Abstract
For economic, agricultural, and environmental reasons, composting is frequently used for organic waste recycling. One approach to limiting the potential risk from bacterial food-borne illnesses is to ensure that soil amendments and organic fertilizers are disinfected. However, more knowledge concerning the microbiological safety of composted substrates other than sludge and manure is necessary. Experimental in-vessel biowaste composts were used to study the survival of seeded Listeria monocytogenes, Salmonella enterica subsp. enterica serotype Enteritidis, and Escherichia coli. Four organic waste mixtures, containing various proportions of paper and cardboard, fruits and vegetables, and green waste, were composted in laboratory reactors with forced aeration. The physicochemical and microbiological parameters were monitored for 12 weeks during composting. The survival of bacteria over a 3-month period at 25°C was assessed with samples collected after different experimental composting times. Strain survival was also monitored in mature sterilized composts. Nonsterile composts did not support pathogen growth, but survival of seeded pathogens was observed. Salmonella serovar Enteritidis survived in all composts, and longer survival (3 months) was observed in mature composts (8 and 12 weeks of composting). Mature biowaste composts may support long-term survival of Salmonella serovar Enteritidis during storage at room temperature. E. coli and L. monocytogenes survival was observed only in 4-week-old composts and never in older composts. Proper composting may prevent long-term survival of E. coli and L. monocytogenes. These results suggest that like composted sewage sludge or manure, domestic waste composts may support pathogen survival. Survival was not related to the physicochemical characteristics of the composts.
The presence of pathogens in food and outbreaks of food-associated diseases can be linked to the poor microbial quality of raw agricultural products (5, 9). Furthermore, the transfer of microbial pathogens, such as Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella enterica serovar Typhimurium, from amended soil to fresh agricultural products has been described (3, 24, 37). Composting is a time-honored biological treatment used for organic wastes; the resulting composts are useful in agriculture and horticulture for improving many soil attributes. However, microbial safety of organic amendments and fertilizers used in agriculture is required to prevent colonization by food-borne illness pathogens, like E. coli O157:H7 and Salmonella spp. (9). In order to reduce the likelihood of pathogen transfer from waste to fresh produce, several international standards for meeting pathogen limits through composting are available (14, 39). According to the U.S. Environmental Protection Agency (US-EPA) standard, class A composts must not exceed maximal limits for either Salmonella spp. (less than 3 most probable numbers [MPN]/4 g) or thermotolerant coliforms (less than 1,000 MPN/g) (39). A draft of a forthcoming European Community standard for the microbial quality of composted biowaste includes the absence of Salmonella spp. and Clostridium perfringens in 50 g and 1 g, respectively (14). Finally, in France, the agricultural use of sewage sludge composts is subject to compliance with French standard NF U 44-095, which sets the following limits: for E. coli, less than 1,000 MPN/g; for C. perfringens, less than 100 CFU/g; for Enterococcus spp., less than 105 CFU/g; and absence of Salmonella spp., viable nematode ova, and L. monocytogenes in 25 g (1).
Some raw organic waste used in composting may contain pathogens. The presence of L. monocytogenes has been reported in sewage sludge (2, 7, 17, 33) and on plants (42, 44), including vegetables (5). Salmonella spp. and nonpathogenic E. coli have been detected in sewage sludge (10, 13, 29), in municipal solid wastes (8, 20), and in animal waste in which E. coli O157:H7 was also present (19). If properly managed, composting can reduce pathogen levels (8, 20, 31, 32). Inactivation of nonpathogenic E. coli, pathogenic E. coli O157:H7, and Salmonella spp. has been reported during composting of several types of waste, such as animal manure and sewage sludge (10, 21, 25, 26, 29, 38). However, persistence of Listeria spp., Salmonella spp., and nonpathogenic E. coli during composting (10-12) and survival of Salmonella spp. and nonpathogenic E. coli in mature composts have been reported (22, 34-36). Most investigations concerning E. coli and Salmonella spp. have focused on manure or sewage sludge composts, but little attention has been paid to other composted substrates, such as green waste and the organic fraction of municipal solid waste. Moreover, in spite of the presence of L. monocytogenes in various raw wastes, the behavior of this organism in composted substrates remains unclear (18). The aim of this study was to evaluate the survival of L. monocytogenes, S. enterica subsp. enterica serotype Enteritidis, and E. coli in four experimental biowaste composts. Laboratory reactors with forced aeration were used to conduct in-vessel composting of four organic waste mixtures containing different proportions of paper and cardboard, fruits and vegetables, and green waste. The physicochemical and microbiological parameters were monitored for 12 weeks during composting. Samples collected during composting were inoculated with rifampin-resistant (Rifr) strains of the bacteria mentioned above and incubated at 25°C for 3 months to simulate storage.
MATERIALS AND METHODS
Composting and sampling procedure.
Four mixtures, mixtures A, B, C, and D, were prepared from 50-mm chopped vegetables (apples, oranges, lettuce, carrots, and zucchini), green waste (20 mm, crushed), and 20- to 50-mm chopped paper and cardboard (1:3 to 2:3, wt/wt) (Table 1). Water was added when necessary in order to obtain the same initial moisture content. These four mixtures were composted in laboratory reactors designated reactors A, B, C, and D using an in-vessel composting method.
TABLE 1.
Initial organic waste proportions and amounts of water added at the beginning of the composting process in the four reactors
| Reactor | Basis | Content (%)
|
|||
|---|---|---|---|---|---|
| Paper and cardboard | Fruits and vegetables | Green waste | Added water | ||
| A | Wet wt | 8 | 31 | 55 | 6 |
| Dry wt | 21 | 8 | 71 | 0 | |
| B | Wet wt | 10 | 54 | 36 | 0 |
| Dry wt | 28 | 17 | 55 | 0 | |
| C | Wet wt | 15 | 54 | 27 | 3 |
| Dry wt | 44 | 17 | 40 | 0 | |
| D | Wet wt | 21 | 54 | 18 | 7 |
| Dry wt | 59 | 16 | 26 | 0 | |
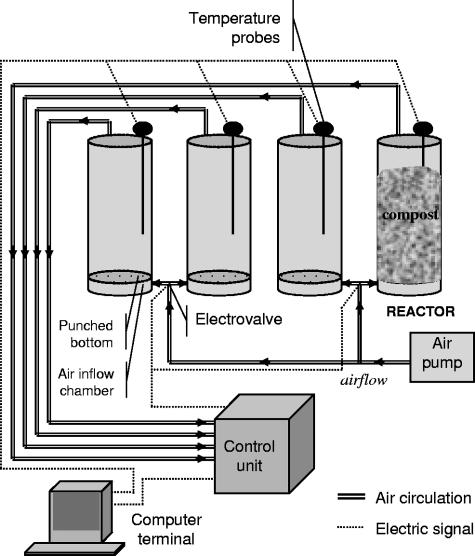
The reactors consisted of polyvinyl chloride cylinders that were 90 cm high and 40 cm in diameter, had a capacity of 170 liters, and were insulated with a layer of glass wool. The composting process lasted 12 weeks, and aeration was provided through the bottom of each reactor (Fig. 1). The airflow decreased from 400 liters h−1 during the first month to 60 liters h−1 at the end of the incubation. The temperature in the core of each reactor was continuously monitored with a 50-cm-long temperature probe. After 1, 2, 4, 6, 8, 10, and 12 weeks, the contents of each reactor were mixed before sampling.
FIG. 1.
In-vessel aerated forced system used for composting.
Physicochemical analysis and organic matter characterization.
Subsamples were freeze-dried at −110°C for 24 h and ground (<1 mm) for physicochemical analysis. Total organic carbon and total nitrogen contents were determined by elementary analysis. Biochemical fractionation of compost organic matter (OM) into soluble organic matter, hemicellulose, cellulose, and lignin was done using crude fiber analysis as described by Van Soest (41), as modified by Robin (30). The compost pH was measured in water (1:5, vol/vol).
Total alkaline-soluble humic substances (fulvic fraction [FA] and humic fraction [HA]) were extracted with 100 ml of 0.1 M NaOH from 2-g dry, ground compost samples over a 2-h period and were recovered by centrifugation (15 min at 8,600 × g). The HA was precipitated overnight in 20 ml of the supernatant at 4°C after the pH was decreased to 1.5 by adding 1.84 ml of 2 M H2SO4. After centrifugation (15 min at 6,300 × g), the acid-soluble FA was separated from the non-acid-soluble humic fraction. The total organic C in the extracts was analyzed using a liquid-phase carbon analyzer (Shimadzu TOC-ASI 5000). The humification index (HA/FA) was used as an indicator of compost maturity (16).
Soil-compost mixtures were incubated for 28 days, during which the kinetics of compost organic matter mineralization were monitored. For these mixtures, soil was taken from the upper layer (0 to 28 cm) of a loamy soil with the following characteristics: clay, 187 g kg−1; silt, 756 g kg−1; sand, 57 g kg−1; pH 6.9; organic carbon content, 11.6 g kg−1; and total N content, 1.19 g kg−1. The soil was sieved with a 5-mm sieve and stored at 4°C until it was used. Three replicates of soil-compost mixtures, each containing the equivalent of 25 g of dry soil and dried ground compost, were incubated in 0.5-liter hermetically sealed jars for 28 days at 28 ± 1°C in the dark. The amounts of incorporated compost were calculated so that all mixtures contained the same proportion of organic carbon (4 g of organic carbon per kg of dry soil). The water content of the mixtures was adjusted to 80% of the soil water-holding capacity (19.11% on wet basis) with ultrapure water and was monitored during the incubation period. Control incubations were performed with only soil. In all jars, both control and experimental, carbon mineralized in the form of CO2 was trapped in 10 ml of 1 M NaOH, which was replaced after 1, 3, 7, 10, 14, 21, and 28 days of incubation, and was analyzed by colorimetry with a continuous-flow analyzer (Skalar, The Netherlands).
Microbial analysis of composts.
For each compost, 10 g (equivalent dry weight) was suspended in 90 ml of 0.1% (wt/vol) sodium pyrophosphate (40) and mechanically shaken (45 min, 320 rpm, 25°C). The resulting slurries were serially diluted, and bacteria were enumerated by pour plating. Total indigenous culturable mesophilic bacteria were recovered on plate count agar (Biokar Diagnostics, Beauvais, France) to which cycloheximide (0.2 g liter−1) was added to inhibit fungi. Spore-forming bacteria were enumerated after heat treatment (80°C, 10 min). The plate count agar plates were incubated for 48 h at 30°C. Total coliform and thermotolerant coliform counts were determined on deoxycholate (0.1%) lactose agar (Biokar Diagnostics, Beauvais, France) after incubation for 24 h at 37°C and 44°C, respectively. Microbial analyses were performed in triplicate. Differences between the four mixtures were identified by one-way analysis of variance, followed by pairwise multiple comparison by the Holm-Sidak method.
Bacterial strains and culture media.
Rifampin-resistant mutants of wild-type strains L. monocytogenes EGD-e, S. enterica subsp. enterica serotype Enteritidis CIP 81.3 (Institut Pasteur Collection, Paris, France), and E. coli K-12 (E. coli Genetic Stock Center, New Haven, Conn.) were used. Rifr mutants were isolated as described by Lung et al. (25), except that rifampin was added at a concentration of 0.2 g liter−1. For each strain, the growth rate and the maximum growth of 10 Rifr colonies were compared to the growth rate and the maximum growth of wild-type strains after incubation in tryptic soy broth (TSB) (tryptone, 17 g liter−1; peptone, 3 g liter−1; NaCl, 5 g liter−1; K2HPO4, 2.5 g liter−1; dextrose, 2.5 g liter−1; pH 7.3) for 24 h at 37°C. Mutants with growth characteristics which were similar to those of the wild-type strains were selected. The stability of the rifampin resistance trait was analyzed by culturing organisms for 100 generations. Based on these criteria, one Rifr strain of L. monocytogenes, one Rifr strain of Salmonella serovar Enteritidis, and one Rifr strain of E. coli were selected.
The selective media for Rifr L. monocytogenes, Salmonella serovar Enteritidis, and E. coli were Palcam medium, Hektoen medium, and Drigalski medium (Biokar Diagnostics, Beauvais, France), respectively, supplemented with rifampin (0.2 g liter−1) and cycloheximide (0.1 g liter−1). Modified Drigalski and Hektoen media were incubated at 37°C for 24 h, and modified Palcam medium was incubated at 37°C for 72 h.
Inoculation of composts and survival test.
Composts were inoculated with the Rifr strain of either L. monocytogenes, Salmonella serovar Enteritidis, or E. coli. Each strain was precultured twice in TSB (8 h at 37°C). The inoculum was prepared after 16 h of incubation at 37°C in TSB. Compost samples collected after 1, 2, 4, 8, and 12 weeks of composting were inoculated (102 CFU g [dry weight]−1) with 2.5-ml bacterial suspensions using appropriate dilutions.
Each inoculated compost sample (30 g [equivalent dry weight]) was placed in a sterile air-tight flask. One 25-ml test tube containing 10 ml of sterile distilled water was added to each flask to maintain the moisture content. The flasks were incubated for 90 days at 25°C, opened once daily to provide O2, and sampled after 3, 8, 30, 60, and 90 days to enumerate the inoculated bacteria. To ensure that compost samples were free of indigenous rifampin-resistant microorganisms, unseeded composts were used as controls during survival tests. When necessary, survival tests were performed with sterile compost treated by ionization. Several 100-g bag-packed samples were hung 20 cm from a 60Co source. Each sample received 50 kGy of radiation and was turned halfway through the treatment. All survival tests were done in triplicate in three independent experiments.
Quantification and detection of inoculated bacteria.
At each sampling time, slurries were prepared by adding 1 g (equivalent dry weight) of compost to 9 ml of 0.1% sodium pyrophosphate (40) and shaking the mixture (320 rpm, 45 min, room temperature) to the resuspend bacteria. The slurries and serial dilutions were plated on selective solid media. In order to reduce the detection limit, selective enrichments were also performed at each sampling time. Enrichment was performed for Rifr L. monocytogenes, Salmonella serovar Enteritidis, and E. coli on enrichment broth supplemented with rifampin (0.1 g liter−1) (Fraser broth [Biokar Diagnostics, Beauvais, France], TSB, and TSB supplemented with 0.1% deoxycholate, respectively). Each enrichment broth was streaked for detection after incubation at 37°C for 24 h.
RESULTS
Temperature during composting.
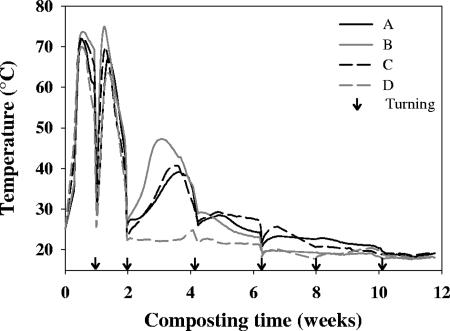
In the four reactors, the temperature remained above 60°C during the first 2 weeks (Fig. 2). Our reactors thus met the US-EPA criteria (39): the temperature was at least 55°C during 3.5 consecutive days in the first week of composting and during 2.5 consecutive days in the second week of composting. After the second turning, the temperature increased to 48, 40, and 39°C in reactors A, B, and C, respectively, but remained 24°C in reactor D. After the third turning, the temperature progressively decreased in all reactors.
FIG. 2.
Temperature during composting of organic wastes in the four reactors. The reactor A waste content was as follows (on a wet weight basis): paper and cardboard, 8%; fruits and vegetables, 31%; and green waste, 55%. The reactor B waste content was 10% paper and cardboard, 54% fruits and vegetables, and 36% green waste; the reactor C waste content was 15% paper and cardboard, 54% fruits and vegetables, and 27% green waste; and the reactor D waste content was 21% paper and cardboard, 54% fruits and vegetables, and 18% green waste.
Physicochemical characteristics during composting.
A decrease in the total dry mass was observed in all reactors (Table 2). The total organic carbon content also decreased, while the total nitrogen content increased, leading to a decrease in the C/N ratio. The larger proportions of paper and cardboard in reactors C and D explained the high initial C/N ratios in these reactors. In reactor C, active composting resulted in a large decrease in the C/N ratio, which was not as pronounced in reactor D.
TABLE 2.
Changes in dry mass and organic matter characteristics of the waste mixtures during composting: total organic C and N, C/N, pH, and proportion of organic C mineralized after 28 days of incubation in soil
| Reactora | Age (weeks) | Dry mass (kg) | Organic C (%, dry wt) | Organic N (g kg−1, dry wt) | C/N | pH | Humification indexb | C mineralization (% of organic C) |
|---|---|---|---|---|---|---|---|---|
| A | 0 | 19 | 33.6 | 9.2 | 36.6 | 6.2 | 0.65 | 20 |
| 1 | 19 | 34.9 | 8.6 | 40.4 | 6.8 | |||
| 2 | 16 | 33.1 | 9.8 | 33.7 | 7.9 | 0.64 | 15 | |
| 4 | 15 | 30.2 | 10.9 | 27.7 | 7.9 | 0.86 | 14 | |
| 8 | 14 | 29.5 | 10.9 | 27.0 | 7.5 | 1.32 | 12 | |
| 12 | 14 | 29.1 | 11.3 | 25.8 | 8.3 | 1.15 | 11 | |
| B | 0 | 18 | 32.5 | 10.1 | 32.2 | 5.9 | 0.33 | 27 |
| 1 | 15 | 36.4 | 8.6 | 42.4 | 7.3 | |||
| 2 | 12 | 35.0 | 10.2 | 34.3 | 8.3 | 0.70 | 18 | |
| 4 | 11 | 29.6 | 11.0 | 26.8 | 8.1 | 1.03 | 17 | |
| 8 | 10 | 30.5 | 13.3 | 22.9 | 8.3 | 1.18 | 11 | |
| 12 | 10 | 30.8 | 13.5 | 22.8 | 8.4 | 1.17 | 11 | |
| C | 0 | 17 | 39.4 | 6.6 | 60.0 | 5.9 | 0.25 | 24 |
| 1 | 14 | 38.6 | 7.5 | 51.6 | 7.6 | |||
| 2 | 12 | 37.6 | 8.3 | 45.4 | 8.2 | 0.70 | 17 | |
| 4 | 10 | 36.4 | 9.7 | 37.4 | 8.1 | 0.81 | 15 | |
| 8 | 10 | 34.2 | 11.1 | 30.7 | 8.3 | 1.13 | 13 | |
| 12 | 9 | 34.2 | 12.0 | 28.5 | 8.4 | 1.18 | 14 | |
| D | 0 | 13 | 39.2 | 6.8 | 57.4 | 6.1 | 0.52 | 25 |
| 1 | 13 | 40.1 | 6.1 | 65.7 | 7.4 | |||
| 2 | 11 | 41.1 | 5.9 | 70.4 | 8.5 | 0.84 | 18 | |
| 4 | 10 | 39.1 | 6.8 | 57.3 | 8.4 | 0.87 | 18 | |
| 8 | 8 | 39.6 | 6.4 | 61.8 | 7.8 | 1.18 | 16 | |
| 12 | 9 | 39.9 | 8.0 | 50.2 | 8.2 | 1.45 | 12 |
The reactor A waste content (on a wet weight basis) was 8% paper and cardboard, 31% fruits and vegetables, and 55% green waste; the reactor B waste content was 10% paper and cardboard, 54% fruits and vegetables, and 36% green waste; the reactor C waste content was 15% paper and cardboard, 54% fruits and vegetables, and 27% green waste; and the reactor D waste content was 21% paper and cardboard, 54% fruits and vegetables, and 18% green waste.
Ratio of C humic acid to C fulvic acid (HA/FA).
Up to 58% of the initial OM was mineralized during composting (Table 3), and the rates of mineralization in reactors B and C (57.2 and 57.8% mineralization, respectively) were higher than those in reactors A and D (44.5 and 37.1% mineralization, respectively). The size of the soluble fraction of OM decreased rapidly during the first 2 weeks of composting and then remained rather stable until the end of composting. The sizes of the cellulose and hemicellulose fractions also decreased rapidly throughout the composting period. A slow decrease in the lignin content was observed, but, due to the low degradation rate, the proportion of lignin in the residual organic matter increased from 14 to 23% of the OM in the initial mixtures to 23 to 34% in the 12-week-old composts. The largest final proportion of lignin was in reactor B.
TABLE 3.
Mineralization of initial OM and changes during composting of biochemical fractions of organic matter in the four reactors
| Reactora | Age (weeks) | % of initial OM
|
||||
|---|---|---|---|---|---|---|
| Mineralized OM | Soluble OM | Hemicellulose | Cellulose | Lignin | ||
| A | 0 | 0 | 25.8 | 16.2 | 34.7 | 23.3 |
| 2 | 23.6 | 12.7 | 9.2 | 32.0 | 22.5 | |
| 4 | 31.4 | 13.6 | 7.5 | 27.6 | 19.9 | |
| 8 | 41.7 | 13.3 | 4.6 | 20.7 | 19.6 | |
| 12 | 44.5 | 15.2 | 4.4 | 17.2 | 18.7 | |
| B | 0 | 0 | 28.7 | 10.9 | 40.2 | 20.1 |
| 2 | 35.8 | 11.5 | 7.5 | 30.5 | 14.7 | |
| 4 | 43.3 | 11.3 | 5.3 | 24.4 | 15.8 | |
| 8 | 55.1 | 12.9 | 5.1 | 12.9 | 14.0 | |
| 12 | 57.2 | 12.7 | 3.7 | 12.7 | 15.6 | |
| C | 0 | 0 | 22.3 | 11.1 | 51.8 | 14.7 |
| 2 | 33.2 | 9.5 | 7.5 | 34.7 | 15.1 | |
| 4 | 49.9 | 11.6 | 5.3 | 20.9 | 12.3 | |
| 8 | 50.8 | 9.6 | 4.4 | 20.5 | 14.7 | |
| 12 | 57.8 | 10.3 | 4.1 | 14.8 | 13.0 | |
| D | 0 | 0 | 17.8 | 10.8 | 57.2 | 14.1 |
| 2 | 17.3 | 8.7 | 7.0 | 51.5 | 15.6 | |
| 4 | 23.5 | 12.5 | 5.9 | 45.8 | 12.2 | |
| 8 | 26.2 | 8.2 | 6.1 | 44.3 | 15.2 | |
| 12 | 37.1 | 10.5 | 6.3 | 31.3 | 14.7 | |
For reactor contents see Table 2, footnote a.
The proportion of organic carbon mineralized during the 28-day incubation in soil was used as an indicator of compost organic matter stabilization during composting (16). In all reactors, 20 to 27% of the carbon present in the initial mixtures was highly biodegradable and mineralized during the short-term incubation in soil. After 12 weeks of composting, only 11 to 14% of the carbon remaining in the mixtures was still highly biodegradable and mineralized during incubation in soil.
The humification index, the ratio of the humic fraction to the fulvic fraction, was used to evaluate the maturity of the composts. Composts are usually considered mature when HA/FA is greater than 1 (16). In the four reactors, the humification index became greater than 1 after 8 weeks of composting, and 8- and 12-week-old composts could be considered mature.
In all reactors the pH increased from pH 5.9 to 6.2 to pH 8.2 to 8.4.
Microbial parameters.
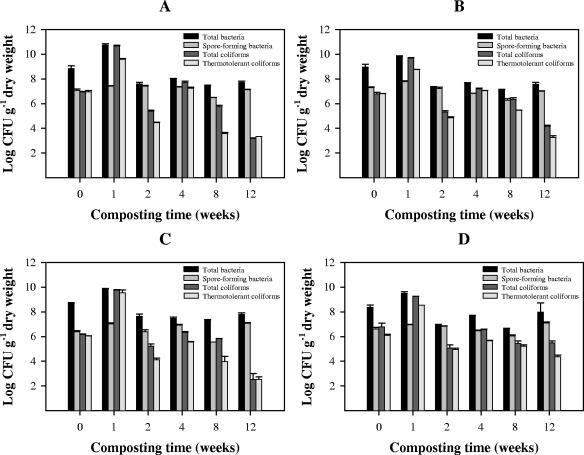
The populations of indigenous total mesophilic bacteria, sporulated bacteria, and total and thermotolerant coliforms in the initial waste and the changes during composting are shown in Fig. 3. Similar patterns were observed in the four composts. The sizes of the populations of indicator microorganisms (total mesophilic bacteria, total and thermotolerant coliforms) increased during the first week of composting and then decreased after 2 weeks of composting. An increase in thermotolerant coliform counts was observed at week 4, but the coliform counts decreased thereafter until the end of composting (12 weeks), when the concentrations were 2.2 × 103, 1.8 × 103, 3.3 × 102, and 2.3 × 104 CFU g−1 in reactors A, B, C, and D, respectively. The concentrations of spore-forming bacteria remained constant throughout.
FIG. 3.
Amounts of indicator microorganisms during four organic waste composting tests (mixtures A, B, C, and D). The reactor A waste content (on a wet weight basis) was 8% paper and cardboard, 31% fruits and vegetables, and 55% green waste; the reactor B waste content was 10% paper and cardboard, 54% fruits and vegetables, and 36% green waste; the reactor C waste content was 15% paper and cardboard, 54% fruits and vegetables, and 27% green waste; and the reactor D waste content was 21% paper and cardboard, 54% fruits and vegetables, and 18% green waste. The error bars indicate standard errors (error bars are not shown when the standard error was not larger than the histogram bar).
Survival in composts during incubation at 25°C.
Seeded composts were sampled after 3, 8, 30, 60, and 90 days of incubation at 25°C. After inoculation, no growth was observed in any compost, but during incubation the inoculated bacteria were detected after broth enrichment. The duration of detection for each seeded strain is shown in Table 4. The behaviors of the three strains tested were different. Survival of seeded L. monocytogenes was observed only in immature composts collected after 1, 2, and 4 weeks of composting in reactor C. In mature compost (8 and 12 weeks), L. monocytogenes was never detected. Survival of seeded E. coli was always observed in the composts sampled after 4 weeks of composting. Seeded Salmonella serovar Enteritidis was recovered in all composts. Survival was shorter in 1-week-old composts sampled during the thermophilic phase. Nevertheless, Salmonella serovar Enteritidis survived longer in mature composts (8 and 12 weeks), in which the strain was still detected at the end of the experiment (90 days), except for mature compost B, in which Salmonella serovar Enteritidis was not detected after 8 days of storage.
TABLE 4.
Survival of L. monocytogenes, E. coli, and Salmonella serovar Enteritidis during 3 months of incubation at 25°C after inoculation into organic waste at various stages of composting
| Composting time (wk) | Last detection daya
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
L. monocytogenes
|
E. coli
|
Salmonella serovar Enteritidis
|
||||||||||
| Reactor Ab | Reactor B | Reactor C | Reactor D | Reactor A | Reactor B | Reactor C | Reactor D | Reactor A | Reactor B | Reactor C | Reactor D | |
| 1 | NDc | ND | 60 | ND | ND | 3 | ND | ND | 3 | 3 | 8 | 3 |
| 2 | ND | ND | 60 | ND | ND | 30 | ND | 8 | 8 | 60 | 30 | 8 |
| 4 | ND | ND | 90 | ND | 90 | 60 | 90 | 90 | 60 | 8 | 8 | 30 |
| 8 | ND | ND | ND | ND | ND | 8 | ND | ND | 90 | 90 | 90 | 90 |
| 12 (mature) | ND | ND | ND | ND | ND | 8 | 8 | 3 | 90 | 8 | 90 | 90 |
Last sampling time with positive selective enrichment (3, 8, 30, 60, or 90 days). The limit of detection was 1 CFU g (dry weight)−1.
For reactor contents see Table 2, footnote a.
ND, not detected after broth enrichment.
Survival in sterilized 12-week-old composts.
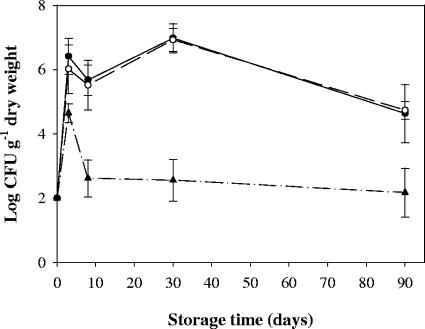
Twelve-week-old composts from reactors B and D were ionized in order to eliminate indigenous microorganisms. Growth of seeded E. coli, Salmonella serovar Enteritidis, and L. monocytogenes was observed during incubation in sterilized 12-week-old reactor D compost (Fig. 4). Within 3 days E. coli and Salmonella serovar Enteritidis grew to concentrations of 3.8 × 106 and 2.6 × 106 CFU g (dry weight)−1, respectively, and the maximal population sizes were reached after 30 days (1.2 × 107 and 1 × 107 CFU g [dry weight]−1, respectively). In the subsequent 2 months of the experiment, the E. coli and Salmonella serovar Enteritidis populations decreased but still contained 1.6 × 105 and 6.2 × 104 CFU g (dry weight)−1, respectively, after 90 days of storage. Similarly, growth of L. monocytogenes was observed within 3 days. The concentration of this organism reached 2.4 × 104 CFU g (dry weight)−1 before it decreased to 2.0 × 102 CFU g (dry weight)−1 after 8 days. The population remained stable thereafter. The behavior of seeded strains was different in the sterilized compost from reactor B. E. coli and Salmonella serovar Enteritidis did not grow but survived at a level of 102 CFU g (dry weight)−1, the initial seeded concentration (data not shown), while in this compost L. monocytogenes was never detected after inoculation, even after enrichment (data not shown).
FIG. 4.
Growth of L. monocytogenes (▴), E. coli (•), and Salmonella serovar Enteritidis (○) in sterilized mature compost (12 weeks of composting) from pilot reactor D during storage at 25°C. The reactor D waste content was 21% paper and cardboard, 54% fruits and vegetables, and 18% green waste. The error bars indicate standard errors.
DISCUSSION
Monitoring of the temperature during waste composting and the changes in the physicochemical characteristics confirmed that satisfactory composting occurred in the four reactors. Indeed, the duration of exposure to a temperature above 55°C met the requirements of the US-EPA standards (three consecutive days or more) (39) for in-vessel composting methods. Intense microbial activity led to organic matter mineralization and water loss, resulting in a decrease in the dry mass in all reactors. Simultaneously, the C/N ratio decreased, which is usually observed during composting. The physicochemical characteristics changed similarly in the reactors, with the exception of reactor D. The large proportion of paper and cardboard in reactor D probably explains why the C/N ratio did not decrease as much as it did in the other reactors. Furthermore, the level of OM mineralization was lower in reactor D, meaning that the initial mixture did not have optimal physicochemical characteristics for microbial activity. This was reflected by the faster temperature drop in this reactor.
Composting is a biological treatment of organic biodegradable wastes that produces a stabilized organic amendment (4). After 8 weeks of composting, compost organic matter became less biodegradable, as revealed by the C mineralization intensity during incubation of soil compost mixtures. All composts reached a high degree of maturity after 8 weeks, as indicated by the humification index used as a maturity indicator (16).
The indigenous microbial flora of each compost was characterized in order to assess composting quality and possible microbial differences between reactors. The microbial parameters of the four mixtures changed in similar ways during composting. At the end of composting (12 weeks), no significant differences between the composts were observed for indicator organisms, except for the total coliform and thermotolerant coliform counts, which were higher in reactor D. The concentrations of thermotolerant coliforms, which are usually used as sanitation indicators of environmental samples (8, 20, 32), were reduced during processing, thus confirming that there was efficient composting. As observed by other authors in composting studies based on composting in windrows (20), resurgent growth of thermotolerant coliforms occurred at 4 weeks, probably due to recontamination or recolonization during turning combined with temperatures that permitted growth. The final thermotolerant coliform concentrations (2.2 × 103, 1.8 × 103, 3.3 × 102, and 2.3 × 104 CFU g−1 in reactors A, B, C, and D, respectively) were similar to concentrations obtained in other studies (6, 8, 20, 29).
Pathogen survival was tested in mature compost to evaluate pathogen behavior in case of postprocessing colonization. Several samples were also collected during composting in order to assess the effect of storage of immature composts on pathogen survival. Rifampin-resistant strains were used to facilitate recovery from unsterilized composts. A low inoculation rate for seeded strains (102 CFU g−1) was chosen to simulate realistic pathogen contamination.
Although the strains of L. monocytogenes, Salmonella serovar Enteritidis, and E. coli did not grow in any compost, we did detect survival of these strains in some composts. However, each strain behaved differently in composted organic waste mixtures. This study showed that Salmonella serovar Enteritidis survived better than the other bacteria tested. Survival of seeded Salmonella serovar Enteritidis was observed in all composts irrespective of their maturity. Indeed, Salmonella spp. have been found in raw sludges (13), commercial compost-based products (36), and sewage sludge composts (22), which reflects the ability of this pathogen to survive in organic substrates even after composting. Although rapid inactivation of seeded Salmonella serovar Typhimurium has been described for composted biosolids (35), in the present study long-term survival (at least 3 months) of Salmonella serovar Enteritidis was observed in the stabilized composts sampled after 8 weeks of composting. Nevertheless, survival for only a short time was observed in the composts sampled during the thermophilic phase, after 1 week of composting. This suggests that proper management of this thermophilic phase could allow satisfactory sanitation of the composts. Nevertheless, contamination with Salmonella serovar Enteritidis during mature compost storage may occur.
Composting is usually efficient for reducing high initial nonpathogenic E. coli levels. However, it has been reported that nonpathogenic E. coli is able to survive in composts (6, 8, 20). In the present study, in all reactors the survival of seeded E. coli depended on the compost age; long-term survival was observed only in the 4-week-old composts.
L. monocytogenes is an environmentally ubiquitous opportunistic pathogen. For example, it can be found on decaying vegetation, in soil (15, 43, 44), and in sewage sludge (2, 7, 33). For composted substrates, the occurrence of Listeria spp. and L. monocytogenes has been described for food scrap composts (11) and for composted biosolids (18), respectively. In this study, long-term survival of seeded L. monocytogenes was observed in immature composts collected from only one of four reactors during the thermophilic phase. However, L. monocytogenes was never detected during incubation in mature composts.
When pathogen survival in nonsterile composts processed in the four pilot reactors was considered, no correlation between survival and any physicochemical characteristics could be established. Thus, the initial percentage of organic wastes did not appear to influence survival in the composted material. Other studies showed that the nature of the organic waste influenced the behavior of Salmonella spp., nonpathogenic E. coli, and pathogenic E. coli O157:H7 (10, 26, 38).
These results suggest that biowaste composts may support long-term survival of Salmonella serovar Enteritidis when sanitation has been unsatisfactory during the thermophilic phase or in the case of colonization during storage of mature compost. Industrial biowaste composts are usually commercialized after at least 4 to 6 months of composting, and other tests with older industrial composts should be done to confirm the potential risk. Mature biowaste composts did not allow L. monocytogenes survival. Only a short survival time was observed for E. coli. However, this study shows that management of the maturation phase is critical for limiting hazards associated with L. monocytogenes. Indeed, survival was observed in 4-week-old immature composts. The decrease in E. coli and L. monocytogenes survival in composts sampled at 4 weeks could be explained by an increase in the antagonistic effects of the indigenous microflora. An increase in microbial diversity after the cooling phase and during the maturation phase has been reported (23, 32). As high microbial diversity should logically increase antagonism, an increase in microbial diversity could result in E. coli and L. monocytogenes inactivation during the maturation phase.
All seeded strains survived better in mature sterile composts (12 weeks of composting) than in nonsterile composts, as was previously observed for survival of Salmonella spp. in composted biosolids by several authors (22, 28, 35). Indigenous microflora play a significant role in suppression of L. monocytogenes, Salmonella serovar Enteritidis, and E. coli regrowth in organic waste composts. Indigenous microflora may limit pathogen survival through limiting the bioavailability of nutrients and through indigenous antimicrobial activities (27, 28). However, survival differences between the sterile reactor B and D composts highlight the complexity of interacting factors affecting bacterial survival. Further investigations are necessary to understand the impact of indigenous microflora dynamics.
Conclusions.
Pathogens have been observed in sludge or manure composts (12, 22, 36). The objective of this work was to study the potential survival of the seeded pathogens L. monocytogenes and Salmonella serovar Enteritidis and nonpathogenic E. coli in biowaste composts. Composting was performed in insulated reactors to allow increases in the temperature in laboratory-scale amounts of organic wastes. The composting process was an in-vessel composting process with frequent turning. The changes in the physicochemical characteristics confirmed that satisfactory composting occurred in the reactors. Survival of the seeded strains was observed in experimental composts, suggesting that composted sewage sludge and manure and domestic waste composts may also contain pathogens. For E. coli and L. monocytogenes, undesirable long-term survival could be prevented by proper management of the maturing phase. No risk of survival during compost storage should occur with L. monocytogenes. The survival time for Salmonella serovar Enteritidis was very short in composts sampled during the thermophilic phase but was longer when organisms were inoculated into mature biowaste compost. These results emphasize the need for proper management of both the thermophilic and maturation phases during the composting process. Since there is a risk of contamination during storage, the results also confirmed the need to take Salmonella spp. into account in the required quality standards for composts. Although waste composition affected pathogen growth in sterile composts, no clear relationship between compost physicochemical parameters and pathogen survival was found. Nevertheless, the indigenous microflora plays a critical role in pathogen control. Further investigations of industrial composts made from green waste, municipal solid waste, and industrial waste should generate interesting data concerning the microbial safety of composts. An increasing number of different types of waste residues are being considered as feedstocks for compost. A better understanding of pathogen survival in these materials and the various procedures and approaches used for the actual composting process is needed to address microbial safety issues.
Acknowledgments
This work was sponsored by the French Agency for Environment and Energy Management (ADEME) and the Conseil Régional de Bourgogne. We thank the Research Center for Environment and Waste (CreeD, Veolia Environment) for supplying composting reactors and for financial support.
REFERENCES
- 1.AFNOR. 2002. French standard U 44-095. Organic soil improvers—composts containing substances essential to agriculture, stemming from water treatment. AFNOR Editions, Saint-Denis-la-Plaine, France.
- 2.Al-Ghazali, M. R., and S. K. Al-Azawi. 1986. Detection and enumeration of Listeria monocytogenes in a sewage treatment plant in Iraq. J. Appl. Bacteriol. 60:251-254. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ghazali, M. R., and S. K. Al-Azawi. 1990. Listeria monocytogenes contamination of crops grown on soil treated with sewage sludge cake. J. Appl. Bacteriol. 69:642-647. [DOI] [PubMed] [Google Scholar]
- 4.Bernal, M. P., M. A. Sanchez-Monedero, C. Paredes, and A. Roig. 1998. Carbon mineralization from organic wastes at different composting stages during their incubation with soil. Agric. Ecosyst. Environ. 69:175-189. [Google Scholar]
- 5.Beuchat, L. R. 1996. Listeria monocytogenes: incidence on vegetables. Food Control 7:223-228. [Google Scholar]
- 6.Christensen, K. K., M. Carlsbaek, and E. Kron. 2002. Strategies for evaluating the sanitary quality of composting. J. Appl. Microbiol. 92:1143-1158. [DOI] [PubMed] [Google Scholar]
- 7.De Luca, G., F. Zanetti, P. Fateh-Moghadm, and S. Stampi. 1998. Occurrence of Listeria monocytogenes in sewage sludge. Zentbl. Hyg. Umweltmed. 201:269-277. [PubMed] [Google Scholar]
- 8.Déportes, I., J.-L. Benoit-Guyod, D. Zmirou, and M.-C. Bouvier. 1998. Microbial disinfection capacity of municipal solid waste (MSW) composting. J. Appl. Microbiol. 85:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Roever, C. 1998. Microbiological safety and recommendations on fresh produce. Food Control 9:321-347. [Google Scholar]
- 10.Droffner, M. L., and W. F. Brinton. 1995. Survival of E. coli and Salmonella populations in aerobic thermophilic composts as measured with DNA gene probes. Zentbl. Hyg. 197:387-397. [PubMed] [Google Scholar]
- 11.Droffner, M. L., and W. F. Brinton. 1996. Occurrence and detection of viable Listeria in food scrap compost. Zentbl. Hyg. 199:51-59. [PubMed] [Google Scholar]
- 12.Droffner, M. L., W. F. Brinton, and E. Evans. 1995. Evidence for the prominence of well characterized mesophilic bacteria in thermophilic (50-70°C) composting environments. Biomass Bioenergy 8:191-195. [Google Scholar]
- 13.Dudley, D. J., M. N. Guentzel, M. J. Ibarra, B. E. Moore, and B. P. Sagik. 1980. Enumeration of potentially pathogenic bacteria from sewage sludges. Appl. Environ. Microbiol. 39:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Community. 2001. Working document—biological treatment of biowaste, 2nd draft. http://europa.eu.int/comm/environment/waste.
- 15.Fenlon, D. R. 1985. Wild birds and silage as reservoirs of Listeria in the agricultural environment. J. Appl. Bacteriol. 59:537-543. [DOI] [PubMed] [Google Scholar]
- 16.Francou, C. 2004. Stabilisation de la matière organique au cours du compostage de déchets urbains: influence de la nature des déchets et du procédé de compostage—recherche d'indicateurs pertinents. Ph.D. thesis. INA-PG, Paris, France. [Online.] http://pastel.rilk.com/archive/00000788/01/These-C%C3%A9dric_Francou.doc.
- 17.Garrec, N., F. Picard-Bonnaud, and A. M. Pourcher. 2003. Occurrence of Listeria sp. and Listeria monocytogenes in sewage sludge used for land application: effect of dewatering, liming and storage in tank on survival of Listeria species. FEMS Immunol. Med. Microbiol. 35:275-283. [DOI] [PubMed] [Google Scholar]
- 18.Garrec, N., L. Sutra, F. Picard, and A. M. Pourcher. 2003. Development of a protocol for the isolation of Listeria monocytogenes from sludge. Water Res. 37:4810-4814. [DOI] [PubMed] [Google Scholar]
- 19.Haapapuro, E. R., N. D. Barnard, and M. Simon. 1997. Animal waste used as livestock feed: dangers to human health. Prev. Med. 26:599-602. [DOI] [PubMed] [Google Scholar]
- 20.Hassen, A., K. Belguith, N. Jedidi, A. Cherif, M. Cherif, and A. Boudabous. 2001. Microbial characterization during composting of municipal solid waste. Bioresource Technol. 80:217-225. [DOI] [PubMed] [Google Scholar]
- 21.Himathongkham, S., S. Bahari, H. Riemann, and D. Cliver. 1999. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 22.Hussong, D., W. D. Burge, and N. K. Enkiri. 1985. Occurrence, growth, and suppression of salmonellae in composted sewage sludge. Appl. Environ. Microbiol. 50:887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii, K., M. Fukui, and S. Takii. 2000. Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J. Appl. Microbiol. 89:768-777. [DOI] [PubMed] [Google Scholar]
- 24.Islam, M., J. Morgan, M. P. Doyle, S. C. Phatak, P. Millner, and X. Jiang. 2004. Fate of Salmonella enterica serovar Typhimurium on carrots and radishes in fields treated with contaminated manure composts or irrigated water. Appl. Environ. Microbiol. 70:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lung, A. J., C. M. Lin, J. M. Kim, M. R. Marshall, R. Nordstedt, N. P. Thompson, and C. I. Wei. 2001. Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. J. Food Prot. 64(9):1309-1314. [DOI] [PubMed] [Google Scholar]
- 26.Makawi, A. A. M. 1973. The survival of salmonellae in compost prepared with straw of different plants and sewage sludge. Zentbl. Bakteriol. Abt. II 128:203-208. [DOI] [PubMed] [Google Scholar]
- 27.Makawi, A. A. M. 1980. The effect of thermophilic actinomycetes isolated from compost and animal manure on some strains of Salmonella and Shigella. Zentbl. Bakteriol. Abt. II 135:12-21. [DOI] [PubMed] [Google Scholar]
- 28.Millner, P. D., K. E. Powers, N. K. Enkiri, and W. D. Burge. 1987. Microbially mediated growth suppression and death of Salmonella in composted sewage sludge. Microb. Ecol. 14:255-265. [DOI] [PubMed] [Google Scholar]
- 29.Pereira-Neto, J. T., E. I. Stentiford, and D. V. Smith. 1986. Survival of faecal indicator organisms in refuse/sludge composting using the aereted static pile. Waste Manag. Res. 4:397-406. [Google Scholar]
- 30.Robin, D. 1997. Intérêt de la caractérisation biochimique pour l'évaluation de la proportion de matière organique stable après décomposition dans le sol et la classification des produits organominéraux. Agronomie 17:157-171. [Google Scholar]
- 31.Russ, C. F., and W. A. Yanko. 1981. Factors affecting salmonella repopulation in composted sludges. Appl. Environ. Microbiol. 41:597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryckeboer, J., J. Mergaert, J. Coosemans, K. Deprins, and J. Swings. 2003. Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 94:127-137. [DOI] [PubMed] [Google Scholar]
- 33.Schwartzbrod, J., O. Papadopoulos, and J. C. Burdin. 1989. Detection and behaviour of Listeria in wastewater sludge. Microbiol. Aliments Nutr. 7:225-232. [Google Scholar]
- 34.Sidhu, J., R. A. Gibbs, G. E. Ho, and I. Unkovich. 1999. Selection of Salmonella typhimurium as an indicator for pathogen regrowth potential in composted biosolids. Lett. Appl. Microbiol. 29:303-307. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu, J., R. A. Gibbs, G. E. Ho, and I. Unkovich. 2001. The role of indigenous microorganisms in suppression of Salmonella regrowth in composted biosolids. Water Res. 35:913-920. [DOI] [PubMed] [Google Scholar]
- 36.Skanavis, C., and W. A. Yanko. 1994. Evaluation of composted sewage sludge based soil amendments for potential risks of salmonellosis. J. Environ. Health 56:19-25. [Google Scholar]
- 37.Solomon, E. B., S. Yaron, and, K. R. Matthews 2002. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 68:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner, C. 2002. The thermal inactivation of E. coli in straw and pig manure. Bioresource Technol. 84:57-61. [PubMed] [Google Scholar]
- 39.U.S. Environmental Protection Agency. 1993. Standards for the use or disposal of sewage sludge (40 Code of Federal Regulations Part 503). U.S. Environmental Protection Agency, Washington, D.C.
- 40.Van Elsas, J. D., K. Smalla, A. K. Lilley, and M. J. Bailey. 2002. Methods of sampling soil microbes, p. 505-515. In C. J. Hurst, R. L. Crawford, G. R. Knudsen, M. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 41.Van Soest, P. J. 1963. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Anal. Chem. 46:829-835. [Google Scholar]
- 42.Weis, J., and H. P. R. Seeliger. 1975. Incidence of Listeria monocytogenes in nature. Appl. Microbiol. 30:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welshimer, H. J. 1960. Survival of Listeria monocytogenes in soil. J. Bacteriol. 80:316-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welshimer, H. J., and J. Donker-Voet. 1971. Listeria monocytogenes in nature. Appl. Microbiol. 21:516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]