Abstract
Microbes have been able to persist in water distribution systems through the development of multicellular communities known as biofilms. This study evaluated the usefulness of the bioelectric effect for the elimination of water distribution system biofilms from annular reactors. The bioelectric effect did not have any bactericidal action either alone or when coupled with free chlorine.
The utilization of adequate water management methods has vastly contributed to lowering human mortality and improving quality of life (4-6, 9). However, a number of microbes have been able to persist in water distribution systems by the development of a multicellular community known as a biofilm (3). A strategy to effectively eliminate biofilms from water distribution systems may be through the application of the bioelectric effect. This effect was initially noted by Costerton et al. and Jass et al. (2, 7), who showed that the efficacy of antibiotics was increased through the application of weak electric fields. While the cause of the bioelectric effect remains unexplained, a number of other researchers have also noted the bioelectric effect (8, 10-12). This study was designed to evaluate the ability of the bioelectric effect to enhance the antimicrobial properties of free chlorine against mixed-population biofilms of drinking water origin.
Biofilms were grown in annular reactors (BioSurface Technologies Corp., Bozeman, MT) that consisted of a stationary outer cylinder with 20 flush-mounted, polycarbonate, removable slides for biofilm sampling on an inner drum. The inner drum rotates, providing a shear stress at the reactor wall that simulated 1 ft/s. The inner drum also contains draft tubes to ensure vertical as well as horizontal mixing. For a carbon source, humic concentrate prepared as described previously (1) was added to the reactors to produce a final concentration of 1.5 ppm carbon in the reactors.
Stainless-steel electrodes (positive and negative) were placed against the walls of the reactor at opposite sides of the inner rotating drum and connected to a power supply that provided a constant flux of 3.7 μA/mm2 through the reactor, as determined by a multitest ammeter. This current flux was previously shown to be effective in producing a significant bioelectric effect (2, 7, 8). In preliminary studies, metal flocs were generated by the exposure of iron-based reactor components to the direct current. Therefore, internal iron surfaces of the reactors were insulated from the electrodes and system water by application of a silicon sealer.
Humic concentrate was prepared by mixing Elliot silt loam soil (120 g) in 0.1 M sterile NaOH (1 liter) for 48 h at room temperature. The mixture was centrifuged (20 min, 10,000 × g), and the supernatant was recovered. The total carbon concentration was determined for the supernatant and diluted to a final concentration of 12.5 ppm. Sterile potassium nitrate and dibasic potassium phosphate were added to the supernatant to provide final concentrations of 1.25 ppm and 0.125 ppm, respectively, and the pH was adjusted to 7.20. This supplemented and pH-adjusted humic concentrate was combined with tap water treated in laboratory-scale granular activated carbon filters filled with filter medium from a biological treatment facility in Laval, Quebec, Canada, at 1 ml/min and 7.33 ml/min, respectively. The end result was a mixture consisting of 1.5 ppm carbon, 0.15 ppm nitrate, and 0.015 ppm phosphate and a residence time of 120 minutes. These reactor conditions represented a scenario in which negligible reactive chlorine and elevated carbon levels existed for an extended period of time in a water delivery system. We were able to define when biofilm growth had attained steady state by taking repeated samples over subsequent days.
While previous experiments evaluating the bioelectric effect have utilized chloride-free media (see above), our system had measurable levels of chloride ions like those of standard water delivery systems. Therefore, the passage of current through a solution containing these ions may have created reactive chlorine-based substances, such as free chlorine (e.g., Cl2, OCl−, and HOCl), chlorine dioxide (ClO2), chlorite (ClO2−), monochloramine (NH2Cl), dichloramine (NHCl2), and trichloramine (NCl3), due to electrolysis.
We then evaluated the ability of the bioelectric effect to enhance the antimicrobial properties of free chlorine against mixed-population biofilms of drinking water origin. Biofilms were grown until they had attained steady state and then were evaluated with a number of control and experimental groups (Table 1). The free-chlorine infeed was derived from household bleach (5.25% [wt/vol] sodium hypochlorite), and infeed concentrations were confirmed by using a standard n,n-diethyl-p-phenyldiamine (DPD) colorimetric method (Hach Inc., Loveland, CO). The two infeed free-chlorine concentrations, 0.5 mg/liter and 5.0 mg/liter, provided residual-chlorine concentrations of approximately 0.06 mg/liter and 1.5 mg/liter, respectively, within the annular reactors.
TABLE 1.
Control and experimental annular reactor groups for the evaluation of the bioelectric effect (with and without free-chlorine addition) to eliminate mixed-species water biofilms
| Group | Treatment applied to annular reactor |
|---|---|
| 1 | No current or free chlorine (untreated control group) |
| 2 | Current application (15.7 mA) proportional to a current flux of 3.7 μA/mm2 |
| 3 | Constant free-chlorine supply of 0.5 mg/liter |
| 4 | Free chlorine (0.5 mg/liter) and current application (15.7 mA) |
| 5 | Constant free-chlorine supply of approximately 5.0 mg/liter |
| 6 | Free chlorine (5.0 mg/liter) and current application (15.7 mA) |
Biofilm and planktonic samples were obtained at 0, 6, 12, 24, 48, and 168 hours after initiation of the experiment. These samples were homogenized, serially diluted, and plated on R2A agar and incubated for 7 days at room temperature. Free- and total-chlorine concentrations were determined for each group by using the DPD colorimetric method. All sampling was performed in triplicate, with the exception of biofilm samples that were collected in duplicate. All differences for average cell counts and average chlorine concentrations between sample points were deemed significant if the P was <0.05 according to Student's t-test.
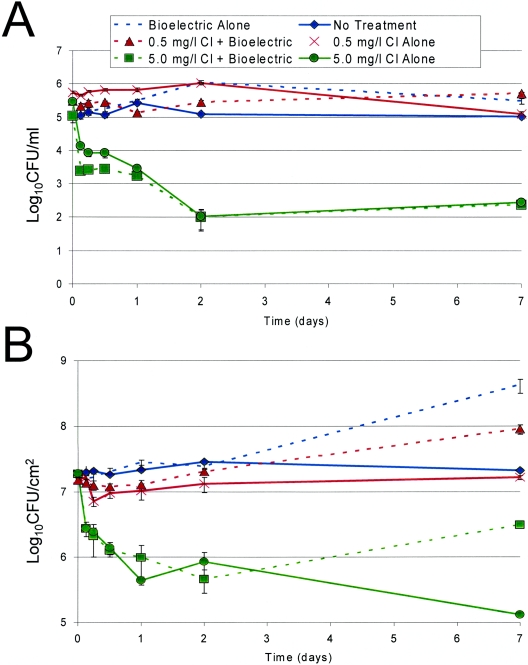
Figure 1A shows planktonic bacterial concentrations in the experimental and control reactors during the 7-day study. Planktonic bacteria were detected at concentrations between 105 and 106 CFU/ml for all groups studied, with the exception of reactors treated with 5.0 mg/liter free-chlorine infeed with and without current application. When compared to each other, these last two groups did not display a significant difference in planktonic bacterial concentrations. Therefore, the application of current had no effect on planktonic bacterial kill rates when current was applied alone to the reactor system or in combination with free-chlorine administration. These trends were also noted in the biofilm bacterial numbers as demonstrated in Fig. 1B. In fact, the application of current significantly increased the number of biofilm bacteria when the current was applied alone and reduced the bactericidal efficacy of the infused free chlorine. This may have been due to the generation of nutrients beneficial to biofilm growth.
FIG. 1.
Evaluation of electric current application (with and without the addition of 0.5 or 5.0 mg/liter chlorine infeed) on the concentrations of (A) planktonic and (B) biofilm-embedded mixed bacterial species in annular reactors. Samples were taken at a number of time points and plated in triplicate.
The residual-chlorine concentrations in the annular reactors were determined for each group (Fig. 2). Residual-chlorine concentrations for reactors that received an infeed of 5.0 mg/liter free chlorine (with or without bioelectric application) are not provided in this figure. However, the concentrations in these reactors ranged between 1.0 and 2.0 mg/liter, and the bioelectric effect-treated and nontreated reactors did not show a significant difference in the detected total chlorine concentrations when the reactor results were compared. All chlorine levels in the four experimental groups and one control group displayed in Table 1 were less than 0.06 mg/liter. Specifically, 90% of the 0.5-mg/liter chlorine infeed was used to satisfy the chlorine demand of the reactors, as shown by the detection of only 0.06 mg/liter of residual chlorine. There was also a demonstrable lack of current-generated reactive chlorine products in the reactor systems. In fact, when current was applied to the reactor receiving 0.5 mg/liter infeed, the observed residual-chlorine concentrations were less than those of reactors that received 0.5 mg/liter infeed alone. The low residual-chlorine levels in the biofilm-containing reactors seen in Fig. 2 may be due to inactivation of the chlorine substances by the biofilm and the adsorbed surface carbon.
FIG. 2.
Biofilms were allowed to grow in the reactors for 3 weeks until steady-state biofilm growth was achieved. Then, total chlorine concentrations were determined in annular reactors at 0, 3, 6, 12, 24, 48, and 168 hours following initiation of current application (3.7 μA/mm2) via stainless-steel electrodes in a solution consisting of 1.5 ppm carbon, 0.15 ppm nitrate, and 0.015 ppm phosphate and after a residence time of 120 minutes. Total and free-chlorine concentrations were determined using a HACH2 spectrophotometer and a modified DPD colorimetric method.
The results of the experiments testing the efficacy of the bioelectric effect ran contrary to those of previous studies that describe a synergistic effect between weak current fields and antimicrobial agents (2, 7, 8, 10, 12). However, those investigators used pure-culture biofilms under well-defined medium conditions. Therefore, we feel that the experiments described in our report more accurately reflect the complex nature of water delivery systems and their biological/chemical constituents. Current application was unable to reduce biofilm and planktonic bacterial concentrations (either with or without free-chlorine addition) and may in fact increase the bacterial numbers of mixed-species water biofilm bacteria. When these results are coupled with the observation that current application resulted in high corrosion rates on the electrodes and metals that were in contact with the fluid component of electrically treated reactors (data not shown), the usefulness of the bioelectric effect as a biofilm clearance strategy is not evident. The bioelectric effect did not prove to be a feasible strategy for the clearance of mixed-species water biofilms in our experimental system.
Acknowledgments
We thank David Davies, Mark Pasmore, and Bruce McLeod for their help and consultation in carrying out the studies described herein. We also thank the American Water Works Association Research Foundation, the Project Advisory Committee, and the CBE Industrial Associates for their advice and cooperation in completing this research.
REFERENCES
- 1.Butterfield, P. W., A. K. Camper, J. A. Biederman, and A. M. Bargmeyer. 2002. Minimizing biofilm in the presence of iron oxides and humic substances. Water Res. 36:3898-3910. [DOI] [PubMed] [Google Scholar]
- 2.Costerton, J. W., B. Ellis, K. Lam, F. Johnson, and A. E. Khoury. 1994. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 38:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 4.Curtiss, J. R., and D. Grahn. 1980. Population characteristics and environmental factors that influence level and cause of mortality. A review. J. Environ. Pathol. Toxicol. 4:471-511. [PubMed] [Google Scholar]
- 5.Esrey, S. A., J. B. Potash, L. Roberts, and C. Shiff. 1991. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull. W. H. O. 69:609-621. [PMC free article] [PubMed] [Google Scholar]
- 6.Gorter, A. C., P. Sandiford, G. D. Smith, and J. P. Pauw. 1991. Water supply, sanitation and diarrhoeal disease in Nicaragua: results from a case-control study. Int. J. Epidemiol. 20:527-533. [DOI] [PubMed] [Google Scholar]
- 7.Jass, J., J. W. Costerton, and H. M. Lappin-Scott. 1995. The effect of electrical currents and tobramycin on Pseudomonas aeruginosa biofilms. J. Ind. Microbiol. 15:234-242. [DOI] [PubMed] [Google Scholar]
- 8.McLeod, B. R., S. Fortun, J. W. Costerton, and P. S. Stewart. 1999. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol. 310:656-670. [DOI] [PubMed] [Google Scholar]
- 9.Murray, C. J., and A. D. Lopez. 1997. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 349:1436-1442. [DOI] [PubMed] [Google Scholar]
- 10.Stewart, P. S., W. Wattanakaroon, L. Goodrum, S. M. Fortun, and B. R. McLeod. 1999. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 43:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoodley, P., D. de Beer, and H. M. Lappin-Scott. 1997. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob. Agents Chemother. 41:1876-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellman, N., S. M. Fortun, and B. R. McLeod. 1996. Bacterial biofilms and the bioelectric effect. Antimicrob. Agents Chemother. 40:2012-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]