Abstract
Different nutrient receptors varied in triggering germination of Bacillus subtilis spores with a pressure of 150 MPa, the GerA receptor being more responsive than the GerB receptor and even more responsive than the GerK receptor. This hierarchy in receptor responsiveness to pressure was the same as receptor responsiveness to a mixture of nutrients. The levels of nutrient receptors influenced rates of pressure germination, since the GerA receptor is more abundant than the GerB receptor and elevated levels of individual receptors increased spore germination by 150 MPa of pressure. However, GerB receptor variants with relaxed specificity for nutrient germinants responded as well as the GerA receptor to this pressure. Spores lacking dipicolinic acid did not germinate with this pressure, and pressure activation of the GerA receptor required covalent addition of diacylglycerol. However, pressure activation of the GerB and GerK receptors displayed only a partial (GerB) or no (GerK) diacylglycerylation requirement. These effects of receptor diacylglycerylation on pressure germination are similar to those on nutrient germination. Wild-type spores prepared at higher temperatures germinated more rapidly with a pressure of 150 MPa than spores prepared at lower temperatures; this was also true for spores with only one receptor, but receptor levels did not increase in spores made at higher temperatures. Changes in inner membrane unsaturated fatty acid levels, lethal treatment with oxidizing agents, or exposure to chemicals that inhibit nutrient germination had no major effect on spore germination by 150 MPa of pressure, except for strong inhibition by HgCl2.
Spores of Bacillus species are germinated by a variety of agents, including chemicals such as specific nutrients, a 1:1 chelate of Ca2+ and pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), and surfactants such as dodecylamine (13, 40). Nutrients trigger germination by binding to receptors located in the spore's inner membrane and encoded by tricistronic operons. There are three such receptors in B. subtilis spores, the GerA receptor responding to l-alanine and the GerB and GerK receptors that together respond to a mixture of l-asparagine, glucose, fructose, and K+. Stimulation of these receptors triggers the release of the spore's large depot of DPA, and this triggers activation of the enzymes CwlJ and SleB, either of which can initiate hydrolysis of the spore's peptidoglycan cortex leading to completion of spore germination (40). Spore germination with exogenous Ca2+-DPA is by activation of CwlJ, whereas dodecylamine causes release of endogenous Ca2+-DPA from the spore core, leading to subsequent events in germination (25, 38).
Spore germination is also triggered by mechanical treatments, including abrasion and high pressures (23, 36). Abrasion activates either CwlJ or SleB (17). In contrast, pressures of 100 to 300 MPa activate the spore's nutrient receptors, while even higher pressures (500 to 800 MPa) release the spore's Ca2+-DPA depot (14, 27, 37, 43, 44). Spore germination by pressures of 500 to 800 MPa also exhibits some differences from nutrient germination, although germination by pressure activation of nutrient receptors appears to be identical to nutrient germination (20, 43). However, the mechanism of nutrient receptor activation by pressure and the factors influencing the rate of spore germination by such pressures are not known. These unknowns are of applied interest given the potential utility of high pressure as an alternative food processing technology, since the stimulation of spore germination contributes to spore killing by high pressure (18, 32). However, the study of spore germination by pressure, in particular via pressure activation of the spore's nutrient receptors, is also warranted since the results of such a study may give new insight into the mechanism of spore germination by nutrients. We report here results of experiments designed to assess the influence of various factors on B. subtilis spore germination with a pressure of 150 MPa.
MATERIALS AND METHODS
B. subtilis strains and spore preparation.
The B. subtilis strains used in the present study are derivatives of and isogenic with strain PS832, a derivative of strain 168 and are listed in Table 1. Some strains were constructed for the present study, and transformation of these strains with selection for appropriate antibiotic resistance was as described previously (28, 29).
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype (phenotype)a | Source or referenceb |
|---|---|---|
| AM1247 | gerB-lacZ (MLSr)c | Anne Moir |
| FB10 | gerBB* | 28 |
| FB20 | ΔgerA::spc (Spr) | 29 |
| FB22 | ΔgerA::spc gerBA* (Spr) | 28 |
| FB58 | PsspB::gerB (Spr) | 30 |
| FB68 | ΔgerK::erm (MLSr) | 29 |
| FB72 | ΔgerA::spc ΔgerB::cat ΔgerK::erm (Cmr MLSr Spr) | 29 |
| FB87 | ΔgerB::cat ΔgerK::erm (Cmr MLSr) | 29 |
| FB122 | ΔsleB::spc ΔspoVF::tet (Spr Tcr) | 25 |
| PS533 | pUB110 (Kmr wild-type) | 41 |
| PS767 | gerA-lacZ (MLSr) | pAAM81d→PS832 |
| PS832 | Wild type | Laboratory stock |
| PS3301 | Δlgt::erm (MLSr) | 15 |
| PS3415 | PsspB::gerBB* (Spr) | 4 |
| PS3476 | PsspD::gerA (MLSr) | 4 |
| PS3477 | PsspD::gerB (Spr) | 4 |
| PS3502 | PsspD::gerBB* (Spr) | 4 |
| PS3608 | ΔgerA::cat (Cmr) | 15 |
| PS3609 | ΔgerA::neo (Kmr) | 15 |
| PS3611 | gerACC18-spc ΔgerB::cat ΔgerK::erm (Cmr MLSr Spr) | 15 |
| PS3612 | gerACA18-spc ΔgerB::cat ΔgerK::erm (Cmr MLSr Spr) | 15 |
| PS3615 | ΔgerA::neo ΔgerB::cat gerKCC21-spc (Cmr Kmr Spr) | 15 |
| PS3616 | ΔgerA::neo ΔgerB::cat gerKCA21-spc (Cmr Kmr Spr) | 15 |
| PS3624 | amyE::Pdes-lacZ-desK::neo Pneo-desR (Kmr ↑Des) | 8 |
| PS3628 | Δdes::erm (MLSr) | 8 |
| PS3651 | ΔgerA::neo ΔgerK::erm | PS3609→FB68 |
| PS3652 | ΔgerA::neo PsspB::gerB (Kmr Spr) | PS3609→FB58 |
| PS3653 | ΔgerA::neo PsspD::gerB (Kmr Spr) | PS3609→PS3477 |
| PS3654 | ΔgerA::neo ΔgerK::erm PsspB::gerB (Kmr MLSr Spr) | FB68→PS3652 |
| PS3655 | ΔgerA::neo ΔgerK::erm PsspD::gerB (Kmr MLSr Spr) | FB68→PS3653 |
| PS3665 | ΔgerA::spc gerBB* ΔgerK::erm (MLSr Spr) | FB20/FB68→FB10 |
| PS3666 | ΔgerA::cat ΔgerK::erm PsspB::gerBB* (Cmr MLSr Spr) | PS3608/FB68→PS3415 |
| PS3667 | ΔgerA::cat ΔgerK::erm PsspD::gerBB* (Cmr MLSr Spr) | PS3608/FB68→PS3502 |
| PS3709 | gerBA-lacZ (MLSr) | AM1247→PS832 |
Abbreviations: Cmr, chloramphenicol (3 μg/ml) resistance; Kmr, kanamycin (10 μg/ml) resistance; MLSr, erythromycin (1 μg/ml) and lincomycin (25 μg/ml) resistance; and Spr, spectinomycin (100 μg/ml) resistance.
Strains to the right of the arrows were transformed with chromosomal DNA from strains to the left of the arrow. If there are two strains to the left of the arrow, the DNA from the strain listed first was used initially, and then the second DNA was used to transform a transformant obtained using the initial DNA.
The gerB-lacZ fusion in this strain is essentially identical to that in strain BC100 (7).
Zuberi et al. (46).
Unless noted otherwise, spores were prepared at 37°C on 2× SG medium plates without antibiotics (24, 26), except for strain PS3624 where kanamycin (10 μg/ml) was present to induce synthesis of the only B. subtilis fatty acid desaturase (Des) (1, 8). Strain FB122 was also sporulated on plates with or without DPA (200 μg/ml); this strain cannot make DPA but can take it up (25). Spores were harvested and cleaned as described previously (26), and all spore preparations used were free (>99%) of germinated spores or growing or sporulating cells as determined by both phase-contrast microscopy and flow cytometry after being stained with Syto 16 (see below). Spores of strain FB122 prepared without or with DPA had <3 or 77%, respectively, of the DPA levels in wild-type (PS533) spores, as found previously (25).
Spore germination by pressure.
Spores whose pressure germination was to be compared were prepared together to minimize effects due to variations in sporulation conditions. Pressure germination used spores at an optical density at 600 nm (OD600) of 1.0 in 50 mM Tris-HCl (pH 7.5). The spores were initially at a temperature of 37°C and were exposed to a pressure of 150 MPa in a PT-1 Research System pressure unit (Avure Technologies, Kent, WA) with water as the pressure medium. The come-up rate when pressure was applied was ∼600 MPa/30 s, and the pressure release time was ∼4 s. A K-type thermocouple passes through the center of the top closure of the pressure chamber, allowing the temperature of the pressure medium to be monitored. After application of a pressure of 150 MPa the peak temperature reached due to adibiatic heating was ∼42°C; this value fell to 41°C after 40 s, to 40°C after 1 min, and to 38°C after 2 min and had returned to 37°C by 3.5 min after application of pressure. After pressure release, aliquots (∼1.3 ml) of the treated spores were frozen in dry ice-ethanol. Freezing of pressure-treated samples had no effect on the percentages of germinated spores determined by flow cytometry (data not shown). Other aliquots of treated spores were tested for viable counts and centrifuged in a microcentrifuge and the OD270 of the supernatant fluid measured to assess release of DPA (4, 38). Control experiments showed that >85% of the OD270 released upon pressure treatments was due to DPA (data not shown).
The frozen pressure-treated samples were thawed, made 0.5 μM in the fluorescent nucleic acid stain Syto 16 (Molecular Probes, Eugene, OR; λmax values for the absorption and fluorescence emissions of the complex with DNA are 488 and 518 nm, respectively) and incubated in the dark for ≥15 min at 23°C. Neither intact dormant spores nor decoated dormant spores that have lost their outer membrane and much of their coat are stained well by nucleic acid stains such as Syto 16, since these stains do not penetrate into the dormant spore core where nucleic acids are located, but germinated spores are stained well (13, 31, 39). Preliminary work using the dyes Syto 9, Syto 11, Syto 12, Syto 13, Syto 14, Syto 15, and Syto 16 (Molecular Probes, Eugene, OR) showed that Syto 16 gave the largest separation in fluorescence between dormant and germinated B. subtilis spores in flow cytometry (data not shown). In order to determine the percentage of germinated spores in a population, ∼105 spores stained with Syto 16 were analyzed in a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) (42). All experiments measuring spore germination by pressure were repeated at least twice, with ≤15% variation in the rates of germination between replicates, and all spores whose rates of germination were to be compared were prepared together and were pressure treated together on the same day.
Spore germination by chemicals.
For analysis of spore germination by chemicals, spores were germinated at an OD600 of 1.0 either with 10 mM l-alanine-20 mM Tris-HCl (pH 8.6) after a heat shock (70°C; 30 min) or without a heat shock with 1 mM dodecylamine-20 mM Tris-HCl (pH 7.4). In both cases spore germination was measured by monitoring DPA release (4, 38). The heat shock synchronizes and increases the rate of spore germination with nutrients but has no effect on spore germination induced by dodecylamine (4, 38).
Other procedures.
Spores were treated with hydrogen peroxide (H2O2), cumene hydroperoxide (CuOOH), or t-butylhydroperoxide (tBHP); the degree of killing was measured; the reagents were inactivated either with catalase (H2O2) or sodium thiosulfate (CuOOH and tBHP); and the spores were washed exhaustively (about eight times) with water prior to pressure treatment (8, 21).
Spores were decoated and subjected to extraction and analysis of fatty acids in the inner membrane as described previously (8). Spores prepared at different temperatures were decoated, extracted, and assayed for β-galactosidase by using methylumbelliferyl-β-d-galactoside (12); values for spores of strain PS832 were subtracted from values for spores of strains carrying lacZ fusions. The latter values were ≥10-fold higher than in spores of strain PS832.
RESULTS
Measurement of spore germination.
Spores are germinated by pressures from ∼100 to 800 MPa, with the higher pressures often giving more rapid and complete germination (27, 43, 44). We used a pressure of 150 MPa in the present study, since preliminary experiments with B. subtilis spores showed that this pressure caused spore germination only via activation of the spore's nutrient receptors (data not shown; see also below). There is also evidence that the spore germination process triggered at a pressure of ∼150 MPa is similar to the nutrient-triggered germination process, whereas this is not the case for germination triggered by higher pressures (27, 43, 44).
We decided to use B. subtilis to analyze factors that influence the pressure germination of spores via activation of the spore's nutrient receptors, since spore germination has been best studied in this organism. In addition, there are many B. subtilis strains with defects in various aspects of spore germination, including strains lacking one, two, or all three of the spore's functional nutrient receptors (29). Most measurements of spore germination induced by pressure have used plate counts with or without a heat treatment to kill germinated spores, but such an assay is not suitable for measuring low levels of spore germination. Consequently, we used flow cytometry, since dormant and germinated spores exhibit very different fluorescence intensities with nucleic acid stains (6, 13, 19, 21, 31, 39, 42), as was the case with spores stained with Syto 16-stained spores germinated by a pressure of 150 MPa (Fig. 1A and B). The major advantages of using flow cytometry are that low levels of spore germination can be measured by using clean dormant spore preparations and removal of spore coat proteins and outer membrane does not result in the staining of spore nucleic acids by Syto 16 (data not shown). In addition, this analysis is not particularly influenced by spore killing. Although with Syto 16 the fluorescence of dead germinated spores was less than that from live germinated spores (see below), it was still easy to distinguish dormant spores and killed germinated spores. In addition, in experiments treating spores with a pressure of 150 MPa at 37°C, there was ≤25% spore killing in up to 10 min (data not shown). To confirm results assessing germination by flow cytometry, we also monitored DPA release, since this is an early step in spore germination induced by either nutrients or high pressure (40). The results using DPA analyses agreed with the flow cytometry results (data not shown), but the flow cytometry was more accurate, in particular with samples exhibiting low levels of germination. In some experiments we also monitored spore germination by phase-contrast microscopy, and again the results agreed with those obtained by flow cytometry (data not shown).
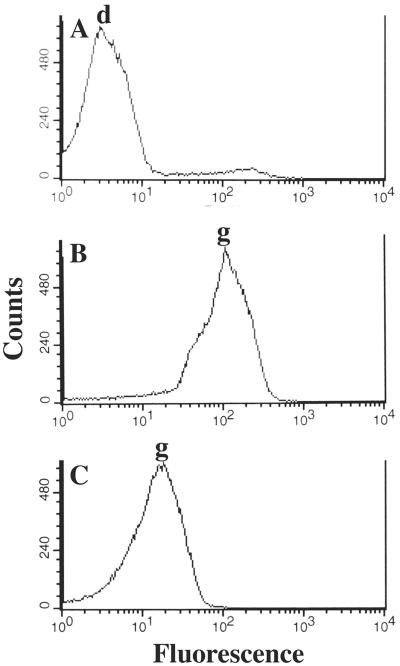
FIG. 1.
Flow cytometry of dormant and germinated spores. Spores of PS533 (wild-type) either dormant (A), treated for 6 min with a pressure of 150 MPa (B), or killed 98% with H2O2 and treated for 7 min with a pressure of 150 MPa (C) were stained with Syto 16 and analyzed by flow cytometry as described in Materials and Methods. Arrows labeled days and g denote dormant and germinated spores, respectively. The fluorescence of dormant spores killed 98% with H2O2 was identical to that of dormant untreated spores, the fluorescence of untreated spores germinated for 30 min with l-alanine was identical to that of pressure-germinated spores, and the fluorescence of spores killed 97 and 98% with CuOOH and tBHP, respectively, and then treated for 7 min with a pressure of 150 MPa was the same as that of pressure-germinated H2O2-killed spores.
Responsiveness of individual nutrient receptors to pressure.
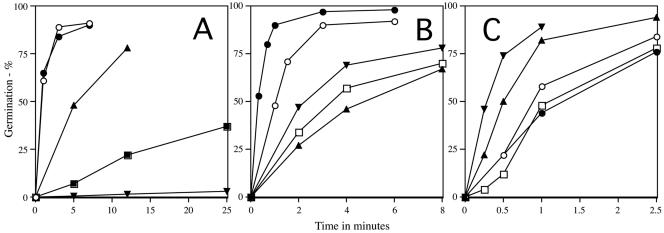
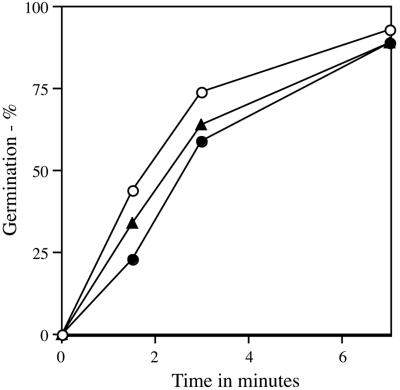
Previous work has shown that germination of B. subtilis spores lacking all nutrient receptors by a pressure of 100 MPa is much slower than is germination of wild-type spores and that much of the germination response of B. subtilis spores to this pressure is due to the GerA and GerB receptors (27, 44). By assessing germination of spores containing only one nutrient receptor, we were able to further quantify the contribution of individual receptors to pressure germination (Fig. 2A). The GerA receptor contributed most to spore germination at a pressure of 150 MPa, since FB87 and PS3611 spores (both gerB gerK) germinated almost as rapidly as wild-type spores (Fig. 2A and Table 2). However, the rates of pressure germination via the GerB or GerK receptors alone (strains PS3615 and PS3651, respectively) were significant, being ca. 20 and 2%, respectively, of the rate of pressure germination via the GerA receptor (Fig. 2A and Table 2). The rate of pressure germination of spores lacking only the GerA receptor (strain FB20) was also essentially identical to that of spores containing only the GerB receptor (data not shown). When no nutrient receptors were present (strain FB72), the rate of spore germination with a pressure of 150 MPa was only ∼0.3% of that given by the GerA receptor alone (Fig. 2A and Table 2). This hierarchy in the rates of pressure germination of spores with different nutrient receptors was seen not only with spores prepared at 37°C but also with spores prepared at 27 and 43°C (see below).
FIG. 2.
Pressure germination of spores with different levels of various nutrient receptors. Spores were treated with a pressure of 150 MPa, and germination was assessed by flow cytometry as described in Materials and Methods. The symbols for the spores of the various strains are as defined as follows. (A) ○, PS533 (wild type); •, FB87 (gerB gerK); ▴, PS3651 (gerA gerK); <▪>, PS3615 (gerA gerB); ▾, FB72 (gerA gerB gerK). (B) ○, PS533 (wild type); •, PS3476 (PsspD::gerA); ▴, PS3651 (gerA gerK); □, PS3655 (gerA gerK PsspD::gerB); ▾, PS3654 (gerA gerK PsspB::gerB). (C) ○, PS533 (wild type); •, FB22 (gerA gerBA*); □, PS3665 (gerA gerK gerBB*); ▾, PS3666 (gerA gerK PsspB-gerBB*); ▴, PS3667 (gerA gerK PsspD::gerBB*).
TABLE 2.
Pressure germination of spores with alterations in diacylglycerylation of nutrient receptorsa
| Strain (relevant genotype) | Germination rate (% of maximum) |
|---|---|
| FB72 (gerA gerB gerK) | 0.3 |
| PS533 (wild type) | 100b |
| PS3301 (lgt) | 8 |
| PS3611 (gerB gerK) | 87 |
| PS3612 (gerAA18gerB gerK) | 2 |
| PS3615 (gerA gerB) | 2 |
| PS3616 (gerA gerB gerKCA21) | 2 |
Spores of various strains were treated with a pressure of 150 MPa, and spore germination was assessed by flow cytometry as described in Materials and Methods.
This value was set at 100%.
One reason for the differences in the rates of pressure germination of spores with different nutrient receptors could be differences in the level of these receptors in spores, and the spore's GerB receptor level is likely to be significantly lower than that of the GerA receptor based on the levels of transcription of the gerA and gerB operons (7, 12). Indeed, the level of β-galactosidase from a gerA-lacZ fusion in spores prepared at 37°C was ∼6-fold higher than from a gerB-lacZ fusion (Table 3). To further determine whether nutrient receptor levels could influence the responsiveness of spores to pressure, we used spores of strains overexpressing the GerA or GerB receptors, the latter in a strain in which GerB is the only nutrient receptor. Overexpression of either the GerA or GerB receptor from the moderately strong, forespore-specific promoter of the sspD gene (PsspD) increased germination rates with pressures 4- and ∼2-fold, respectively, whereas use of the stronger forespore-specific promoter of the sspB gene (PsspB) increased the germination rate via the GerB receptor 3.5-fold (Fig. 2B). PsspB could not be used to drive GerA expression, since this strain does not sporulate (4).
TABLE 3.
Spore levels of β-galactosidase from gerA- and gerB-lacZ fusionsa
| Strain (relevant genotype) | β-Galactosidase (relative sp act)b at sporulation temp:
|
||
|---|---|---|---|
| 27°C | 37°C | 43°C | |
| PS832 (no lacZ fusion) | 1 | 0.8 | 0.7 |
| PS767 (gerA-lacZ) | 245 | 63 | 59 |
| PS3709 (gerB-lacZ) | 51 | 10 | 9.5 |
Spores of various strains were decoated, extracted, and assayed for β-galactosidase as described in Materials and Methods.
The specific activities of β-galactosidase are the amount of substrate hydrolyzed by extracts from 1 ml of spores at an OD600 of 1.0 and are expressed relative to the value in spores of PS832 prepared at 27°C. The latter value was set at 1.
An alternative and not mutually exclusive reason for differences in the ability of individual nutrient receptors to respond to pressure could be intrinsic differences in the pressure sensitivities of various receptors. To determine whether this might be the case, we analyzed spores containing only one of either of two variant GerB receptors with single amino acid changes in either this receptor's A (GerBA) or B (GerBB) proteins (termed the GerBA* or GerBB* receptors, respectively) that allow spore germination in a variety of nutrients not recognized by the wild-type GerB receptor (28). At least the GerBB* receptor is present in spores at the same level as the GerB receptor (4, 30). Spores whose only nutrient receptor was GerBA* or GerBB* germinated as fast as wild-type spores with pressure, and overexpression of the GerBB* receptor from PsspD or PsspB gave spores that germinated 2.5- and 4-fold faster, respectively, with pressure than did spores with the GerBB* receptor expressed from its own promoter (Fig. 2C).
Effect of lipid addition to nutrient receptors on pressure germination.
Covalent addition of diacylglycerol to a cysteine residue in the N-terminal region of the C proteins of some nutrient receptors is essential for receptor function in nutrient germination (15, 40). To assess the role of this diacylglycerylation in pressure germination, we used spores of a strain that lacked the only lipoprotein diacylglycerol transferase, the product of the lgt gene, (also termed gerF [15, 35]). The rate of pressure germination of lgt spores was 8% that of wild-type spores (Table 2), suggesting that diacylglycerylation is needed for nutrient receptors to respond to pressure. Analysis of spores with alanine replacing diacylglycerylated cysteine residues indicated that the responsiveness of the GerA receptor to pressure was almost abolished by this change (in cysteine 18 of GerAC), whereas this change (in cysteine 21 of GerKC) did not alter the GerK receptor's responsiveness to pressure (Table 2).
Effect of inhibitors of nutrient germination on pressure germination.
Since the germinant receptors, in particular GerA, are essential for rapid germination of spores in response to moderate pressures (27), it was of interest to examine the effect of various inhibitors of nutrient receptor function on pressure germination. Previous work has shown that inhibitors of nutrient germination such as HgCl2 strongly (≥95%) inhibit germination of B. subtilis spores by pressures of ∼100 MPa (14, 37, 44). We found that HgCl2 also inhibited germination of spores of strain FB87 that contain only the GerA receptor (Table 4). Unfortunately, it is not known whether HgCl2 inhibits nutrient germination of spores by interacting with the receptors or with some other component of the germination apparatus. Three other compounds—ethanol, octanol, and o-chlorocresol—that strongly inhibit nutrient germination via the GerA receptor but are much less effective at inhibiting nutrient germination via the GerB or GerB* receptors (9) gave either no inhibition of pressure germination of FB87 spores (ethanol and octanol) or only ∼30% inhibition (o-chlorocresol) (Table 4). The ion channel blocker amiloride that blocks nutrient germination via the GerA and GerB receptors (9) also had no effect on pressure germination of FB87 spores (Table 4). Similar results with these inhibitors were obtained when germination of PS533 spores (wild-type) was tested at a pressure of 150 MPa (data not shown).
TABLE 4.
Effects of inhibitors on pressure germination of sporesa
| Inhibitor (concn)b | Germination rate (% of maximum) |
|---|---|
| None | 100c |
| Amiloride (1 mM) | 102 |
| o-Chlorocresol (2 mM) | 67 |
| Ethanol (5%) | 97 |
| HgCl2 (2 mM) | 6 |
| Octanol (0.006%) | 104 |
Spores of strain FB87 (gerB gerK) were treated with a pressure of 150 MPa, and spore germination was assessed by flow cytometry as described in Materials and Methods.
These concentrations of inhibitors gave >85% (amiloride), >90% (HgCl2), and >95% (o-chlorocresol, ethanol, and octanol) inhibition of the rate of spore germination with l-alanine (9).
This value was set at 100%.
Effect of spore DPA content on pressure germination.
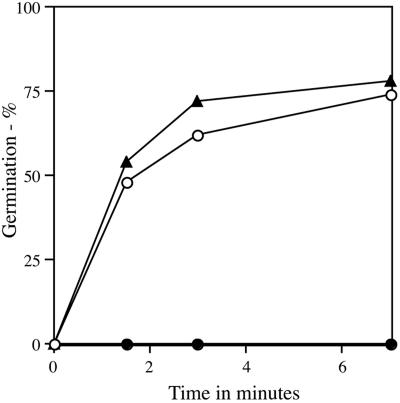
Another small molecule that has a large influence on spore germination with nutrients is DPA, a molecule that normally comprises ≥20% of the dry weight of the spore core. Spores that lack DPA can be generated by using a strain lacking the spoVF operon encoding DPA synthetase (25). Although DPA-less spoVF spores are unstable and germinate spontaneously, stable DPA-less spores are obtained if the sleB gene that encodes one of the spore's two redundant cortex lytic enzymes is also deleted (25). These sleB spoVF spores germinate extremely poorly in nutrients (25), and this was also the case with pressure (Fig. 3). However, if spores of this strain contained near-wild-type DPA levels (obtained by sporulation with added DPA), they germinated normally with pressure (Fig. 3).
FIG. 3.
Effect of spore DPA content on pressure germination. Spores of strains PS533 (wild type) or FB122 (sleB spoVF) prepared with or without DPA were treated with 150 MPa of pressure, and germination was assessed by flow cytometry as described in Materials and Methods. Symbols: ○, PS533 spores; •, FB122 spores prepared without DPA containing <3% of wild-type spore DPA levels; ▴, FB122 spores prepared with DPA and containing 77% of wild-type spore DPA levels.
Effects of inner membrane unsaturated fatty acid content and sporulation temperature on pressure germination.
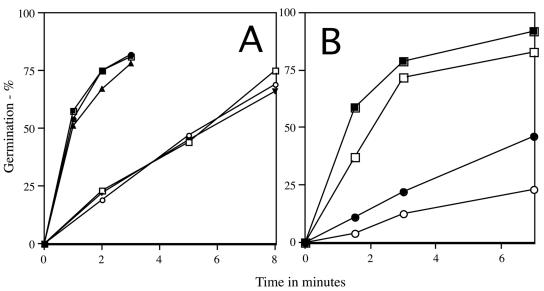
Since the nutrient receptors are in the spore's inner membrane, it was of interest to determine whether changes in this membrane might influence the rate of pressure germination. Previous work has shown that either overproduction or the absence of the B. subtilis fatty acid desaturase, Des, has large effects on levels of unsaturated fatty acids in the B. subtilis spore's inner membrane, in particular at low sporulation temperatures, as well as on levels of fatty acids (1, 8). However, when prepared at the same temperature, wild-type spores and spores lacking or with elevated levels of Des exhibited identical rates of pressure germination (Fig. 4A), despite levels of inner membrane unsaturated fatty acids that in spores made at 27°C ranged from <0.2% (des spores), 0.6% (wild-type spores), and 4.5% (↑Des strainspores), as well as other differences in fatty acid levels (8, 10; data not shown).
FIG. 4.
Effects of changes in unsaturated fatty acid composition and sporulation temperature on pressure germination. Spores of different strains were prepared at various temperatures and treated with 150 MPa of pressure, and germination was assessed by flow cytometry as described in Materials and Methods. (A) Spores prepared at 27°C (○, □, and ▾) and spores prepared at 40°C (•, ▪, and ▴). Circles, PS533 (wild type); squares, PS3624 (↑Des); triangles, PS3628 (Des). (B) PS533 spores prepared at 23°C (○), 30°C (•), 37°C (□), and 44°C (▪).
Another variable that can alter the properties of the spore's inner membrane is the sporulation temperature, since the permeability of the inner membrane decreases and the fatty acid composition changes as the sporulation temperature is increased (10). Although spores of the wild-type, ↑Des, and des strains made at the same temperature germinated equally well with pressure, spores made at 40°C germinated ∼5-fold faster than 27°C spores (Fig. 4A). In contrast, germination with l-alanine was ∼1.5-fold slower in 40°C spores (10; data not shown), although again wild-type, des, and ↑Des spores made at the same temperature germinated at the same rate with l-alanine (±15%; data not shown). With wild-type spores made over an even wider temperature range, spores made at 23°C were 10-fold slower in pressure germination than spores made at 44°C (Fig. 4B and Table 5). Spores with only a single nutrient receptor prepared at 43°C also germinated much faster with pressure than spores of the same strain made at 27°C, as was also the case for spores lacking all nutrient receptors (Table 5). Pressure germination via the GerB or GerK receptors was ∼20-fold faster with 43°C spores but was only ∼6-fold faster via the GerA receptor (Table 5). Strikingly, levels of β-galactosidase from gerA- and gerB-lacZ fusions were four- to fivefold lower in spores made at 43°C compared to levels in 27°C spores (Table 2).
TABLE 5.
Effect of sporulation temperature on pressure germination of spores of various strainsa
| Strain (relevant genotype) | Germination rate (% of maximum) at sporulation temp:
|
|
|---|---|---|
| 27°C | 43°C | |
| PS533 (wild type) | 16 | 100b |
| FB72 (gerA gerB gerK) | <0.1 | 0.4 |
| FB87 (gerB gerK) | 14 | 86 |
| PS3615 (gerA gerB) | 0.1 | 2.5 |
| PS3651 (gerA gerK) | 0.6 | 11 |
Spores of various strains prepared at 27 and 43°C were treated with a pressure of 150 MPa, and spore germination was assessed by flow cytometry as described in Materials and Methods. Values are the averages of results from two separate experiments.
This value was set at 100%.
Effect of spore pretreatment with oxidizing agents on pressure germination.
Previous studies have shown that spore populations killed 90 to 99% by a variety of oxidizing agents have defects in nutrient germination, although they actually germinate faster with the surfactant dodecylamine (8). These and other results have led to the suggestion that such oxidizing agents kill spores by damaging the spore's inner membrane, although this is not by oxidation of unsaturated fatty acids (8). Spore populations killed 96 to 99% by H2O2, CuOOH, or tBHP germinated normally with high pressure (Fig. 5) (also data not shown). However, the germinated killed spores were significantly less fluorescent upon Syto 16 staining than were germinated spores that had not been killed (Fig. 1B and C). The lower fluorescence of the germinated killed spores with Syto 16 may be because these spores are dead, or because germination does not proceed to completion, since at least H2O2-killed spores initiate but do not complete germination (21).
FIG. 5.
Pressure germination of spores killed by oxidizing agents. Spores of strain PS533 (wild type), either untreated or killed with H2O2 or tBHP, were exposed to a pressure of 150 MPa, and spore germination was assessed by flow cytometry as described in Materials and Methods. Symbols: ○, untreated spores; •, spores killed 96% with H2O2; ▴, spores killed 98% with tBHP. Spores killed 97% with CuOOH and exposed to a pressure of 150 MPa germinated at the same rate as the tBHP-treated spores.
DISCUSSION
A major conclusion from the results presented here is that many requirements for the triggering of B. subtilis spore germination through pressure activation of nutrient receptors are similar if not identical to requirements for nutrient activation of these receptors. Thus, diacylglycerylation of the nutrient receptors appears to play a similar role in these receptor's activation by either pressure or nutrients. The GerA receptor's responsiveness to nutrients has an almost absolute requirement for diacylglycerylation of this receptor's C protein (GerAC) (15), and spores containing only the GerA receptor that could not be diacylglycerylated were ∼50-fold slower in pressure germination than were spores with the wild-type GerA receptor. In contrast, the GerK receptor's response to nutrients does not require diacylglycerylation (15), and this was also the case for pressure germination. Although we did not directly test the pressure germination of spores containing only the GerB receptor that was not diacylglycerylated, the rate of pressure germination of lgt spores was 8% that of wild-type spores. Subtracting the contributions of GerA and GerK that are not diacylglycerylated from this value indicates that GerB retains ∼20% of its function in pressure germination when not diacylglycerylated, a value similar to the ∼15% function in nutrient germination retained by GerB that is not diacylglycerylated (15).
A second similarity in nutrient and pressure germination is in the requirement for endogenous DPA for germination of sleB spoVF spores. In wild-type spores nutrient receptor activation by either nutrients or pressure causes the release of the spore's depot of Ca2+-DPA, and this in turn triggers cortex hydrolysis by CwlJ directly and by SleB indirectly (25, 40). In DPA-less spores that lack SleB, cortex hydrolysis cannot be triggered by nutrient receptor activation, since there is no endogenous Ca2+-DPA to be released and activate CwlJ (25). However, if sleB spoVF spores contain DPA they germinate normally with nutrients (25). Consequently, the lack of pressure germination of DPA-less sleB spoVF spores and the rapid pressure germination of sleB spoVF spores that contain DPA were not surprising.
A third similarity in nutrient and pressure germination is the effects on rates of spore germination of overexpression of various nutrient receptors. Since activation of nutrient receptors is the mechanism by which pressures of ∼100 MPa trigger spore germination (27, 44), it was not surprising that increasing the level of the GerA, GerB, or GerBB* receptors increased the rate of germination by such a pressure. Levels of the GerB and GerB* receptors in spores are increased 20- and ∼200-fold when the genes encoding these proteins are under the control of PsspD and PsspB, respectively (4, 30; data not shown). The level of overexpression of the GerA receptor when gerA is under the control of PsspD is not known but seems likely to be ≥5-fold (4; data not shown). Consequently, it was surprising that overexpression of these receptors did not give much larger stimulations of pressure germination. However, the increases in rates of pressure germination upon overexpression of these nutrient receptors were almost identical to those found for rates of nutrient germination of spores with similar elevated receptor levels (4). Why increases in the rates of germination with pressure and nutrients are not proportional to increases in levels of specific nutrient receptors is not clear. Possible explanations for this apparent anomaly are that (i) many of the overexpressed receptors are not functional, although some clearly are and also appear to be properly localized (4, 30; data not shown), and (ii) some other component of the germination apparatus becomes rate limiting for germination of spores with elevated levels of nutrient receptors (4). It is also notable that if levels of β-galactosidase from gerA- and gerB-lacZ fusions are a true reflection of the levels of the GerA and GerB receptors (and see below), then levels of these nutrient receptors may actually be lower in spores prepared at 43°C compared to levels in 27°C spores, yet the 43°C spores germinate more rapidly with pressure of 150 MPa. Clearly, there are factors other than the levels of nutrient receptors that influence the rate of pressure germination.
A fourth similarity in pressure and nutrient germination was in the rates of germination via different nutrient receptors, since the hierarchy in rates of germination by a complex mixture of nutrients due to individual nutrient receptors, GerA > GerB > GerK (29), was also seen for pressure germination. Some of the differences in rates of spore germination triggered by a single nutrient receptor appear due to differences in the levels of these nutrient receptors, since the relative levels of β-galactosidase in spores of strains with gerA and gerB-lacZ fusions paralleled the relative pressure responsiveness of spores with only the GerA or GerB receptors; unfortunately, there is no comparable information for the GerK receptor. Both the gerA- and gerB-lacZ fusions used in the present study are transcriptional fusions with the same promoterless lacZ gene (7, 12), but gerA and gerB mRNAs differ in their translational signals, including both the ribosome-binding site (more optimal in gerA) and the translation start codon (UUG for gerA and AUG for gerB), and thus we cannot be sure that relative levels of β-galactosidase from transcriptional fusions are a precise reflection of relative levels of the GerA and GerB receptors. There are 20 to 25 molecules of the GerBA protein and thus no more than this number of GerB receptors in spores made at 37°C (28), but comparable information on GerA receptor levels in spores is not available.
Although it seems likely that nutrient receptor levels in spores are important in determining the rate of spore germination with pressure, different receptors also may differ in their responsiveness to pressure. This was seen most dramatically with the GerB* receptor variants whose levels are identical to that of their wild-type counterparts (4) but that are ≥5-fold more responsive to pressure. The GerB* receptor variants were selected to have a relaxed specificity for nutrient germinants, and spores with only the GerBA* or GerBB* receptors germinate with d-alanine alone, even better with d-alanine plus d-glucose, with l-asparagine alone and also with other nutrients normally not stimulatory for germination via the GerB receptor (28; K. Ragkousi, J.-L. Sanchez Salas, D. E. Cortezzo, and P. Setlow, unpublished data). The increased responsiveness of spores with the GerBA* and GerBB* receptors to both nutrients and pressure thus suggests that the single amino acid changes in these receptor variants have greatly increased their overall responsiveness to a variety of stimuli, although how this is achieved is not clear. The larger increases in the rates of pressure germination via the GerB and GerK receptors compared to the increase seen in germination via the GerA receptor as spore preparation temperature increased from 27 to 43°C further suggests that different nutrient receptors can respond differently to pressure.
In addition to the similarities between nutrient and pressure germination, there were also some differences. Amiloride, o-chlorocresol, ethanol, and octanol at concentrations that almost completely inhibit nutrient germination, in particular via the GerA receptor, had little if any effect on pressure germination, suggesting that these chemicals may inhibit nutrient germination by interacting with some hydrophobic site on the nutrient receptors, such as the l-alanine binding site on the GerA receptor (45), and not by blocking receptor function directly, although it is possible that alterations in receptor structure at high pressure may preclude inhibitor binding. However, HgCl2, an agent that inhibits spore germination with all nutrients (9), did block pressure germination. Perhaps this chemical inhibits the function of either the nutrient receptors or a protein that acts subsequent to the receptors in the germination process.
Another difference in spore germination with nutrients and a pressure of 150 MPa was in the response of germination rates to spore preparation temperature. The rate of pressure germination of spores prepared at 44°C was ∼10-fold higher than that of spores made at 23°C, and spores with only a single germinant receptor also exhibited large increases in pressure germination as the sporulation temperature increased. These increases are consistent with previous work showing that spores of several species prepared at lower temperatures are more resistant to killing by pressures of 100 to 300 MPa than are spores prepared at higher temperatures (16, 33, 34), since the triggering of germination by pressure is likely the rate-limiting step in pressure killing of spores. In contrast to these results with pressure, the rate of germination with l-alanine decreases 1.5- to 2-fold in B. subtilis spores prepared at 44°C compared to 23°C; rates of germination with dodecylamine also decrease ∼10-fold in spores prepared at 44° compared to 23°C spores (10; data not shown). Spores prepared at higher temperatures are also more resistant to a number of chemicals that kill spores by DNA damage, perhaps because of the decrease in inner spore membrane permeability as sporulation temperature increases (10, 22).
One explanation for the increase in pressure germination with increasing sporulation temperature is that levels of nutrient receptors are higher in spores made at higher temperatures. However, this seems unlikely, since levels of β-galactosidase from gerA- and gerB-lacZ fusions were lower in spores made at higher temperature. Alternative explanations for the effect of sporulation temperature on pressure germination are that the effect is not on nutrient receptors themselves but on (i) properties of the inner spore membrane where these receptors are located or (ii) another component involved in spore germination. We cannot decide between these latter alternatives but favor the first one because sporulation temperature does alter some properties of the spore's inner membrane (10). We focus on the inner membrane not only because the nutrient receptors are located there but also because removal of the spore's coat and outer membrane has no effect on spore germination or killing by pressure (27). In contrast, changes in the inner membrane can alter spore sensitivity to a variety of agents, as well as to some germinants (8, 10). The inner membrane's fatty acid composition changes considerably in spores prepared between 23 and 44°C (8), although current work shows that changes in levels of inner membrane unsaturated fatty acids have little if any effect on pressure germination. Previous work has shown that although the permeability of the spore's inner membrane is quite low, this permeability is even lower in spores made at higher temperatures (10). This further suggests that the fluidity of the spore's inner membrane decreases as the temperature of spore preparation increases, and membrane fluidity affects the response of biological membranes and membrane proteins to pressure (2, 3, 5). Unfortunately, it is difficult to formulate a model of how sporulation temperature and inner membrane fluidity affect the pressure responsiveness of nutrient receptors, since neither how these receptors function nor the likely unusual structure of the spore's inner membrane (11) are currently known.
Acknowledgments
This study was supported by a grant from the U.S. Department of Agriculture (2003-35201-13553) to D.G.H. and P.S. E.P.B. is a recipient of a Traveling Scholarship in Food Science and Technology from the National University of Ireland and is also supported from grant funds to A. L. Kelly and G. F. Fitzgerald at University College Cork.
We are grateful to A. Moir for strain AM1247.
REFERENCES
- 1.Altabe, S. G., P. S. Aguilar, G. M. Cabellero, and D. de Mendoza. 2003. The Bacillus subtilis acyl lipid desaturase is a Δ5 desaturase. J. Bacteriol. 185:3228-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, D. H. 2002. Pressure effects on in vivo microbial processes. Biochim. Biophys. Acta 1595:367-381. [DOI] [PubMed] [Google Scholar]
- 3.Braganza, L. F., and D. L. Worcester. 1986. Structural changes in lipid bilayers and biological membranes caused by hydrostatic pressure. Biochemistry 25:7484-7488. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadei, M. A., P. Mañas, G. Niven, E. Needs, and B. M. Mackey. 2002. Role of membrane fluidity in pressure resistance of Escherichia coli NCTC8164. Appl. Environ. Microbiol. 68:5965-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comas-Riu, J., and J. Vives-Rego. 2002. Cytometric monitoring of growth, sporogenesis, and spore cell sorting in Paenibacillus polymyxa (formerly Bacillus polymyxa). J. Appl. Microbiol. 92:475-481. [DOI] [PubMed] [Google Scholar]
- 7.Corfe, B. M., A. Moir, D. Popham, and P. Setlow. 1994. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology 140:3079-3083. [DOI] [PubMed] [Google Scholar]
- 8.Cortezzo, D. E., K. Koziol-Dube, B. Setlow, and P. Setlow. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes the spores to subsequent stress. J. Appl. Microbiol. 97:838-852. [DOI] [PubMed] [Google Scholar]
- 9.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96:725-741. [DOI] [PubMed] [Google Scholar]
- 10.Cortezzo, D. E., and P. Setlow. 2005. Analysis of factors influencing the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J. Appl. Microbiol. 98:606-617. [DOI] [PubMed] [Google Scholar]
- 11.Cowan, A. E., E. M. Olivastro, D. E. Koppel, C. A. Loshon, B. Setlow, and P. Setlow. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are immobile. Proc. Natl. Acad. Sci. USA 101:7733-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feavers, I. M., J. Foulkes, B. Setlow, D. Sun, W. L. Nicholson, P. Setlow, and A. Moir. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol. Microbiol. 4:275-282. [DOI] [PubMed] [Google Scholar]
- 13.Gould, G. W. 1969. Germination, p. 397-444. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, London, England.
- 14.Gould, G. W., and A. J. H. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi, T., B. Setlow, M. Paidhungat, and P. Setlow. 2004. Analysis of the effects of a gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 186:2984-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igura, N., Y. Kamimura, M. S. Islam, M. Shimoda, and I. Hayakawa. 2003. Effects of minerals on resistance of Bacillus subtilis spores to heat and hydrostatic pressure. Appl. Environ. Microbiol. 69:6307-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, C. A., N. L. Padula, and P. Setlow. Effect of mechanical abrasion on the viability, disruption and germination of spores of Bacillus subtilis. J. Appl. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 18.Knorr, D. 1999. Novel approaches in food-processing technology: new technologies for preserving foods and modifying function. Curr. Opin. Biotechnol. 10:485-491. [DOI] [PubMed] [Google Scholar]
- 19.Laflamme, C., S. Lavigne, J. Ho, and C. Duchaine. 2004. Assessment of bacterial endospore viability with fluorescent dyes. J. Appl. Microbiol. 96:684-692. [DOI] [PubMed] [Google Scholar]
- 20.Margosch, D., M. G. Gänzle, M. A. Ehrmann, and R. F. Vogel. 2004. Pressure inactivation of Bacillus endospores. Appl. Environ. Microbiol. 70:7321-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melly, E., A. E. Cowan, and P. Setlow. 2002. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J. Appl. Microbiol. 93:316-325. [DOI] [PubMed] [Google Scholar]
- 22.Melly, E., P. C. Genest, M. E. Gilmore, S. Little, D. L. Popham, A. Driks, and P. Setlow. 2002. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 92:1105-1115. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, England.
- 25.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paidhungat, M., B. Setlow, W. B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of initiation of germination of spores of Bacillus subtilis by pressure. Appl. Environ. Microbiol. 68:3172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paidhungat, M., and P. Setlow. 2000. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragkousi, K., A. E. Cowan, M. A. Ross, and P. Setlow. 2000. Analysis of nucleoid morphology during germination and outgrowth of spores of Bacillus species. J. Bacteriol. 182:5556-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raso, J., and G. Barbosa-Canovas. 2003. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food Sci. Nutr. 43:265-285. [DOI] [PubMed] [Google Scholar]
- 33.Raso, J., G. Barbosa-Canovas, and B. G. Swanson. 1998. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J. Appl. Microbiol. 85:17-24. [DOI] [PubMed] [Google Scholar]
- 34.Raso, J., M. M. Gongora-Nieto, G. V. Barbosa-Canovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 35.Robinson, C., C. Rivolta, D. Karamata, and A. Moir. 1998. The product of the yvoC (gerF) gene of Bacillus subtilis is required for spore germination. Microbiology 144:3105-3109. [DOI] [PubMed] [Google Scholar]
- 36.Rode, J., and J. W. Foster. 1960. Mechanical germination of bacterial spores. Proc. Natl. Acad. Sci. USA 46:118-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sale, A. J. H., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 38.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 39.Setlow, B., C. A. Loshon, P. C. Genest, A. E. Cowan, C. Setlow, and P. Setlow. 2002. Mechanisms of killing of spores of Bacillus subtilis by acid, alkali, and ethanol. J. Appl. Microbiol. 92:362-375. [DOI] [PubMed] [Google Scholar]
- 40.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 41.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature-sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71-77. [DOI] [PubMed] [Google Scholar]
- 43.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressure. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wuytack, E. Y., J. Soons, F. Poschet, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuda-Yasaki, Y., S. Namiki-Kanie, and Y. Hachisuka. 1978. Inhibition of Bacillus subtilis spore germination by various hydrophobic compounds: demonstration of hydrophobic character of the l-alanine receptor site. J. Bacteriol. 136:484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuberi, A. R., A. Moir, and I. M. Feavers. 1987. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene 51:1-11. [DOI] [PubMed] [Google Scholar]