Abstract
The prevalence of Campylobacter jejuni in commercial feedlot cattle was monitored throughout the feeding period by repeated bacteriologic culture of feces. Fecal pats (n = 10) in 20 feedlot pens were sampled at 2-weeks interval beginning at entry into the feedlot and continuing until slaughter. The least-squares mean C. jejuni prevalence increased from 1.6% at the first sampling to 61.3% at the final sampling just prior to slaughter. Diverse C. jejuni pulsed-field gel electrophoresis macrorestriction profiles (MRP) were identified among the cattle isolates, but five prevalent MRP and minor variants accounted for >80% of all typed isolates. Chlorination of the water supplied to the water troughs of half of the pens did not affect C. jejuni prevalence in the cattle. Overall, the least-squares mean C. jejuni prevalences were 45.6 and 43.6% in chlorinated and nonchlorinated feedlot pens, respectively. The results of this study demonstrate apparent transmission of C. jejuni among feedlot cattle during the feeding period, unaffected by water chlorination, resulting in a high prevalence of C. jejuni excretion by cattle approaching slaughter.
Campylobacter spp., especially Campylobacter jejuni, are among the most frequent causes of laboratory confirmed acute diarrheal disease in the United States. For example, in 2003 a Campylobacter sp. was confirmed as the cause of 12.6 cases per 100,000 population in the Centers for Disease Control and Prevention FoodNet active surveillance program and was the most frequent agent identified in six of the nine FoodNet sentinel states (1). Gastrointestinal colonization of poultry, resulting in contamination of poultry meats and eggs, is frequently implicated as the major source of human infection (6). However, cattle and other food animal species also frequently carry C. jejuni and C. coli (29), as well as other Campylobacter spp., including C. coli, C. hyointestinalis, and C. fetus, and the DNA of these and other species, including prominently C. lanienae, have been detected by PCR (4, 15). Unpasteurized bovine milk and milk products are frequently incriminated vehicles of outbreaks of campylobacteriosis (27). It is likely that cattle carcasses are contaminated at slaughter by direct or indirect fecal contamination and retail meat (beef) contamination with C. jejuni has also been reported, albeit usually at a much lower prevalence (1% or lower in beef versus 23 to 77% in poultry) and at a lower concentration compared to poultry meats (12, 20, 25, 26, 33). Whether by direct contamination of foods of bovine origin (unpasteurized milk and milk products and possibly beef products) or by environmental shedding of Campylobacter spp. resulting in contamination of water sources, there is concern about bovine carriage of this agent resulting in human infection (29).
Strain typing studies have contributed to concerns about bovine origin Campylobacter sp. as a risk for human infection. Several recent studies have examined the strain types of thermophilic Campylobacter spp. isolated from cattle feces or from contaminated bovine origin food products and strain types indistinguishable from those isolated from human disease have been identified (19, 22, 24). Therefore, contamination of cattle origin food products is a potential mechanism of human infection with C. jejuni and the frequency of colonization of cattle destined for beef production may affect the risk of food-borne campylobacteriosis in humans.
Numerous investigations of C. jejuni prevalence in cattle populations on farms report prevalences varying by age group, cattle type (beef versus dairy), and season (7-10, 13, 14, 21, 23, 30, 32). Relatively few longitudinal studies of the temporal dynamics of C. jejuni within individual farms or feedlot groups have been performed, and the effects of interventions to reduce transmission of this agent within cattle populations have rarely been addressed. Therefore, the present study was designed to accomplish the following three objectives: (i) to determine the prevalence of isolation of C. jejuni from feces of feedlot cattle longitudinally throughout the feeding period, (ii) to determine the effect of chlorination of cattle drinking water sources on the prevalence of fecal isolation of C. jejuni from the fed cattle, and (iii) to use macrorestriction profiles (MRP) of bovine fecal C. jejuni to investigate potential C. jejuni transmission among feedlot cattle.
MATERIALS AND METHODS
Study site.
The present study was performed in a large (>50,000 cattle on feed) commercial feedlot. Twenty pens were longitudinally sampled every other week from the time the cattle entered the feedlot until they were removed for slaughter. The study involved pens in two sections of the feedlot, and each study section included 10 pens separated by solid fenceline contact in a five by two configuration. The two study sections were separated by a third section of the feedlot of identical size flanked by two roadways. All study pens were dirt-floored and of identical size (270 by 270 feet) and stocking density (500 cattle per pen). Pens were scraped or cleaned approximately annually but had not been scraped to remove manure between the departure of the previous cohort, and the arrival of the study cohort. The feedlot water supply was drawn from a surface reservoir. The water supply pipe serving the chlorinated pens was fitted with a proportional sodium hypochlorite injector to provide 3 ppm of chlorinated water, while the control pens were served by unchlorinated water. The injector was adjusted weekly as needed to maintain chlorine residuals of approximately 1 ppm in the chlorinated pen feedlot section water troughs. Actual chlorine concentration recorded in the water troughs of the chlorinated pens was 0.89 ± 0.68 ppm (mean ± the standard deviation, n = 197). Each pen contained a single 120-gallon, 90-by-36-by-25 in. water trough (Hedstrom Model 90 Super; Hedstrom Concrete Products, Woodbine, IA). All water troughs were cleaned routinely at approximately 10-day intervals by draining, removal of accumulated sediments, vigorous brushing, rinsing, and refilling. The study cohort cattle originated from approximately 120 different farms. Groups of cattle from each source farm were divided equally to chlorinated and control feedlot pens. Stocking of the study pens began the second week of April 2000, and all 20 pens were stocked within a 21-day period.
Sampling protocol.
Sampling was conducted every other week, beginning April 15, 2000 when the first six study pens had been stocked with cattle and continuing through August 21 and 24 when the final Campylobacter culture specimens and water trough coliform specimens were obtained, respectively. The eight pens still stocked on August 24 were slaughtered by September 1. Within each feedlot (treatment) section, this schedule resulted in sampling at nine occasions for six pens and eight occasions for the remaining four pens. Sampling consisted of the following. (i) Bovine feces (10 g) were collected with a sterile tongue depressor into sterile Whirl-pac bags (Nasco Sampling Products, Ft. Atkinson, WI) from 10 different fresh fecal pats from each occupied study pen for bacteriologic culture for C. jejuni as a measure of the prevalence of fecal excretion of this pathogen (all sampling visits). (ii) Unstirred water trough water samples (50 ml) were obtained from each occupied study pen for determination of total and residual chlorine concentration (second and subsequent sampling visits). (iii) Unstirred water trough water samples (100 ml) were obtained from each occupied study pen for quantitation of total coliform bacteria as a measure of the efficacy of water chlorination (second and subsequent sampling visits). Stirred sediments would be expected to include relatively large numbers of coliform bacteria protected from the effects of residual chlorine in the water column, confounding the use of coliform counts as an indicator of efficacy of chlorination. (iv) Vigorously stirred trough water (50 ml) was obtained from each occupied study pen for bacteriologic culture for C. jejuni as a measure of the prevalence of water trough contamination with this pathogen (second and subsequent sampling visits). Troughs were stirred prior to sampling for C. jejuni culture to resuspend water trough sediments, which are more likely to yield pathogenic bacteria than the overlying water column (18). (v) Water from the reservoir, which was the source of the feedlot water trough water supply (two to eight 50-ml aliquots of water from the water column excluding sediments were collected within 1 m of shore and within 50 m of the feedlot supply inlet), was sampled for bacteriologic culture for C. jejuni (second and subsequent sampling visits).
Water samples for bacteriologic cultures were collected directly into sterile 100-ml polystyrene vials containing 15 to 30 mg of sodium thiosulfate to neutralize free chlorine. Samples for chlorine determination and total coliform counts were processed immediately in the feedlot laboratory; all other samples were shipped on ice in insulated containers by overnight parcel service to the Washington State University Field Disease Investigation Laboratory for analysis. One half of a single shipment from the July 24 sampling was delayed in transit beyond 24 h; these delayed specimens were discarded and replaced with overnight delivered fresh specimens.
Bovine fecal culture for C. jejuni detection.
Swab subsamples from each 10-g fecal pat sample were inoculated directly into Campy-thio broth (Remel, Inc., Lenexa, KS) and incubated (4°C, 48 h) for selective survival of Campylobacter spp. After incubation, a sterile cotton-tipped swab was used to inoculate ca. 0.1 ml of the Campy-thio broth into cefoperazone-vancomycin-amphotericin agar plates (CVA; Remel), which were streaked for isolation and incubated (42°C, 48 to 96 h) in a microaerophilic environment (Campy-packs; Remel) As a positive control, C. jejuni (ATCC 29428; American Type Culture Collection, Manassas, VA) was plated simultaneously and incubated together with each set of fecal or water culture plates. A second plate of ATCC 29428 was incubated in air (42°C, 48 to 96 h) to confirm its requirement for a modified atmosphere. From each CVA plate, up to three colonies with morphology consistent with C. jejuni were microscopically screened for bacterial morphology following Victoria Blue 4R staining (Pfaltz & Bauer, Waterbury CT) and were tested for hippurate hydrolysis (hippurate disks and ninhydrin reagent; Remel) according to methods recommended by the manufacturer. Every tenth isolate was additionally confirmed as C. jejuni by a combination of methods including colony blot hybridization with a hipO DNA probe (2), hipO PCR (3), and lpxA multiplex PCR (2, 31). From each positive specimen, only the first C. jejuni isolate identified in this manner was included in the study.
After identification, C. jejuni isolates were grown on blood agar in a microaerophilic environment for 48 h, and a colony was suspended in proteose peptone broth (1% [wt/vol])-glycerol (10% [vol/vol]) and stored at −70°C for future analysis. After completion of the fecal culture phase, it was discovered that this storage method gave an unsatisfactory rate of recovery of the banked isolates and systematic recovery of all banked isolates was attempted. A laboratory error during this process destroyed all isolates originating from the 26 June 2000 sampling date. Excluding the 26 June isolates, 551 of 720 isolates (76.5%) were recovered and rebanked by using a modified procedure. Two modifications to the banking procedure were made: the isolates were banked after 24 h of growth on blood agar in a microaerophilic environment (versus at 48 h in the unmodified procedures), and the amount of growth suspended in the banking medium was increased ∼10-fold (from one colony in the unmodified procedure to a full loop in the modified procedure).
Enumeration of fecal coliforms and Escherichia coli in water troughs.
Most probable numbers of fecal coliform bacteria were determined in each sample by using a commercially available test kit (Quanti-Tray 2000; Idexx Laboratories, Westbrook, ME) according to the manufacturer's recommendations. This test kit utilizes the chemical indicator ONPG (o-nitrophenyl-β-d-galactopyranoside) as a marker of coliform bacteria.
Water culture for C. jejuni detection.
C. jejuni organisms were isolated and identified from water sources by using centrifugation, selective broth incubation, selective agar plating, and differential testing as follows. First, 40-ml aliquots of water were transferred to sterile polycarbonate screw-top conical tubes and centrifuged (23,000 × g, 10 min, room temperature). After centrifugation, the pellets were used to inoculate Campy-thio tubes (Remel), which were incubated (4°C, 48 h) and subcultured onto CVA agar (Remel). Subsequent isolation and identification steps were as previously described for fecal samples.
MRP determinations.
Pulsed-field gel electrophoresis (PFGE) of SmaI-digested DNA was used to determine the MRP as described by Ribot et al. (28) on all viable C. jejuni isolates obtained from four contiguous pens each in the chlorinated and nonchlorinated feedlot sections. A C. jejuni standard isolate was included on each PFGE gel to aid subsequent analysis as described below. Gel images were scanned into a commercial software program (Bio-Numerics; Applied Maths, Inc., Austin, TX), normalized to a lambda concatemer size standard (Bio-Rad, Hercules CA) and similarity of the banding patterns of all isolates was compared by UPGMA (unweighted pair group method with averages) using the Dice coefficient. The tolerance parameter was set by choosing the minimum value (1.0%) required to group the standard isolates run on each gel as identical on the UPGMA analysis. Each unique banding pattern was then assigned a code.
Some isolates were unavailable for PFGE analysis due to nonviability resulting from faulty banking procedures described previously. Of the total 348 C. jejuni isolates (342 fecal, 6 water) obtained from the eight PFGE study pens, 204 fecal and 3 water isolates (59.5% of the total or 68.1% of isolates from dates other than 26 June 2000) were recovered for PFGE analysis.
Statistical analysis.
For statistical analysis of the effects of chlorination, the unit of observation was the pen, and the dependent variable was the fecal prevalence of C. jejuni. The data were analyzed by a repeated-measures analysis of variance with the C. jejuni pen prevalence as the dependent variable and a factorial analysis of the independent factors chlorine treatment and sample visit and their interaction as the independent variables (Proc Mixed [using first-order autoregressive covariance estimation]; SAS Institute, Inc., Cary, NC). The effect of chlorination on the frequency of isolation of C. jejuni from water troughs was analyzed by using the Fisher exact test (Proc Freq; SAS). The effect of chlorination on fecal coliform concentrations in water trough water was analyzed by repeated measures analyses of variance with the log-transformed water coliform concentrations as the dependent variable and a factorial analysis of the independent factors chlorine treatment and sample visit and their interaction (Proc Mixed [first-order autoregressive covariance estimation]). Simpson's index of diversity was calculated for each pen in which PFGE MRP were determined, and the Mann-Whitney rank sum test was used to test for differences in the index between the chlorinated and nonchlorinated feedlot section pens (Proc Npar1way; SAS).
RESULTS
Prevalence of C. jejuni in feedlot cattle feces.
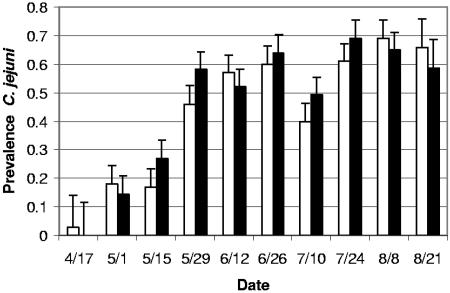
Repeated-measures analysis of variance indicated a significant effect of sampling date (P < 0.0001) but no significant effect of the chlorination treatment and no interaction between sampling date and chlorination treatment. Least-squares mean data are presented in Fig. 1; the sampling date effect is apparent from the fecal prevalences of C. jejuni detected, ranging from 1.6% at the first sampling (within 2 weeks of the placement of the cohort in the feedlot) to 62.2% at the final sampling (within 2 weeks of slaughter). Least-squares mean fecal prevalence of C. jejuni in cattle in water chlorination treatment feedlot sections (45.6%) was very similar that in nonchlorinated control feedlot sections (43.6%, Fig. 1).
FIG. 1.
Least-squares mean pen prevalence of C. jejuni by date in cattle watered from chlorinated (▪) and nonchlorinated (□) water troughs. The first sample date is within 2 weeks of arrival of the cattle at the feedlot, and the final sample date is within 2 weeks of slaughter. Error bars depict standard errors of the mean.
Water trough chlorination and fecal coliform contamination of cattle water troughs.
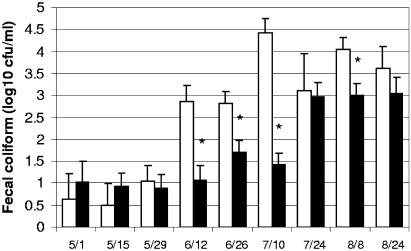
Fecal coliform bacterial concentrations were determined in order to determine the efficacy of water chlorination in improving the bacteriological quality of the water supply for the feedlot cattle. Repeated-measures analysis of variance indicated the presence of a significant interaction between sampling date and chlorination in the analysis of the log-transformed water fecal coliform concentrations (P < 0.001, Fig. 2). Significant reductions in total coliform concentrations in the chlorinated water troughs were detected on four of the nine sampling dates, as indicated in Fig. 2. During the final three sampling dates, including August 8 when a significant reduction was associated with chlorination, the total coliform concentrations in the chlorinated water troughs approached or exceeded 1,000 CFU/ml, indicating very highly contaminated water and demonstrating that the ability of chlorination in improving the water quality in these intensively used cattle water troughs was quite limited. Some observations that may explain the lack of efficacy of chlorination included the presence of large amounts of sediments, which appeared to consist primarily of cereal grains and other cattle feedstuffs, in the water troughs despite routine cleaning at approximately 10 days intervals (data not shown). In addition, during the hottest summer weather, cattle were observed on occasion physically standing in the water troughs (data not shown).
FIG. 2.
Log10 concentrations of coliform bacteria in water troughs filled with chlorinated (▪) and nonchlorinated (□) water. Error bars depict the standard error of the mean log10 CFU/milliliter. Asterisks indicate sampling dates on which the chlorinated and nonchlorinated troughs differed significantly (P < 0.01) in log10 coliform bacterial concentrations.
Prevalence of C. jejuni in feedlot water sources.
The surface water reservoir which provided water to the feedlot consistently tested negative for Campylobacter contamination (n = 45 specimens cultured, including from two to eight subsamples at each sampling date beginning 1 May 1 2000). Each study pen contained a single water trough, and each pen's water trough was sampled for C. jejuni culture at each sampling date. C. jejuni was isolated from 18 of 166 (10.8%) of pen water trough samples through the course of the present study, including one, two, and three positive troughs in eleven, two, and one pens, respectively. C. jejuni was never cultured from the six other pens' water troughs. The positive water trough cultures were obtained from troughs in both nonchlorinated (n = 6) and chlorinated (n = 12, P = 0.211 [Fisher exact test]) sections of the feedlot. Positive water cultures were obtained on 1 May (n = 1), 15 May (n = 2), 12 June (n = 7), 26 June (n = 5), 10 July (n = 1), and 8 August (n = 2).
PFGE analysis of MRP of C. jejuni.
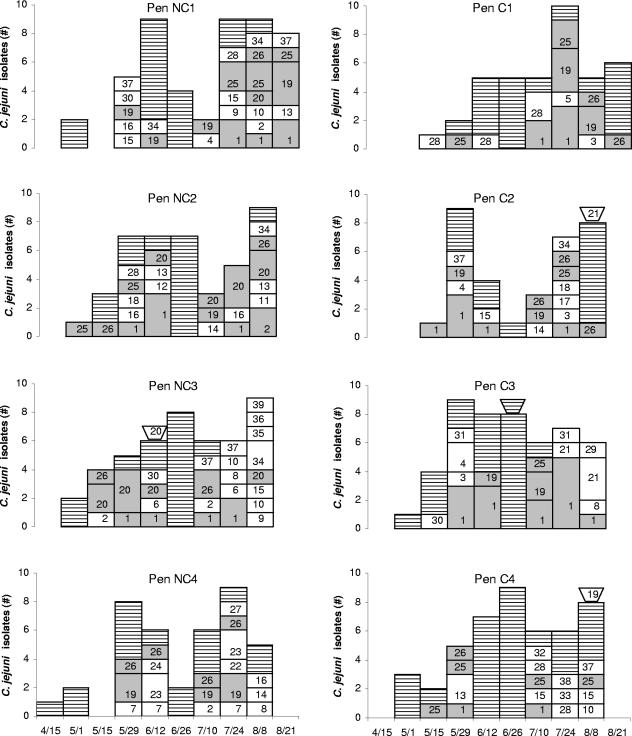
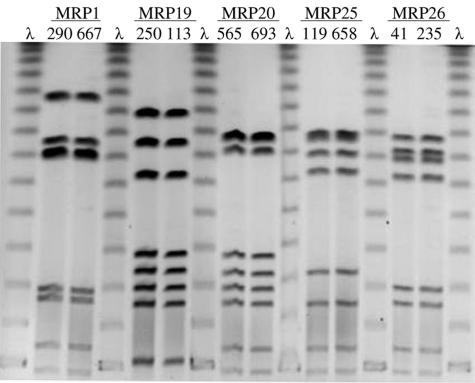
A total of 205 isolates, which included all recoverable isolates from four contiguous pens each in the chlorinated and nonchlorinated areas of the study feedlot, were analyzed for MRP by PFGE after digestion with SmaI. Thirty-nine different MRP were detected among the isolates analyzed (Fig. 3). Five common MRP (MRP 1, 19, 20, 25, and 26) (Fig. 4), each accounting for >8% of the isolates, cumulatively accounted for >55% of all isolates analyzed. These five predominant strain types were very similar (one or two band differences) to additional MRP, such that together the predominant and very similar MRP accounted for over 80% of the cattle fecal isolates and two of the three water isolates typed. The MRP distribution within each pen, as described by Simpson's index of diversity, ranged from 6.64 to 9.48 in nonchlorinated pens and from 2.47 to 9.26 in chlorinated pens (P = 0.49 [Mann-Whitney rank sum test]), indicating that both treatment and control feedlot sections showed similar diversity of C. jejuni genotypes. Three water trough isolates from the eight PFGE study pens were analyzed; two of these three exhibited an MRP that had never been identified among the fecal isolates from the pen of origin, although these MRP were identified in fecal isolates from adjacent and distant pens (Fig. 3).
FIG. 3.
Distribution of MRP of C. jejuni isolates from four adjacent (fenceline contact) pens each in the nonchlorinated (NC) and chlorinated (C) water sections of the feedlot. The NC and C sections were separated by two alleyways and three other rows of pens. Shaded bars (░⃞) indicate the five major MRP (defined in Fig. 4) and close variants. Horizontally lined bars (▤) represent isolates that could not be recovered for PFGE analysis. Trough symbols atop bars denote isolates from water troughs. Numbers within bar segments indicate the MRP determined by SmaI PFGE.
FIG. 4.
PFGE gel of the five most common SmaI MRP identified among eight pens of feedlot cattle over a 4-month feeding period. The MRP values represent >55% of all isolates tested. λ, concatemers of lambda used for molecular size normalization. Numbers over the horizontal lines are the MRP designations. Numbers immediately over the lanes are isolate bank numbers. The gel photograph was inverted for clarity.
DISCUSSION
The prevalence of C. jejuni detected in the present study falls within the broad range of bovine prevalences reported in the literature, although comparisons of prevalence studies are difficult due to the varied isolation methods, specimen types, farm and husbandry systems, seasons, ages of animals, and specimen types. The relatively low (<5%) prevalence reported here in animals newly arrived at study feedlot is somewhat surprising in view of the fact that most of these cattle are thought to have originated in intensive stocked backgrounding feedlots. However, backgrounding feedlot feeding practices are typically forage intensive, and the low incoming C. jejuni prevalence is consistent with the low prevalence reported for pastured cattle by others (9, 13). In contrast, our previous study (2) found much higher C. jejuni prevalence in pastured beef cattle. The high prevalence of C. jejuni in cattle after 2 months or longer in the feedlot is similar to findings reported by others (7, 8, 21, 32) for intensively stocked slaughter or dairy cattle. In a longitudinal study in Ireland, Minihan et al. (21) sampled feedlot cattle monthly through the feeding period and documented average monthly prevalences of 12, 52, 74, and 76% in a cohort fed from November through February. Clearly, the pattern Minihan et al. observed in a single pen of 133 cattle is very consistent with the prevalence of C. jejuni reported here in 20 pens feeding 10,000 cattle.
Seasonal variation in the prevalence of bovine fecal Campylobacter shedding has been observed (30), with peaks in shedding that tended to occur in the spring and fall seasons. Since the present study was initiated in the early summer and since all of the cohorts of cattle were fed simultaneously, it is impossible to exclude the possibility that the marked increase in prevalence in C. jejuni observed here was partly or completely due to a seasonal effect rather than a days-on-feed effect. However, others have observed summertime to be a season with lower, rather than higher prevalence (30). Nevertheless, we cannot exclude a different seasonal effect, with increased Campylobacter prevalence in hot summer weather, as an alternative explanation to the increasing prevalence observed here at the end of the feeding period.
The observed pattern of increasing prevalence of C. jejuni with duration of stay in the feedlot raises a question that has been previously discussed (29) with regard to seasonal fluctuations in prevalence: whether the observed increased prevalence represents new infections or recrudescence of previously undetectable infections. In the present study, we analyzed the MRP observed after PFGE resulting from all viable C. jejuni isolates from four adjacent cattle pens each in two different areas of the study feedlot to address this question. Specifically, the diversity of MRP strain types was hypothesized to differ depending on whether most infections resulted from epidemic transmission within the feedlot (where a relatively few strain types would be expected) or from recrudescent infections preexisting the cattle movement to the feedlot (where a much more diverse set of strains would be expected due to their diverse herd and geographic origins). The results of the strain typing exercise tend to favor the former hypothesis, in that a few MRP types accounted for the majority of the isolates characterized, especially relative to the size of the study population (approximately 4,000 cattle in the eight pens whose isolates were subjected to PFGE). Moreover, the MRP types circulating in the two study areas of the feedlot sampled were largely similar, despite the physical separation of these areas by nonstudy pens containing several thousand additional cattle of differing origins. A significant number of isolates (ca. 25% for most sampling dates) were lost prior to MRP determination due to a faulty banking procedure, and this may have affected our estimate of MRP diversity. However, even if these nonviable isolates had been significantly more genetically diverse than those that were recovered for typing, the observation of a high proportion of shared MRP types between pens and feedlot sections would have remained and supported the same argument.
Fitzgerald et al. (5) also evaluated MRP of cattle isolates of C. jejuni and demonstrated diversity of MRP in a diverse collection of isolates from humans and farm animals, including some genotypes which were apparently restricted to specific hosts, whereas others were broadly distributed across time and hosts. The overall diversity Fitzgerald et al. observed was broad (71 SmaI MRP in 315 isolates versus 39 MRP in 205 isolates in the present study). In contrast, Nielsen et al. (23) investigated SmaI MRP in dairy herds, which would typically have far lower animal turnover rates than feedlots, and found only a single MRP in 45% of the study herds, with the most diverse herd having only five C. jejuni MRP. Therefore, the diversity observed in the present study is similar to that of the highly diverse regional sources of C. jejuni observed by Fitzgerald et al. and greater than the relatively restricted diversity within individual small dairy herds observed by Nielsen.
Given the apparent transmission of C. jejuni within the study population, the routes and vehicles of transmission are of great interest since they represent potential points where interventions could be made to block the within-farm transmission. A great many possibilities exist for vehicles and routes of transmission, including direct contact and mutual grooming, wildlife (rodent and avian) reservoirs, aerosols, and fomites. For fecal-oral transmitted agents, including C. jejuni and others, a contaminated water trough water represents one very plausible potential mode of transmission (11, 14, 16, 17, 21). Therefore, a significant component of the present study was an attempt to evaluate the efficacy of chlorination of water trough water supplies in preventing contamination of water troughs with these agents.
Water is frequently a vehicle of Campylobacter transmission to humans and has been suggested as a likely vehicle of cattle infection as well. Hanninen et al. (11) observed markedly higher C. jejuni prevalences in dairy cattle when seasonally grazed with access to (C. jejuni contaminated) surface waters compared to when housed with access only to mains (i.e., municipal) chlorinated water. Humphrey and Beckett (14) observed in a study of 12 dairy herds that all 10 herds with access to contaminated surface water sources were positive for cattle shedding C. jejuni, whereas the 2 herds with access only to well or mains water supplies were both negative for C. jejuni. More recently, Minihan et al. (21) identified an association between contamination of water trough surfaces with C. jejuni and increased incidence of C. jejuni fecal shedding. Interestingly, this latter study found that the contamination of the water trough water itself and sediments at the bottom of the troughs were not clearly associated with C. jejuni fecal shedding by the cattle, possibly due to the chlorinated water supply. Wesley et al. (32) also evaluated water chlorination as a risk (protective) factor in an observational study including 80 herds and found no association with C. jejuni fecal prevalence; however, the power of that study was limited by a relatively small number of study herds with chlorinated water supplies.
Three lines of evidence from the present study indicated that water chlorination did not significantly affect the transmission of C. jejuni. (i) The fecal prevalence of C. jejuni in cattle drinking from chlorinated water troughs was very similar to that of cattle watered with nonchlorinated water. (ii) Chlorinated water troughs were themselves detectably contaminated with C. jejuni, as were control water troughs. (iii) The diversity of C. jejuni isolated from cattle as assessed by Simpson's index applied to PFGE MRP did not differ among isolates from cattle receiving chlorinated and nonchlorinated water. These results represent a much more powerful test of the chlorination of water supplies than was previously available due to the prospective balanced study design, the monitored hypochlorite residuals in the water column, the large numbers of experimental animals, and the concurrent matched-origin control groups of cattle on the same farm and receiving the same feeds. The results clearly show that chlorination alone is insufficient to provide detectable control of C. jejuni transmission or recrudescence and strongly suggest that other interventions to reduce organic matter contamination of water troughs are an essential precondition to more effective chlorination. A previous report (17) of fecal E. coli O157:H7 conducted in parallel with the present study provided similar conclusions regarding the lack of effect of chlorination for prevention of bovine infection with that agent.
In summary, cattle entering a high stocking density feedlot shed C. jejuni in their feces at low prevalence but the prevalence increased markedly during the feeding period. This increase in prevalence was attributed primarily to epidemic spread of a relatively small number of common strain types. Furthermore, the provision of chlorinated water supply did not significantly affect the frequency of contamination of the cattle trough specimens or the prevalence of C. jejuni isolation from the feces of the cattle receiving the chlorinated water supply.
Acknowledgments
This project was funded in part by the U.S. Department of Agriculture National Research Initiative program Epidemiological Approach to Food Safety grant 1999-04281 and by the Agricultural Animal Health Program at Washington State University, Pullman, WA.
REFERENCES
- 1.Anonymous. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—selected sites, United States, 2003. Morb. Mortal. Wkly. Rep. 53:338-343. [PubMed] [Google Scholar]
- 2.Bae, W., K. N. Kaya, D. D. Hancock, D. R. Call, Y. H. Park, and T. E. Besser. 2004. Prevalence and antimicrobial resistance of thermophilic Campylobacter spp. from cattle farms in Washington State. Appl. Environ. Microbiol. 71:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang, D. D., A. Wedderkopp, K. Pedersen, and M. Madsen. 2002. Rapid PCR using nested primers of the 16S rRNA and the hippuricase (hipO) genes to detect Campylobacter jejuni and Campylobacter coli in environmental samples. Mol. Cell Probes 16:359-369. [DOI] [PubMed] [Google Scholar]
- 4.Busato, A., D. Hofer, T. Lentze, C. Gaillard, and A. Burnens. 1999. Prevalence and infection risks of zoonotic enteropathogenic bacteria in Swiss cow-calf farms. Vet. Microbiol. 69:251-263. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald, C., K. Stanley, S. Andrew, and K. Jones. 2001. Use of pulsed-field gel electrophoresis and flagellin gene typing in identifying clonal groups of Campylobacter jejuni and Campylobacter coli in farm and clinical environments. Appl. Environ. Microbiol. 67:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman, C. R., R. M. Hoekstra, M. Samuel, R. Marcus, J. Bender, B. Shiferaw, S. Reddy, S. D. Ahuja, D. L. Helfrick, F. Hardnett, M. Carter, B. Anderson, and R. V. Tauxe. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38(Suppl. 3):S285-S296. [DOI] [PubMed] [Google Scholar]
- 7.Garcia, M. M., H. Lior, R. B. Stewart, G. M. Ruckerbauer, J. R. R. Trudel, and A. Skljarevski. 1985. Isolation, characterization and serotyping of Campylobacter jejuni and Campylobacter coli from slaughter cattle. Appl. Environ. Microbiol. 49:667-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacoboni, G. I., K. Itoh, K. Hirayama, E. Takahashi, and T. Mitsuoka. 1993. Comparison of fecal Campylobacter in calves and cattle of different ages and areas in Japan. J. Vet. Med. Sci. 55:555-559. [DOI] [PubMed] [Google Scholar]
- 9.Grau, F. H. 1988. Campylobacter jejuni and Campylobacter hyointestinalis in the intestinal tract and on the carcasses of calves and cattle. J. Food Prot. 51:857-861. [DOI] [PubMed] [Google Scholar]
- 10.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanninen, M.-L., M. Niskanen, and L. Korhonen. 1998. Water as a reservoir for Campylobacter jejuni infection in cows studied by serotyping and pulsed-field gel electrophoresis (PFGE). J. Vet. Med. B 45:37-42. [DOI] [PubMed] [Google Scholar]
- 12.Harris, N. V., D. Thompson, D. C. Martin, and C. M. Nolan. 1986. A survey of Campylobacter and other bacterial contaminants of pre-market chicken and retail poultry and meats, King County, Washington. Am. J. Public Health 76:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoar, B. R., E. R. Atwill, C. Elmi, and T. B. Farver. 2001. An examination of risk factors associated with beef cattle shedding pathogens of potential zoonotic concern. Epidemiol. Infect. 127:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey, T. J., and P. Beckett. 1987. Campylobacter jejuni in dairy cows and raw milk. Epidemiol. Infect. 98:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inglis, G. D., L. D. Kalischuk, and H. W. Busz. 2003. A survey of Campylobacter species shed in faeces of beef cattle using polymerase chain reaction. Can. J. Microbiol. 49:655-661. [DOI] [PubMed] [Google Scholar]
- 16.LeJeune, J. T., T. E. Besser, and D. D. Hancock 2001. Cattle water troughs as reservoirs of Escherichia coli O157. Appl. Environ. Microbiol. 67:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeJeune, J. T., T. E. Besser, D. H. Rice, J. L. Berg, R. P. Stilborn, and D. D. Hancock. 2004. Longitudinal study of fecal shedding of Escherichia coli O157:H7 in feedlot cattle: predominance and persistence of specific clonal types despite massive cattle population turnover. Appl. Environ. Microbiol. 70:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeJeune, J. T., T. E. Besser, D. H. Rice, and D. D. Hancock. 2001. Methods for the isolation of waterborne Escherichia coli O157. Lett. Appl. Microbiol. 32:316-320. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz, E., A. Lastovica, and R. J. Owen. 1998. Subtyping of Campylobacter jejuni Penner serotypes 9, 38, and 63 from human infections, animals and water by pulsed field gel electrophoresis and flagellin gene analysis. Lett. Appl. Microbiol. 26:179-182. [DOI] [PubMed] [Google Scholar]
- 20.Madden, R. H., L. Moran, and P. Scates. 1998. Frequency of occurrence of Campylobacter spp. in red meats and poultry in Northern Ireland and their subsequent subtyping using polymerase chain reaction-restriction fragment length polymorphism and the random amplified polymorphic DNA method. J. Appl. Microbiol. 84:703-708. [DOI] [PubMed] [Google Scholar]
- 21.Minihan, D., P. Whyte, M. O'Mahoney, S. Fanning, K. McGill, and J. D. Collins. 2004. Campylobacter spp. in Irish feedlot cattle: a longitudinal study involving pre-harvest and harvest phases of the food chain. J. Vet. Med. B 51:28-33. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen, E. M., J. Engberg, V. Fussing, L. Petersen, C. H. Brogren, and S. L. On. 2000. Evaluation of phenotypic and genotypic methods for subtyping Campylobacter jejuni isolates from humans, poultry, and cattle. J. Clin. Microbiol. 38:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen, E. M. 2002. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Lett. Appl. Microbiol. 35:85-89. [DOI] [PubMed] [Google Scholar]
- 24.On, S. L., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono, K., and K. Yamamoto. 1999. Contamination of meat with Campylobacter jejuni in Saitama, Japan. Int. J. Food Microbiol. 47:211-219. [DOI] [PubMed] [Google Scholar]
- 26.Osano, O., and S. M. Arimi. 1999. Retail poultry and beef as sources of Campylobacter jejuni. East Afr. Med. J. 76:141-143. [PubMed] [Google Scholar]
- 27.Pebody, R. G., M. J. Ryan, and P. G. Wall. 1997. Outbreaks of Campylobacter infection: rare events for a common pathogen. Commun. Dis. Rep. CDR Rev. 7:R33-R37. [PubMed] [Google Scholar]
- 28.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley, K., K. Jones. 2003. Cattle and sheep farms as reservoirs of Campylobacter. J. Appl. Microbiol. 94:104S-113S. [DOI] [PubMed] [Google Scholar]
- 30.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480. [DOI] [PubMed] [Google Scholar]
- 31.Werno, A. M., J. D. Klena, G. M. Shaw, and D. R. Murdoch. 2002. Fatal case of Campylobacter lari prosthetic joint infection and bacteremia in an immunocompetent patient. J. Clin. Microbiol. 40:1053-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wesley, I. V., S. J. Wells, K. M. Harmon, A. Green, L. Schroeder-Tucker, M. Glover, and I. Siddique. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 66:1994-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the greater Washington, D.C., area. Appl. Environ. Microbiol. 67:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]