplast genome, the existence of such a complex seems very unlikely (5). By using a pharmacological approach (18a), a single-subunit flavine containing NADH dehydrogenase (type 2 NADH dehydrogenase) was recently proposed to be involved in both PQ reduction and H2 production. Several limitations of this electron transfer chain, such as the low abundance of the NAD(P)H-PQ oxidoreductase or the formation of a proton gradient, have been discussed (5) and should be considered potential targets for biotechnological modification attempts.
An important issue for future applications of H2 production by microalgae lies in the design of H2 production protocols avoiding the use of organic supply to culture media. We found that in photoautotrophic conditions, cells are able to produce H2 in significant amounts via the PSII-independent pathway, provided that starch accumulated and anoxia was reached. However, strong inhibition of PSII was required in these conditions to maintain anoxic conditions. This was performed by adding DCMU, which is an irreversible method. In the future, reversible methods should be designed, including, for instance, the control of photosynthesis by light intensity to maintain photosynthesis below respiration. In addition, initial removal of O2 from the medium consumes a lot of internal reserves in photoautotrophic conditions. This can be minimized by flushing N2 (as performed in this study). Another possibility would be to limit O2 accumulation by conducting the starch accumulation phase in an open system rather than in a closed system. One can also imagine adding calibrated amounts of acetate when the establishment of anoxia is requested. Thus, H2 production from autotrophic cultures through a sulfur deprivation protocol is possible, but a strong optimization must be achieved before it can be considered relevant. As evidenced in the present study, the main points on which future research efforts should be focused include the kinetics of starch accumulation, the control of PSII activity, the transition toward anoxia compatible with the preservation of starch pools, and the optimization of the PSII-independent H2 production pathway.
Acknowledgments
This work was supported by CNRS (program ENERGIE) and by the European Commission (6th FP, NEST STRP SOLAR-H contract 516510).
We thank especially Patrick Carrier for his help in this study, notably in the maintenance of the mass spectrometer, and Véronique Cardettini for technical assistance. We thank R. Surzycki and B. Geneletti for their help in improving the English writing of the manuscript.
REFERENCES
- 1.Antal, T. K., T. E. Kredeleva, T. V. Laurinavichene, V. V. Makarova, M. L. Ghirardi, A. B. Rubin, A. Tsygankov, and M. Seibert. 2003. The dependence of algal H2 production on photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim. Biophys. Acta 1607:153-160. [DOI] [PubMed] [Google Scholar]
- 2.Benemann, J. R., J. Berenson, N. Kaplan, and M. Kauren. 1973. Hydrogen evolution by a chloroplast-ferredoxin-hydrogenase system. Proc. Natl. Acad. Sci. USA 70:2317-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, F., and M. R. Johns. 1996. Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochem. 31:601-604. [Google Scholar]
- 4.Chen, F., and M. R. Johns. 1994. Substrate inhibition of Chlamydomonas reinhardtii by acetate in heterotrophic culture. Process Biochem. 29:245-252. [Google Scholar]
- 5.Cournac, L., F. Mus, L. Bernard, G. Guedeney, P. Vignais, and G. Peltier. 2002. Limiting steps of hydrogen production in Chlamydomonas reinhardtii and Synechocystis PCC 6803 as analysed by light-induced gas exchange transients. Int. J. Hydrogen Energy 27:1229-1237. [Google Scholar]
- 6.Florin, L., A. Tsokoglou, and T. Happe. 2001. A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J. Biol. Chem. 276:6125-6132. [DOI] [PubMed] [Google Scholar]
- 7.Gaffron, H., and J. Rubin. 1942. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 26:219-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghirardi, M. L., Z. Huang, M. Forestier, S. Smolinski, M. Posewitz, and M. Seibert. 2000. Development of an efficient algal H2-production system, p. 1-10. In Proceedings of the 2000 U.S. DOE Hydrogen Program Review NREL/CP-570-28890, San Ramon, California. National Renewable Energy Laboratory, Golden, Colo.
- 9.Ghirardi, M. L., R. K. Togasaki, and M. Seibert. 1997. Oxygen-sensitivity of algal H2 production. Appl. Biochem. Biotechnol. 63:141-151. [DOI] [PubMed] [Google Scholar]
- 10.Ghirardi, M. L., L. Zhang, J. W. Lee, T. Flynn, M. Seibert, E. Greenbaum, and A. Melis. 2000. Microalgae: a green source of renewable H2. Trends Biotechnol. 18:506-511. [DOI] [PubMed] [Google Scholar]
- 11.Godde, D., and A. Trebst. 1980. NADH as electron donor for the photosynthetic membrane of Chlamydomonas reinhardtii. Arch. Microbiol. 127:245-252. [Google Scholar]
- 12.Grossman, A. 2000. Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist 151:201-224. [DOI] [PubMed] [Google Scholar]
- 13.Happe, T., B. Mosler, and J. Naber. 1994. Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 222:769-774. [DOI] [PubMed] [Google Scholar]
- 14.Happe, T., and J. Naber. 1993. Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 214:475-481. [DOI] [PubMed] [Google Scholar]
- 15.Klein, U., and A. Betz. 1978. Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol. 61:953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melis, A., and T. Happe. 2001. Hydrogen production. Green algae as source of energy. Plant Physiol. 127:740-748. [PMC free article] [PubMed] [Google Scholar]
- 17.Melis, A., L. Zhang, M. Forestier, M. L. Ghirardi, and M. Seibert. 2000. Sustained photobiological hydrogen gas upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 122:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura, Y. 1995. Hydrogen production by biophotolysis based on microalgal photosynthesis. Process Biochem. 30:1-7. [Google Scholar]
- 18a.Mus, F., L. Cournac, V. Cardetitini, A. Caruana, and G. Peltier. 2005. Inhibitor studies on non-photochemical plastoquinone reduction and H2 photoproduction in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1708:322-332. [DOI] [PubMed] [Google Scholar]
- 19.Posewitz, M. C., S. L. Smolinski, S. Kanakagiri, A. Melis, M. Seibert, and M. L. Ghirardi. 2004. Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell 16:2151-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stirnberg, M., and T. Happe. 2004. Identification of a cis-acting element controlling anaerobic expression of the hydA-gene from Chlamydomonas reinhardtii, p. 117-127. In J. Miyake, Y. Igarashi, and M. Rögner (ed.), Biohydrogen III: renewable energy system by biological solar energy conversion. Elsevier Science, Amsterdam, The Netherlands.
- 21.Winkler, M., A. Hemschemeier, C. Gotor, A. Melis, and T. Happe. 2002. [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int. J. Hydrogen Energy 27:1431-1439. [Google Scholar]
- 22.Wykoff, D. D., J. P. Davies, A. Melis, and A. R. Grossman. 1998. The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 117:129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]