Abstract
We used flow cytometry to examine seasonal variations in basin-scale distributions of bacterioplankton in Lake Biwa, Japan, a large mesotrophic freshwater lake with an oxygenated hypolimnion. The bacterial communities were divided into three subgroups: bacteria with very high nucleic acid contents (VHNA bacteria), bacteria with high nucleic acid contents (HNA bacteria), and bacteria with low nucleic acid contents (LNA bacteria). During the thermal stratification period, the relative abundance of VHNA bacteria (%VHNA) increased with depth, while the reverse trend was evident for LNA bacteria. Seasonally, the %VHNA was strongly positively correlated (r = 0.87; P < 0.001) with the concentration of dissolved inorganic phosphorus, but not with the concentration of chlorophyll a. The growth of VHNA bacteria was significantly enhanced by addition of phosphate or phosphate plus glucose but not by addition of glucose alone. Although the growth of VHNA and HNA bacteria generally exceeded that of LNA bacteria, our data also revealed that LNA bacteria grew faster than and were grazed as fast as VHNA bacteria in late August, when nutrient limitation was presumably severe. Based on these results, we hypothesize that in severely P-limited environments such as Lake Biwa, P limitation exerts more severe constraints on the growth of bacterial groups with higher nucleic acid contents, which allows LNA bacteria to be competitive and become an important component of the microbial loop.
In pelagic ecosystems, heterotrophic bacteria comprise the most important trophic level for processing dissolved organic matter (DOM) and consume a substantial fraction (40 to 60%) of the primary production (1, 6, 7, 31). Generally, previous studies have treated bulk bacterial communities as a homogeneous pool (1, 6, 7, 31), even though they consist of diverse subgroups that differ in metabolic state (14), DOM use (9), growth rate (10, 46), susceptibility to grazing (17), and phylogenetic affiliations (15). One of the contemporary challenges for aquatic microbial ecology is to clarify variations and regulation of different bacterial subgroups in order to facilitate establishing ecologically useful functional units with which the internal dynamics of the bacterioplankton “black box” can be better understood.
Flow cytometry has become a powerful tool for discriminating bacterial subgroups based on cellular nucleic acid content (13, 26, 37). Recent studies using flow sorting have revealed that bacteria with high nucleic acid contents (HNA bacteria) and bacteria with low nucleic acid contents (LNA bacteria) differ in phylogenetic composition (49), although Servais et al. (36) found that common phylotypes are present in both subgroups. Some investigators have suggested that HNA and LNA bacteria represent active and less active components, respectively (14, 22), leading to the proposition that the percentage of HNA bacteria relative to the total bacterial abundance (%HNA) can be an indicator of the fraction of active cells in bacterial communities (14, 19). Other studies, however, have reported conflicting results, suggesting that the biomass-specific activities (48) and growth (21) of LNA bacteria can exceed those of HNA bacteria in marine waters. These results suggest that LNA bacteria can become competitively superior to HNA bacteria under certain environmental conditions. However, the factors that affect the relative growth rates of LNA and HNA bacteria are not well understood.
Previous studies have shown that the %HNA varies greatly depending on the environment (range, 3 to 90%) (2, 20, 26). Li et al. (26) found that the %HNA was positively correlated with the chlorophyll a (Chl a) concentration for diverse marine systems, suggesting that the %HNA increases with increasing organic supply over a large scale. However, this generalization would not be true for a depth profile of bacteria. In the Gulf of Mexico, Jochem (20) found that there was a systematic increase in the %HNA with depth (range, 0 to 200 m), which was inconsistent with the expectation that the %HNA is higher in more productive surface environments. The dynamics of bacterial communities may be influenced not only by the organic substrate supply but also by other factors, including the availability of inorganic resources (4, 5, 41) and mortality (17, 42). How different forces affect spatiotemporal variations in bacterial composition has yet to be elucidated.
Here we describe our data on bacterioplankton composition distinguished by flow cytometry in a large freshwater lake, Lake Biwa. The vertical distributions of bacterial composition and biogeochemical variables were examined in different seasons. Incubation experiments were conducted to examine variations among different bacterial groups in growth and grazing mortality rates and responses to nutrient additions. Our data suggest that the availability of P could be a major factor that affects the composition of bacterioplankton in P-limited environments such as Lake Biwa.
MATERIALS AND METHODS
Study site and water sampling.
Lake Biwa is a large (surface area, 674 km2), deep (maximum depth, 104 m), tectonic lake located in the central part of Honshu Island, Japan. This study was conducted in the monomictic north basin, which has a water residence time of 5.5 years. Previous studies have reported the gross primary production (910 to 1,460 mg C m−2 day−1) (33), bacterial production (ca. 30% of the primary production) (29), and microbial trophic interactions (16, 30, 31) in the pelagic environment of this mesotrophic basin.
Samples were obtained at seven stations along the major axis of the basin (see the supplemental material) at bimonthly intervals between June 2001 and June 2002. Due to rough weather, station 1 was not visited in June 2001, whereas stations 1 and 5 were not visited in December 2001. Each transect survey was completed within 3 or 4 days. At selected stations (stations 4 and 6), additional samples were obtained at bimonthly intervals until December 2002. Depth profiles of water temperature were obtained with a conductivity-temperature-depth probe (SBE 911plus; Sea-Bird Electronics, Bellevue, WA). Water samples were collected with clean 5-liter Niskin-X bottles (General Oceanics, Miami, FL) at predetermined depths. Water samples for flow cytometry were placed in cryovials, fixed with a formaldehyde solution (final concentration, 2%), frozen in liquid nitrogen, and stored at −80°C for later analysis. Water samples were also collected for measurement of biogeochemical variables, including dissolved organic carbon (DOC), dissolved organic nitrogen, dissolved organic phosphorus, and inorganic constituents (NO3−, NO2−, NH4+, PO43−, O2), as well as particulate organic carbon (POC), particulate organic nitrogen, and particulate organic phosphorus (see below).
Incubation experiments.
Surface water samples were collected at either station 4 (26 June, 25 July, 22 August, and 31 October) or station 7 (11 June) in 2003. The water samples were filtered through 0.8-μm-pore-size Nuclepore filters (Whatman) with gentle pressure (<20 mm Hg) in order to eliminate protozoan grazers. The filtrates were dispensed into acid-washed polycarbonate bottles (250ml; Nalgene) and incubated at the in situ temperature in the dark. Triplicate bottles were prepared for each sample. Subsamples were withdrawn from each bottle at the beginning of incubation and after incubation for 48 h. The subsamples were fixed with a 2% formaldehyde solution, frozen in liquid nitrogen, and stored at −80°C for later analysis by flow cytometry. Growth rates of individual bacterial subgroups (μc) were estimated by assuming that there was exponential growth, as follows: μc = (1/t) ln(Nend/Nin), where t is the incubation time and Nend and Nin are the bacterial abundance after incubation for 48 h and at the beginning of incubation, respectively. Time course measurements confirmed that bacterial abundance generally increased exponentially during the first 48 h (data not shown).
In order to examine the effect of nutrient addition on bacterial growth, either glucose (final concentration, 10 μmol liter−1) or phosphate (KH2PO4) (final concentration, 0.5 μmol liter−1) or both were added to incubation bottles prepared as described above. Although kinetic data on phosphate uptake are not available for our samples, the level of phosphate addition probably represented the saturation level for lake bacterial communities (8). Triplicate bottles were prepared for each addition. Growth rates of individual bacterial subgroups were calculated as described above.
To estimate grazing mortality, we measured the apparent growth rates of bacterial subgroups in water samples prefiltered through a 25-μm-mesh screen. The filtrate was dispensed into triplicate polycarbonate bottles (250 ml) and incubated at the in situ temperature in the dark, as described above for measurement of bacterial growth. The apparent growth rates in the 25-μm filtrate, calculated by assuming that there was exponential growth, were then compared with bacterial growth rates in the 0.8-μm filtrate (μc). Grazing mortality was estimated by determining the difference between μc and the apparent growth rate in the 25-μm filtrate (30).
Flow cytometric analyses of heterotrophic bacteria.
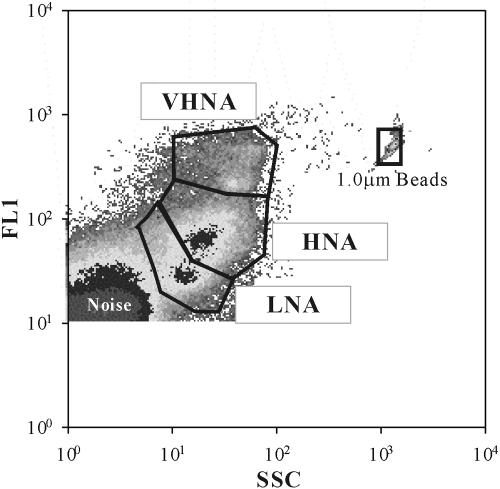
The frozen samples were thawed, stained with SYBR Green I (1:10,000 dilution of commercial stock; Molecular Probes), incubated for 20 min, and analyzed with a Becton Dickinson FACScalibur flow cytometer equipped with a laser emitting at 488 nm (28, 37). For each sample, fluorescent beads (diameter, 1 μm; Molecular Probes) were added as an internal standard at a final concentration of approximately 105 particles ml−1. The concentration of the fluorescent beads was calibrated with TruCounts (Becton Dickinson) for every 20 to 30 runs (37). Samples were run at a speed of approximately 30 μl min−1 to acquire around 50,000 counts for each sample. The data were collected in list mode files and then analyzed using WinMDI 2.8 (http://facs.scripps.edu/software.html). Bacteria were detected in a plot of green fluorescence (FL1) versus side angle light scatter (SSC). Bacterial clusters distinguished in FL1-SSC plots were counted using polygonal gating. The same gating regions were used for most (>95%) samples; the only exceptions were samples in which slight adjustment in gating resulted in more reasonable separation.
Chemical analyses.
Concentrations of Chl a were determined fluorometrically using a Turner Designs fluorometer (10-AU) after extraction with 90% acetone (43). Dissolved oxygen (DO) concentrations were measured by the Winkler method (43). Concentrations of POC and particulate organic nitrogen were determined with a CHN analyzer (PE-2400II; Perkin-Elmer). Concentrations of NO3−, NO2−, and PO43− were determined spectrophotometrically using an autoanalyzer (AACS II; Bran+Luebee), whereas the NH4+ concentration was determined by the OPA method (18). Total dissolved nitrogen and total dissolved phosphorus concentrations were measured using an autoanalyzer after wet oxidation (35). Dissolved organic nitrogen and dissolved organic phosphorus concentrations were determined by determining differences between the total dissolved nitrogen and total dissolved inorganic nitrogen (DIN) (NO3− plus NO2− plus NH4+) concentrations and between total dissolved phosphorus and dissolved inorganic phosphorus (DIP) (PO43−) concentrations, respectively. DOC concentrations were determined with a total organic carbon analyzer (TOC-5000; Shimazu).
RESULTS
Environmental variables.
The ranges of the water temperature in the epi- and hypolimnion were 10.4 to 30.2°C and 6.8 to 9.9°C, respectively. The concentrations of Chl a ranged from 0.2 to 8.8 μg liter−1 in the epilimnion, where the nutrient pool was characterized by low DIP concentrations (average, 0.009 ± 0.009 μmol liter−1) compared to the DIN concentrations (5.3± 3.9 μmol liter−1). The annual minimum DO concentration was 3.0 mg liter−1 in the hypolimnion and was associated with the accumulation of DIP (maximum level, 0.43 μmol liter−1). The annual ranges for other biogeochemical variables are shown in Table 1.
TABLE 1.
Summary of the variability in environmental and bacterial variablesa
| Parameter | Units | Whole water layers
|
Epilimnion
|
Hypolimnion
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SD | n | Range | Mean | SD | n | Range | Mean | SD | n | ||
| Total bacteria | 106 cells ml−1 | 0.6-8.2 | 2.3 | 1.4 | 571 | 1.0-8.2 | 3.6 | 1.8 | 168 | 0.6-2.5 | 1.2 | 0.3 | 212 |
| Temp | °C | 6.5-30.2 | 11.8 | 6.2 | 571 | 10.4-30.2 | 19.8 | 5.5 | 168 | 6.8-9.9 | 7.7 | 0.7 | 212 |
| DO | mg liter−1 | 3.0-11.5 | 8.8 | 1.8 | 571 | 6.5-10.4 | 8.9 | 0.9 | 168 | 3.0-10.3 | 7.5 | 1.7 | 212 |
| Chl a | μg liter−1 | 0.1-11.0 | 1.8 | 1.7 | 568 | 0.2-8.8 | 3.2 | 1.6 | 166 | 0.1-5.0 | 0.5 | 0.5 | 211 |
| POC | μmol liter−1 | 4.3-71.1 | 20.8 | 12.1 | 571 | 11.2-70.8 | 32.2 | 9.9 | 168 | 4.3-54.0 | 14.5 | 9.3 | 212 |
| PON | μmol liter−1 | 0.3-9.3 | 2.4 | 1.4 | 568 | 0.8-7.6 | 3.6 | 1.2 | 168 | 0.3-3.8 | 1.5 | 0.8 | 209 |
| POP | μmol liter−1 | 0.015-0.249 | 0.101 | 0.042 | 571 | 0.015-0.249 | 0.128 | 0.045 | 168 | 0.021-0.208 | 0.077 | 0.035 | 212 |
| DOC | μmol liter−1 | 72.6-138.4 | 94.1 | 13.1 | 561 | 90.1-138.4 | 109.4 | 9.3 | 166 | 72.6-133.3 | 84.5 | 6.9 | 206 |
| DON | μmol liter−1 | 1.2-18.1 | 7.2 | 1.7 | 568 | 1.2-15.0 | 8.3 | 1.4 | 167 | 3.3-8.1 | 5.9 | 1.2 | 210 |
| DOP | μmol liter−1 | 0-0.212 | 0.054 | 0.030 | 562 | 0-0.212 | 0.071 | 0.032 | 165 | 0-0.179 | 0.036 | 0.028 | 208 |
| DIN | μmol liter−1 | 0-24.3 | 14.4 | 6.9 | 570 | 0-20.7 | 5.3 | 3.9 | 167 | 10.2-24.3 | 20.5 | 1.9 | 211 |
| DIP | μmol liter−1 | 0-0.425 | 0.061 | 0.096 | 570 | 0-0.044 | 0.009 | 0.009 | 167 | 0.001-0.425 | 0.140 | 0.121 | 211 |
The epi- and hypolimnion data are from the thermal stratification period (June, August, October, and December). Abbreviations: PON, particulate organic nitrogen; POP, particulate organic phosphorus; DON, dissolved organic nitrogen; DOP, dissolved organic phosphorus.
Bacterial variables.
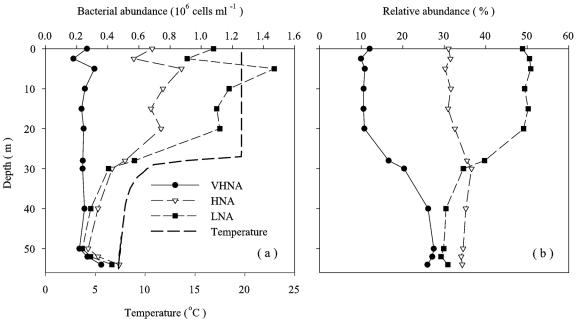
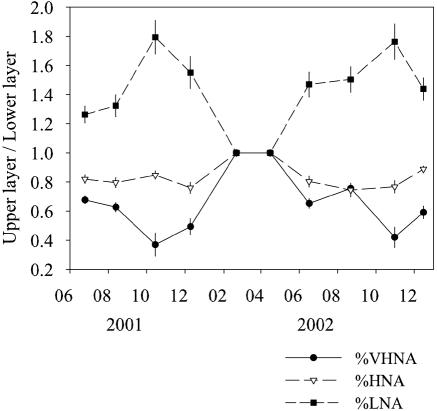
The total bacterial abundance ranged from 0.6 × 106 to 8.2 × 106 cells ml−1 (Table 1). On the basis of SSC and FL1, we discriminated three subgroups of bacteria, which were designated the bacteria with very high nucleic acid contents (VHNA bacteria), the HNA bacteria, and the LNA bacteria (Fig. 1). Significant vertical and seasonal variations were observed for all bacterial variables during the thermal stratification period, whereas horizontal variations were less pronounced (as determined by three-way analysis of variance [ANOVA]) (Table 2). Notably, there were very consistent trends in the vertical distribution patterns of bacterial subgroups during the thermal stratification period (Fig. 2; also see the supplemental material). The depth-dependent decrease in cell abundance was most pronounced for the LNA bacteria, followed by the HNA bacteria, whereas the abundance of the VHNA bacteria varied little with depth except that, on occasion, the abundance of all the subgroups increased significantly near the bottom (Fig. 2a; also see the supplemental material). Because of this difference in the vertical distribution patterns among the different subgroups, the %VHNA in the upper layer (10.8% ± 0.7% in the case of the profile shown in Fig. 2b) was much lower than that in the lower layer (23.9% ± 4.4%), whereas the reverse was observed for the %LNA, for which high values (49.8% ± 0.8%) and low values (32.5% ± 4.2%) were obtained for the upper and lower layers, respectively. The %HNA tended to increase with depth, although the vertical differences in the %HNA (range, 30.2 to 36.6%) were less pronounced than those for other subgroups (Fig. 2b). The trends described above for vertical differences in the composition of bacteria were consistently observed over the seasons except for February and April, when compositional differences with depth were minimal because of vertical mixing (Fig. 3).
FIG. 1.
Example of a flow cytogram of bacterioplankton in Lake Biwa (the sample was collected on 15 October 2001 at a depth of 40 m at station 5). Side scatter (SSC) and fluorescence (FL1) emitted from SYBR Green I bound to bacterial nucleic acid differentiated noise and bacteria enclosed by the gray gate. Bacteria were further divided into three subgroups, the VHNA, HNA, and LNA bacteria, according to the positions in the cytogram. Beads were added as internal standards.
TABLE 2.
Results of the three-way ANOVA to test the significance of variations in bacterial abundance and the relative abundance of bacterial groups (%VHNA, %HNA, and %LNA) associated with seasonal (month [June 2001, August 2001, October 2001, December 2001, and June 2002]), horizontal (station [stations 4, 5, 6, and 7]), and vertical (depth [upper and lower layers as defined in the text]) dimensions during the stratification period
| Variable | Source of variation | DF | F | P |
|---|---|---|---|---|
| Total bacteria | Month | 4 | 59 | <0.001 |
| Station | 3 | 2 | 0.173 | |
| Depth | 1 | 475 | <0.001 | |
| %VHNA | Month | 4 | 9 | 0.001 |
| Station | 3 | 2 | 0.207 | |
| Depth | 1 | 256 | <0.001 | |
| %HNA | Month | 4 | 43 | <0.001 |
| Station | 3 | 23 | <0.001 | |
| Depth | 1 | 355 | <0.001 | |
| %LNA | Month | 4 | 30 | <0.001 |
| Station | 3 | 25 | <0.001 | |
| Depth | 1 | 552 | <0.001 |
FIG. 2.
(a) Depth profiles of temperature and abundance of VHNA, HNA, and LNA bacterial cells at station 7 on 19 October 2001. (b) Depth profiles of contributions of different subgroups to the total bacterial abundance. The symbols are the same as those in panel a.
FIG. 3.
Seasonal changes in the ratio of the contribution of a cytometric group (VHNA, HNA, or LNA) in the upper layer to the contribution in the lower layer. The ratios were calculated for stations 4 and 6, and the averages of two stations are shown for individual groups. The error bars indicate the ranges of the values obtained at the two stations.
Correlations between bacterial and environmental variables.
We conducted Pearson's correlation analyses to examine the relationships between cytometric compositions of bacteria and environmental variables. The statistical analysis using all the data (n = 561 to 571) revealed that the contributions of different subgroups (%VHNA, %HNA, and %LNA) were significantly correlated (P < 0.001) with all the environmental variables except the DO concentration (the range for the absolute correlation coefficient was 0.15 to 0.74), which reflected strong covariations in the vertical dimension among multiple factors.
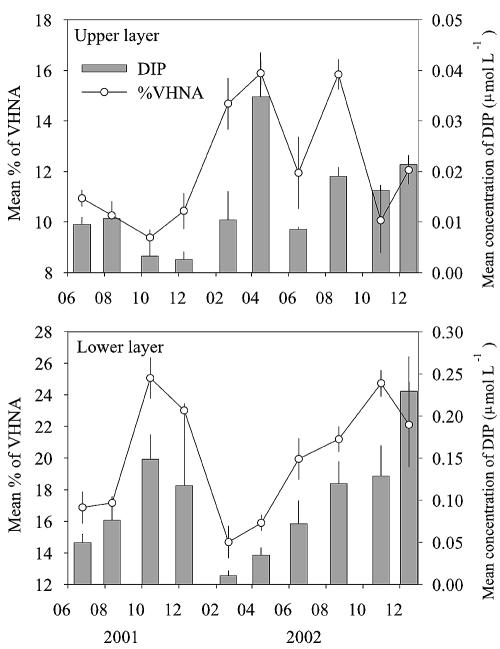
To minimize correlations due to vertical gradients, we divided the stratified water columns into upper and lower layers at the depth of the maximum thermal gradient and performed the correlation analysis for each layer (for the data obtained during the circulation period [February and April], we used all the data obtained throughout the water columns without distinguishing “upper” and “lower” layers). Except for a weak (r = 0.211; P < 0.01) positive correlation between DOC and %VHNA in the upper layer, we found no tendency of the %VHNA and %HNA to increase with increasing concentrations of Chl a, POC, and DOC (indices of carbon availability). Rather, %VHNA and %HNA were correlated negatively with Chl a in both the upper and lower layers (P < 0.001) (Table 3). The results also showed that both %VHNA and %HNA were significantly (P < 0.001) positively correlated with DIP concentration in both the upper and lower layers. In order to examine these relationships more closely, we compared the seasonal patterns of depth-weighted averages of %VHNA and %HNA with those of Chl a and DIP concentrations in each layer at stations 4 and 6 (for the data obtained during the circulation period, the values averaged for the whole water column were used for both the upper and lower layers). We found negative correlations between %VHNA and Chl a in the lower layer (r = −0.75; P = 0.01) and between %HNA and Chl a in the upper layer (r = −0.67; P = 0.036). These negative correlations might reflect the responses of VHNA and HNA bacteria to the DOM supply associated with algal mortality (32), although we failed to detect significant correlations (P > 0.05) when the data were limited to the growing season (June to December). %VHNA and DIP concentration were significantly positively correlated in both layers (r = 0.79 and P = 0.006 and r = 0.68 and P = 0.03 for the upper and lower layers, respectively) (Fig. 4). For the data combined for both the upper and lower layers, %VHNA was highly significantly correlated with DIP concentration (r=0.87; P < 0.001; n = 18), indicating that a substantial portion (71%) of the seasonal variation in %VHNA was accounted for by the DIP concentration (Fig. 5).
TABLE 3.
Pearson's correlation coefficients indicating significant (P < 0.01) correlations between the relative abundance of bacterial groups (%VHNA, %HNA, and %LNA) and environmental variables in the upper layer (n = 169 to 171) and lower layer (n = 247 to 254)c
| Variable | Temp | DO | Chl a | PON | POP | DOC | DOP | DIN | DIP |
|---|---|---|---|---|---|---|---|---|---|
| Upper layer | |||||||||
| %VHNA | −0.153a | 0.211b | 0.456a | ||||||
| %HNA | −0.277a | −0.459a | −0.388a | 0.255a | 0.263a | ||||
| %LNA | 0.245b | 0.306a | 0.304a | −0.291a | −0.295a | ||||
| Lower layer | |||||||||
| %VHNA | −0.679a | −0.326a | −0.595a | −0.304a | −0.292a | −0.294a | 0.487a | 0.505a | |
| %HNA | −0.245a | −0.243a | −0.332a | −0.307a | 0.251a | 0.385a | |||
| %LNA | 0.615a | 0.36a | 0.615a | 0.316a | 0.262a | 0.254a | −0.498a | −0.586a |
P < 0.001.
P < 0.01.
Abbreviations: PON, particulate organic nitrogen; POP, particulate organic phosphorus; DOP, dissolved organic phosphorus.
FIG. 4.
Seasonal changes in depth-integrated averages of %VHNA and DIP concentration in the upper and lower layers at stations 4 and 6. The average values of the data collected at the two stations are shown, and the error bars indicate the ranges.
FIG. 5.
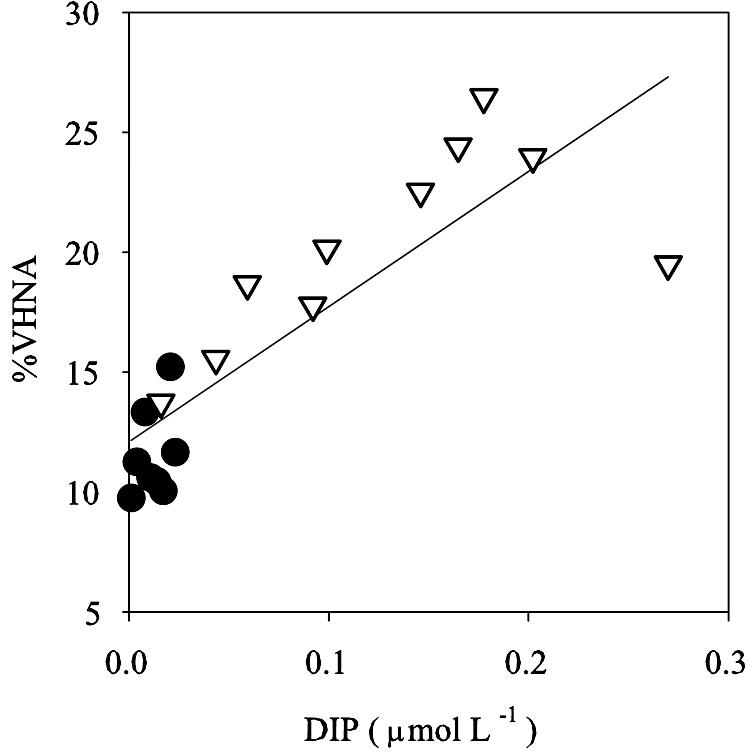
Relationship between depth-integrated averages of %VHNA and DIP concentration in the upper layer (circles) and lower layer (triangles). All the data were used to draw the regression line: %VHNA = 12.1 + 56.4 × DIP (r2 = 0.71; P < 0.01; n = 18).
Growth rates and responses to nutrient enrichment of bacterial subgroups.
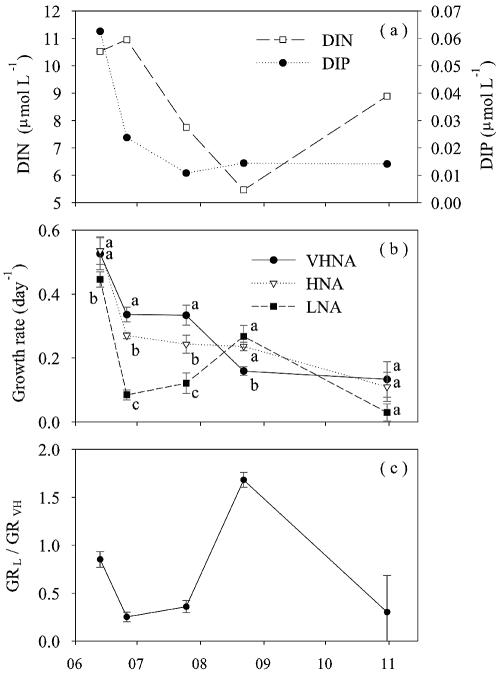
Incubation experiments were conducted during the period when there was increasing thermal stability (June to August) associated with depletion of nutrients in the surface water, followed by the period when there was partial mixing (October), which appeared to result in relaxation of the nutrient limitations, as indicated by the increase in the concentration of nitrate (Fig. 6a). The ranges of the growth rates of the VHNA bacteria (GRVH), the HNA bacteria (GRH), and the LNA bacteria (GRL) were 0.08 to 0.58, 0.08 to 0.59, and 0.01 to 0.47 day−1, respectively (Fig. 6b). Seasonal variations of GRVH and GRH were characterized by the highest values (0.53 and 0.54 day−1 for GRVH and GRH) in June, with a decreasing trend over the season to the minimum values (0.13 and 0.11 day−1 for GRVH and GRH) in October. The seasonal pattern of GRL differed greatly from that of GRVH and GRH, exhibiting the second highest value in August (0.26 day−1), when GRL exceeded GRVH by 1.7-fold (Fig. 6c). In other experiments, GRL was significantly (P < 0.05) lower than GRVH (Fig. 6b and c).
FIG. 6.
Seasonal changes in the concentrations of DIN and DIP (a), the growth rates of bacterial cytometric groups (b), and the ratio of GRL to GRVH (c) in surface water collected in the pelagic area of the north basin of Lake Biwa. The error bars indicate the standard errors (n = 3). Different letters indicate that growth rates differed significantly (P < 0.05; ANOVA with Bonferroni’s correction) among the treatments.
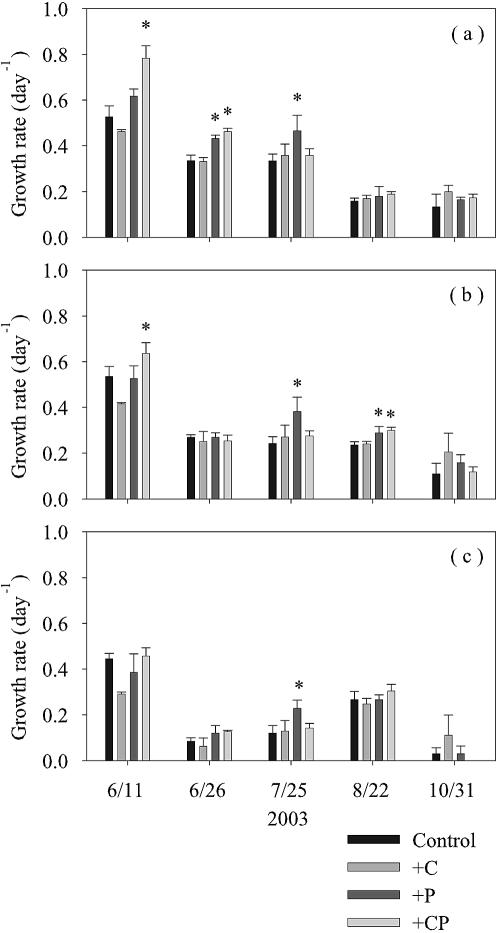
The effects of addition of nutrients (glucose and phosphate) on different subgroups of bacteria were examined by comparing the growth rates of nonsupplemented controls and cultures supplemented with glucose alone, phosphate alone, or glucose plus phosphate (using ANOVA with Bonferroni's correction). Our results do not support the hypothesis that the surface bacterial communities were C limited; addition of glucose alone did not result in a significant increase in the growth rate of any subgroup (Fig. 7) regardless of the level of Chl a (an index of the organic substrate supply) in water samples (2.2 to 5.0 μg liter−1). In contrast, significant increases (P < 0.05) in the growth rates in response to addition of phosphate alone suggested that there was P limitation of VHNA bacteria (26 June and 25 July), HNA bacteria (25 July and 22 August), and LNA bacteria (25 July). Colimitation by P and C was suggested by the results of the 11 June experiment, in which GRVH and GRH increased in response to addition of phosphate plus glucose (but not in response to addition of phosphate alone or glucose alone). It is notable that the responses of the LNA bacteria to nutrient additions were much less pronounced than those of the VHNA and HNA bacteria; in four of five cases, nutrient additions enhanced either GRVH or GRH or both, whereas a significant response of GRL was detected on only one occasion (25 July).
FIG. 7.
Growth rates of VHNA bacteria (a), HNA bacteria (b), and LNA bacteria (c) in the 0.8-μm filtrate enriched with either glucose (+C), phosphate (+P), or glucose and phosphate (+CP). The growth rates in enriched samples were compared with those in nonsupplemented controls (Control). An asterisk indicates that the rate in the enriched sample was significantly greater than that in the control (P < 0.01, as determined by ANOVA with Bonferroni's correction). The error bars indicate standard errors (n = 3).
Grazing mortality of bacterial subgroups.
In the July experiment, the grazing mortality rates of VHNA bacteria (0.54 ± 0.03 day−1) and HNA bacteria (0.47 ± 0.04 day−1) did not differ significantly (P > 0.05), but these rates were significantly higher than that of LNA bacteria (0.19 ± 0.06 day−1) (P < 0.05, as determined by ANOVA with Bonferroni's correction). In contrast, in the August experiment, there was no significant difference (P > 0.05) in grazing mortality rates between different groups (0.37 ± 0.01, 0.36 ± 0.01, and 0.36 ± 0.02 day−1 for VHNA, HNA, and LNA bacteria, respectively), indicating that grazers did not distinguish bacterial groups. In the October experiment, the grazing mortality rates were low (0.09± 0.01, 0.10 ± 0.01, and 0.06 ± 0.01 day−1, for the VHNA, HNA and LNA bacteria, respectively) and did not differ significantly for the different groups (P > 0.05).
DISCUSSION
Our measurements were obtained over a full seasonal cycle to cover a wide range of environmental conditions in the north basin of Lake Biwa. They provided, for the first time, an extensive data set that allowed us to explore plausible environmental factors that affect the relative abundance of cytometric subgroups of freshwater bacterial communities. We distinguished three subgroups of bacteria that exhibited distinctive vertical distribution patterns. During the thermal stratification period, LNA bacteria dominated in the epilimnion, whereas the VHNA and HNA subgroups prevailed in hypolimnetic communities. During the circulation period, the HNA bacteria were the most abundant group throughout the water column. A notable feature of the variations in the cytometric composition during the thermal stratification period was the increase in %VHNA and the decrease in %LNA with depth.
The vertical dimension in stratified water bodies represents dramatically steep gradients in environmental conditions for heterotrophic bacterial communities. In the euphotic zone, a substantial fraction (ca. 50%) of photosynthetically fixed organic carbon is released as DOM by several mechanisms and fuels bacterial production in the zone (32), while the depletion of inorganic nutrients, depending on the environment, may result in severe competition for the use of these nutrients between phytoplankton and bacteria, as well as among bacterial subgroups (39, 40). In deeper aphotic layers, bacterial production is largely constrained by the limited supply of DOM, whereas the amounts of inorganic nutrients available for bacterial use are typically high. Few studies have examined vertical variation of bacterial community composition determined by flow cytometry with concomitant measurement of environmental variables. The available data have shown that the vertical distribution of %HNA is either variable (19, 45) or can be characterized by an increasing trend with depth (20); the latter possibility is consistent with our results obtained for Lake Biwa. At first glance, it is odd that the %HNA (or %VHNA) in euphotic layers (more productive layers) does not generally exceed that in aphotic layers (less productive layers) given that %HNA (or %VHNA) has been considered an index of bacterial activity that increases with increasing supply of organic matter (19, 26). In the discussion below, we use our empirical and experimental data collected for Lake Biwa to develop hypotheses to explain these and other variations in the relative abundance of bacterial subgroups in aquatic systems.
Covariation of multiple factors in the vertical dimension hampered coherent examination of individual correlations among variables when we used all the data obtained throughout the water columns. However, when correlation analyses were conducted for different depth horizons (i.e., the upper and lower layers), a striking feature emerged: there was a strong tendency toward an increase in the %VHNA with increasing concentrations of DIP. For the depth-averaged seasonal data, we found that 71% of the variation in %VHNA was accounted for by the DIP concentration. Although positive correlations do not necessarily indicate that there is a causal relationship between two variables, the results described above suggest that P limitation may have an effect on %VHNA.
Our results obtained in incubation experiments using surface water provided evidence which supports the proposition described above. We found that the growth rates of the VHNA and HNA bacteria were enhanced by addition of phosphate or phosphate plus glucose but not by addition of glucose alone. The LNA bacteria were much less responsive to addition of phosphate (and phosphate plus glucose) than the other groups. These results suggest that (i) the growth of bacterial communities in surface water of Lake Biwa is primarily limited by P rather than by C and (ii) addition of P selectively enhances the growth of bacterial groups with higher nucleic acid contents (VHNA and HNA bacteria). Consistent with our data, Bouvy et al. (2) reported that in a West African reservoir, P addition enhanced the growth of HNA bacteria but not the growth of LNA bacteria. Importantly, these results suggest that increasing the availability of P selectively enhances the growth of HNA (or VHNA) bacteria in P-limited systems.
Note that our calculation of the growth rate could have included errors due to the movement of bacterial cells from one group to another during incubation. Because bacterial subgroups distinguished by nucleic acid content are not necessarily composed of specific phylogenetic groups (36) and because the nucleic acid content determined by the method that we used (SYBR Green I staining) may reflect not only the content of DNA but also the content of RNA (24), the movement across different subgroups could be associated with changes in the physiological state of bacterial cells. Although it is imperative for future studies to examine the possibilities described above in order to fully understand the control of individual groups, our data are consistent with the hypothesis that P availability can be a factor that controls the composition of bacteria determined by flow cytometry.
Previous studies have suggested that %HNA increases with increasing Chl a concentration on a large scale, presumably because of the increasing supply of organic matter (26), although Chl a alone would not fully explain the variation in %HNA (26). Investigators have found high values for %HNA (70 to 80%) typically in coastal marine systems, where P limitation is probably not severe (e.g., the %HNA was 65% in the coastal Atlantic Ocean off Delaware [26], 58 to 78% in the Grand Banks [25], and 63 to 70% in the coastal Mediterranean Sea [36]). Lower values for %HNA (<40%) have often been reported for environments such as the eastern Mediterranean Sea (average, 35%) (26), the central North Atlantic Ocean (average, 36%) (26), and the Gulf of Mexico (38% ± 12.8% for the sum of group III and group IV of Jochem et al. [21]), where P limitation of plankton communities might have been severe. Our data obtained for Lake Biwa (the sum of %HNA and %VHNA was on average 39.9% in the upper layer during the stratification period) are close to the lower end of the range of %HNA reported by other workers previously. In addition, the similar vertical patterns for %HNA with increasing depth in Lake Biwa and the Gulf of Mexico (20) are consistent with the P-limitation hypothesis since the DIP profiles are characterized by severe depletion in the surface and accumulation in deeper layers not only in Lake Biwa but also in the Gulf of Mexico (11). We have found that growing bodies of evidence suggest that P limitation of bacterial growth is a widespread, rather than exceptional, phenomenon in both freshwater environments (4, 12) and marine environments (34, 44, 47). Several studies have demonstrated that P addition enhances growth (12) and production (4) of bulk bacterial communities. A theory has been developed (40) and stoichiometric examinations have been made (27, 38) which suggest possible consequences of P limitation on resource competition and ecological stoichiometry, yet the role of P availability in shaping bacterial community structure remains largely unexplored. Although differences among researchers in analytical procedures and criteria for discrimination of cytometric groups make it difficult to draw a conclusive picture about cross-system patterns in %HNA distributions, the results described above suggest that it would be fruitful for future studies to examine if P availability affects flow cytometrically distinguished bacterial community compositions in diverse aquatic environments.
Because the abundance of each subgroup of bacteria at steady state is determined as a balance between growth and loss, variations in mortality among different subgroups of bacteria can affect the community composition. Previous studies performed in coastal environments have suggested that bacterivorous flagellates preferentially graze on HNA bacteria rather than LNA bacteria (14), a result which is consistent with the notion that flagellates selectively graze larger cells (17). However, in the Gulf of Mexico, Jochem et al. (21) have obtained variable results; the grazing mortality rates for HNA bacteria were higher than, equal to, or lower than the corresponding rates for LNA bacteria. Our data obtained for Lake Biwa agree with the results of Jochem et al.; we found that the grazing mortality of VHNA bacteria was 2.8-fold higher than that of LNA bacteria in the 25 July experiment, whereas LNA bacteria were grazed at a rate close to that of VHNA bacteria in the 22 August experiment, suggesting that LNA bacteria play a significant role in the microbial food chain.
There are conflicting views on the growth characteristics and ecological role of LNA bacteria. Some previous studies have claimed that LNA bacteria represent less active, dormant, or even dead cells (3, 14, 23, 36). However, other studies have suggested a different view. Based on sorting [35S]methionine-labeled cells, Zubkov et al. (48) suggested that the growth rates (biomass specific uptake rates) of LNA bacteria are equivalent to or even higher than those of HNA bacteria in the Celtic Sea. Jochem et al. (21) found that in the Gulf of Mexico the growth rates of LNA bacteria on occasion exceeded those of bacteria containing more nucleic acid. Our results partially support the latter view. We found that the growth rates of LNA bacteria were equivalent to (21 June) or even exceeded (22 August) those of bacteria with high nucleic acid contents. These data led us to derive the following hypothetical scenario. In late summer, when increasing thermal stability and calm conditions severely limit the supply of P to surface communities, LNA bacteria become competitively superior to VHNA bacteria, possibly because of LNA bacterial traits, including higher nutrient uptake efficiencies and lower requirements for P (7). The growth of VHNA bacteria, on the other hand, is largely constrained by the availability of P during the period of severe P limitation, with ambient P concentrations of <15 nmol liter−1, values that are less than the half-saturation constant of phosphate uptake kinetics displayed by lake bacterial communities (20 to 200 nmol liter−1) (8). An important implication of this scenario is that LNA bacteria, under severe P-limitation conditions, largely represent an “active” subgroup that outcompetes VHNA bacteria and may play an important role in the functioning of the microbial loop.
In conclusion, our extensive data on seasonal and depth-dependent variations in the bacterial composition in pelagic environments of Lake Biwa revealed a novel feature of the relationships between environmental forces and the relative abundance of bacterial subgroups. The most striking pattern is the dominance of LNA bacteria in surface water but not in deep water, accompanied by a strong positive correlation between %VHNA and the DIP concentration. The experimental results provided evidence which supports the hypothesis that P limitation exerts more severe constraints on the growth of bacterial groups with higher nucleic acid contents, which allows LNA bacteria to be competitive and become an important component of the microbial loop in P-limited environments. Future studies should test the general applicability of this hypothetical model of the regulation by P of bacterial composition distinguished by flow cytometry.
Supplementary Material
Acknowledgments
We thank K. Koitabashi, T. Miyano, T. Yokokawa, and K. Yoshiyama for support during the field sampling.
This study was supported by MEXT/JSPS grants 12800016 and 13308029, by the Nippon Life Insurance Foundation, and by the 21st Century COE program of Kyoto University (A14). Financial support was also provided by the Basic Research Program (CREST type-Strategic Sector, R&D of Hydrological Modeling and Water Resources System) of the Japan Science and Technology Agency.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Azam, F. 1998. Microbial control of oceanic carbon flux: the plot thickens. Science 280:694-696. [Google Scholar]
- 2.Bouvy, M., M. Troussellier, P. Got, and R. Arfi. 2004. Bacterioplankton responses to bottom-up and top-down controls in a West African reservoir (Selingue, Mali). Aquat. Microb. Ecol. 34:301-307. [Google Scholar]
- 3.Button, D. K., B. R. Robertson, and F. Juttner. 1996. Microflora of a subalpine lake: bacterial populations, size and DNA distributions, and their dependence on phosphate. FEMS Microbiol. Ecol. 21:87-101. [Google Scholar]
- 4.Carlsson, P., and D. A. Caron. 2001. Seasonal variation of phosphorus limitation of bacterial growth in a small lake. Limnol. Oceanogr. 46:108-120. [Google Scholar]
- 5.Chrzanowski, T. H., and J. P. Grover. 2001. Effects of mineral nutrients on the growth of bacterio- and phytoplankton in two southern reservoirs. Limnol. Oceanogr. 46:1319-1330. [Google Scholar]
- 6.Cole, J. J., S. Findlay, and M. L. Pace. 1988. Bacterial production in fresh and saltwater ecosystems—a cross-system overview. Mar. Ecol. Prog. Ser. 43:1-10. [Google Scholar]
- 7.Cotner, J. B., and B. A. Biddanda. 2002. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105-121. [Google Scholar]
- 8.Cotner, J. B., and R. G. Wetzel. 1992. Uptake of dissolved inorganic and organic phosphorus-compounds by phytoplankton and bacterioplankton. Limnol. Oceanogr. 37:232-243. [Google Scholar]
- 9.Cottrell, M. T., and D. L. Kirchman. 2000. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 66:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottrell, M. T., and D. L. Kirchman. 2004. Single-cell analysis of bacterial growth, cell size, and community structure in the Delaware estuary. Aquat. Microb. Ecol. 34:139-149. [Google Scholar]
- 11.El-Sayed, S. Z., W. M. Sackett, L. M. Jeffrey, A. D. Fredericks, R. P. Saunders, P. S. Conger, G. A. Fryxell, K. A. Steidinger, and S. A. Earle. 1972. Chemistry, primary productivity, and benthic algae of the Gulf of Mexico, p. 1-29. In Serial atlas of the marine environment, vol. 22>. American Geographical Society, New York, N.Y. [Google Scholar]
- 12.Elser, J. J., L. B. Stabler, and R. P. Hassett. 1995. Nutrient limitation of bacterial-growth and rates of bacterivory in lakes and oceans—a comparative study. Aquat. Microb. Ecol. 9:105-110. [Google Scholar]
- 13.Gasol, J. M., and P. A. Del Giorgio. 2000. Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci. Mar. 64:197-224. [Google Scholar]
- 14.Gasol, J. M., U. L. Zweifel, F. Peters, J. A. Fuhrman, and A. Hagstrom. 1999. Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl. Environ. Microbiol. 65:4475-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glockner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haga, H., T. Nagata, and M. Sakamoto. 1995. Size-fractionated NH4+ regeneration in the pelagic environments of two mesotrophic lakes. Limnol. Oceanogr. 40:1091-1099. [Google Scholar]
- 17.Hahn, M. W., and M. G. Hofle. 2001. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35:113-121. [DOI] [PubMed] [Google Scholar]
- 18.Holmes, R. M., A. Aminot, R. Kerouel, B. A. Hooker, and B. J. Peterson. 1999. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56:1801-1808. [Google Scholar]
- 19.Jellett, J. F., W. K. W. Li, P. M. Dickie, A. Boraie, and P. E. Kepkay. 1996. Metabolic activity of bacterioplankton communities assessed by flow cytometry and single carbon substrate utilization. Mar. Ecol. Prog. Ser. 136: 213-225. [Google Scholar]
- 20.Jochem, F. J. 2001. Morphology and DNA content of bacterioplankton in the northern Gulf of Mexico: analysis by epifluorescence microscopy and flow cytometry. Aquat. Microb. Ecol. 25:179-194. [Google Scholar]
- 21.Jochem, F. J., P. J. Lavrentyev, and M. R. First. 2004. Growth and grazing rates of bacteria groups with different apparent DNA content in the Gulf of Mexico. Mar. Biol. 145:1213-1225. [Google Scholar]
- 22.Lebaron, P., P. Servais, H. Agogue, C. Courties, and F. Joux. 2001. Does the high nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems? Appl. Environ. Microbiol. 67:1775-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebaron, P., P. Servais, A. C. Baudoux, M. Bourrain, C. Courties, and N. Parthuisot. 2002. Variations of bacterial-specific activity with cell size and nucleic acid content assessed by flow cytometry. Aquat. Microb. Ecol. 28:131-140. [Google Scholar]
- 24.Lebaron, P., N. Parthuisot, and P. Catala. 1998. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl. Environ. Microbiol. 64:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, W. K. W., and P. M. Dickie. 2001. Monitoring phytoplankton, bacterioplankton, and virioplankton in a coastal inlet (Bedford Basin) by flow cytometry. Cytometry 44:236-246. [DOI] [PubMed] [Google Scholar]
- 26.Li, W. K. W., J. F. Jellett, and P. M. Dickie. 1995. DNA distributions in planktonic bacteria stained with TOTO or TO-PRO. Limnol. Oceanogr. 40:1485-1495. [Google Scholar]
- 27.Makino, W., J. B. Cotner, R. W. Sterner, and J. J. Elser. 2003. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C:N:P stoichiometry. Funct. Ecol. 17:121-130. [Google Scholar]
- 28.Marie, D., F. Partensky, S. Jacquet, and D. Vaulot. 1997. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl. Environ. Microbiol. 63:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata, T. 1987. Production rate of planktonic bacteria in the north basin of Lake Biwa, Japan. Appl. Environ. Microbiol. 53:2872-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata, T. 1988. The microflagellate-picoplankton food linkage in the water column of Lake Biwa. Limnol. Oceanogr. 33:504-517. [Google Scholar]
- 31.Nagata, T. 1990. Contribution of picoplankton to the grazer food chain of Lake Biwa, p. 526-539. In M. M. Tilzer and C. Serruya (ed.), Large lakes—ecological structure and function. Springer Verlag, Berlin, Germany.
- 32.Nagata, T. 2000. Production mechanisms of dissolved organic matter, p. 121-152. In D. L. Kirchman (ed.), Microbial ecology of the oceans. John Wiley & Sons, New York, N.Y.
- 33.Nakanishi, M., Y. Tezuka, T. Narita, O. Mitamura, K. Kawabata, and S. Nakano. 1992. Phytoplankton primary production and its fate in a pelagic area of Lake Biwa. Arch. Hydrobiol. 35:47-67. [Google Scholar]
- 34.Pakulski, J. D., R. Benner, T. Whitledge, R. Amon, B. Eadie, L. Cifuentes, J. Ammerman, and D. Stockwell. 2000. Microbial metabolism and nutrient cycling in the Mississippi and Atchafalaya River plumes. Estuar. Coast. Shelf Sci. 50:173-184. [Google Scholar]
- 35.Pujo-pay, M., and P. Raimbault. 1994. Improvement of the wet-oxidation procedure for simultaneous determination of particulate organic nitrogen and phosphorus collected on filters. Mar. Ecol. Prog. Ser. 105:203-207. [Google Scholar]
- 36.Servais, P., E. O. Casamayor, C. Courties, P. Catala, N. Parthuisot, and P. Lebaron. 2003. Activity and diversity of bacterial cells with high and low nucleic acid content. Aquat. Microb. Ecol. 33:41-51. [Google Scholar]
- 37.Sherr, B., E. Sherr, and P. del Giorgio. 2001. Enumeration of total and highly active bacteria. Methods Microbiol. 30:129-159. [Google Scholar]
- 38.Tezuka, Y. 1990. Bacterial regeneration of ammonium and phosphate as affected by the carbon-nitrogen-phosphorus ratio of organic substrates. Microb. Ecol. 19:227-238. [DOI] [PubMed] [Google Scholar]
- 39.Thingstad, T. F. 2000. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 45:1320-1328. [Google Scholar]
- 40.Thingstad, T. F., and H. Havskum. 1999. Bacteria-protist interactions and organic matter degradation under P-limited conditions: analysis of an enclosure experiment using a simple model. Limnol. Oceanogr. 44:62-79. [Google Scholar]
- 41.Torreton, J. P., V. Talbot, and N. Garcia. 2000. Nutrient stimulation of bacterioplankton growth in Tuamotu atoll lagoons. Aquat. Microb. Ecol. 21:125-137. [Google Scholar]
- 42.Weinbauer, M. G., and F. Rassoulzadegan. 2004. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 43.Wetzel, R. G., and G. E. Likens. 2000. Limnological analysis, 3rd ed. Springer Verlag, Berlin, Germany.
- 44.Wu, J. F., W. Sunda, E. A. Boyle, and D. M. Karl. 2000. Phosphate depletion in the western North Atlantic Ocean. Science 289:759-762. [DOI] [PubMed] [Google Scholar]
- 45.Yanada, M., T. Yokokawa, C. W. Lee, H. Tanaka, I. Kudo, and Y. Maita. 2000. Seasonal variation of two different heterotrophic bacterial assemblages in subarctic coastal seawater. Mar. Ecol. Prog. Ser. 204:289-292. [Google Scholar]
- 46.Yokokawa, T., T. Nagata, M. T. Cottrell, and D. L. Kirchman. 2004. Growth rate of the major phylogenetic bacterial groups in the Delaware estuary. Limnol. Oceanogr. 49:1620-1629. [Google Scholar]
- 47.Zohary, T., and R. D. Robarts. 1998. Experimental study of microbial P limitation in the eastern Mediterranean. Limnol. Oceanogr. 43:387-395. [Google Scholar]
- 48.Zubkov, M. V., B. M. Fuchs, P. H. Burkill, and R. Amann. 2001. Comparison of cellular and biomass specific activities of dominant bacterioplankton groups in stratified waters of the Celtic Sea. Appl. Environ. Microbiol. 67:5210-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zubkov, M. V., B. M. Fuchs, G. A. Tarran, P. H. Burkill, and R. Amann. 2002. Mesoscale distribution of dominant bacterioplankton groups in the northern North Sea in early summer. Aquat. Microb. Ecol. 29:135-144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.