Abstract
The apparently rapid increase in the prevalence of amphibian limb deformities has led to substantial interest from ecologists and public health professionals. Hypotheses proposed to explain the deformities fall into two broad categories: chemical contaminants and trematode infection. Although there are convincing experimental demonstrations that certain factors can lead to some deformities, the causes for recent increases in amphibian malformation remain controversial. Moreover, no experimental studies on amphibian deformities have been conducted in the field, and no studies have attempted to examine the synergistic effects of trematode infection and exposure to chemical contaminants. Here, I present the results of field and laboratory experiments that link increased trematode infection, and increased limb deformities, to pesticide exposure. Field experiments conclusively demonstrated that exposure to trematode infection was required for the development of limb deformities in wood frogs, Rana sylvatica. However, deformities were more common at sites adjacent to agricultural runoff. Laboratory experiments corroborated the association between pesticide exposure and increased infection with pesticide-mediated immunocompetency as the apparent mechanism. Given the conservative contaminant exposure levels used [Environmental Protection Agency (EPA) drinking water standards] and the widespread use of many pesticides, these negative impacts may help to explain pathogen-mediated amphibian declines in many regions.
Amphibian populations from around the world have apparently declined or experienced severe range reductions (1–6). In addition, for some populations, unprecedented mortality has been documented (7–11). Included within these reports are accounts of increases in developmental deformities (12–14). Amphibian deformities, in particular those related to limb development, have now been reported in 43 states in the U.S. and in five Canadian Provinces, as well as in several other countries around the world (13). The widespread nature and apparent increase in the prevalence of deformities have led to substantial interest from scientists and the general public. This concern stems in part from the recent increase of disease in human and wildlife populations linked with patterns of environmental change (15). Hypotheses proposed to explain the deformities fall into two broad camps: chemical contaminants (16, 17) and trematode infection (18–21). Although preliminary laboratory experiments suggest that infection by some species of trematode or exposure to certain contaminants can cause some deformities, there are often inconsistencies between observations of deformities occurring under natural conditions and those that result from laboratory manipulations (13). Moreover, no experimental manipulations have been conducted in the field, and no studies have attempted to examine synergistic effects of trematode infection and exposure to chemical contaminants.
To examine the relationship between trematode-mediated limb deformities and chemical contaminants, I quantified limb deformities in relation to trematode infection at natural breeding sites in the context of variation in exposure to agricultural runoff. I used field and laboratory experimentation to test four predictions regarding trematode-associated limb deformities of wood frogs, Rana sylvatica: (i) limb deformities are associated with trematode infections at natural breeding sites; (ii) trematode-mediated limb deformities at natural breeding sites are a function of proximity to agricultural runoff; (iii) exposure to chemical contaminants increases rates of trematode infection; (iv) contaminant-mediated increases in trematode infection are consistent with a decrease in the ability of developing amphibians to elicit an immune response that would prevent infection.
Materials and Methods
Field Experiment.
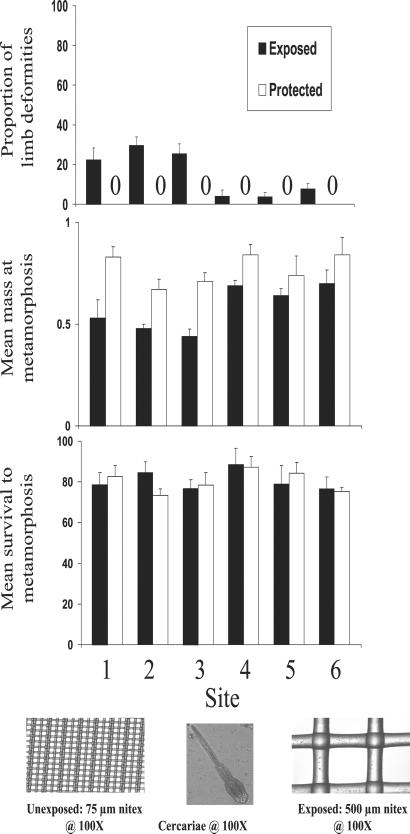
The field experiment used six ponds (all within 10 km of one another) that varied in exposure to agricultural runoff. All six ponds had populations of the snail Planorbella campanulata (snail density in no. of snails/liter ± 1 SE and prevalence of infection with Ribeiroia sp.: Site 1 = 0.163 ± 0.065, 42.4%; Site = 0.114 ± 0.074, 36.7%; Site 3 = 0.231 ± 0.034, 28.6%; Site 4 = 0.234 ± 0.045, 31.7%; Site 5 = 0.211 ± 0.87, 42.1%; Site 6 = 0.173 ± 0.013, 21.4%), which were infected with a trematode, Ribeiroia sp., previously shown to cause limb deformities (20). Three of the sites were in close proximity to agricultural fields, and all three had evidence of exposure to agricultural runoff (insecticides and herbicides; see below). During spring 2000, I placed six field enclosures at each of the six sites. Three enclosures per site allowed trematode cercariae (the larval infective stage of the life cycle) to enter from the pond, whereas the other three prevented entry of the cercariae. Enclosures consisted of wooden frames (0.64 × 0.64 × 0.71 m) with an open bottom. Enclosures had a lower edge of aluminum flashing, and the sides were covered with either 75-μm nitex that prevented the entry of cercariae or 500-μm nitex that allowed entry of cercariae (Fig. 1).
Figure 1.
Summary of the effects of proximity to agricultural runoff (sites 1–3 received inputs of contaminants; sites 4–6 had no detectable levels of contaminants) and exposure to trematode infection (exposed and protected) on limb deformities (proportion exhibiting limb deformities), survival (proportion reaching metamorphosis), and mass (mass at metamorphosis) of wood frogs raised in field enclosures. Each bar represents the mean + 1 SE of responses from three replicate enclosures. (Inset) Schematic of nitex screens used in field enclosure arrays.
Wood frogs were collected on April 28, 2000, as embryos from three ponds located in Centre County, PA (two clutches from each pond) and were allowed to hatch in outdoor pools (1.2-m diameter; 120-liter volume). After hatching, larvae were fed ad libitum a diet of pressed alfalfa pellets. Larvae from different ponds were mixed together and then randomly assigned to experimental enclosures. All focal animals used in the experiment were Gosner stage 25 (22), and all animals were size matched before being added to enclosures (x ± SD mass in grams = 0.112 ± 0.012, n = 30).
On May 16, 2000, 10 hatchling wood frog tadpoles were added to each of the 36 enclosures. Once the first metamorphic frog was observed (after 29 days), I monitored the enclosures daily and removed individuals from the enclosures as they metamorphosed (front-limb emergence; ref. 22). Metamorphs were brought back to the lab, weighed to the nearest milligram, and preserved for later determination of limb deformities and assessment of the number of metacercarial cysts.
Measurement of Pesticides at Field Sites.
Pond water samples were tested for the presence of insecticides and herbicides listed under EPA standard method 8081 (Organochlorine Pesticides, 28 compounds) and 8141a (Organophosphorus Pesticides, 51 compounds). In three ponds (ponds 4–6), tests failed to detect any biocides; however, in three ponds (ponds 1–3), detectable levels of both organochlorine pesticides and organophosphorus compounds (e.g., Atrazine and Malathion) were detected. Analyses were performed by Centre Analytical Laboratories Incorporated, State College, PA.
Infection Assay.
I assessed the level of infection in tadpoles by clearing and staining metamorphs and searching for metacercariae. When animals were removed from the ponds, they were fixed in 10% buffered formalin for 24 hr, rinsed in distilled water, and postfixed in 70% EtOH. Specimens were then stained with Alcian blue and cleared with methyl salicylate (23). Subsequently, each animal was examined under a dissecting microscope and the number and identity, where possible to species, of metacercarial cysts were counted as an index of parasite load (number of cysts per individual). Most digenetic trematodes infect three successive host species (21, 23). Eggs of the trematode exit through the feces of the definitive host and either are consumed by the first intermediate host (typically a snail) or hatch into free-living miracidial larvae that penetrate and infect snails. During the time the snail is infected, the trematode typically undergoes an embryonic amplification that results in the production of thousands of cercariae. Cercariae emerge from the snail and seek, penetrate, and form a metacercarial cyst in the second intermediate tadpole hosts. The parasite's life cycle is completed when the infected secondary intermediate host is ingested by the definitive host, typically a vertebrate.
Limb-Deformity Assay.
I scored metamorphic wood frogs for both the number and type of abnormalities. Each metamorph was scored independently, so that an individual frog could have more than one abnormality, but abnormalities that were part of other abnormalities were not considered. For example, a frog exhibiting polymelia (multiple limb segments) and polydactyly (extra digit) was counted only as an extra limb. Abnormality categories were adapted from published descriptions (16, 21).
Laboratory Experiments.
To further examine the interaction of exposure to contaminants and increased infection, I conducted a series of laboratory experiments to evaluate potential links between contaminant exposure, immunocompetency, and trematode infection. In these experiments, I examined the impact of low concentrations of three commonly used pesticides (two insecticides, one herbicide) on susceptibility to infection and a measure of immune response of larval wood frogs. I tested the effects of the triazine herbicide Atrazine, the organophosphate insecticide Malathion, and the synthetic pyrethroid insecticide Esfenvalerate. Atrazine is one of the most widely used herbicides in the U.S. (24). The uses of Atrazine include control of broad leaf and grass weed species in numerous crop species. Malathion is commonly used to control mosquitoes and insect pests of crop plants (24). Esfenvalerate is used to control a variety of insect pests of crops. Synthetic pyrethroids have increased in popularity in recent years, because they have retained high insecticidal activity and low avian and mammalian toxicity (24).
Tadpoles used in the experiments were from the same clutches used for the field experiment. Individual tadpoles were raised in polyethylene tubs (30 × 15 × 10 cm) containing 3.5 liters of dechlorinated tap water. The tubs were placed on laboratory shelves arranged in three spatial blocks under a 16:8 light/dark cycle. Tubs were randomly assigned to one of four treatments (each replicated 12 times). The treatments consisted of a negative control (water addition), a solvent control, and two levels of contaminant addition for each contaminant tested. Exposure levels were chosen to reflect conservative conditions that animals may experience under field situations. Thus, the lower concentration of each contaminant tested was based on EPA maximum contaminant levels for drinking water (24). Wood frog tadpoles were exposed to Atrazine at 3 and 30 μg/liter; to Malathion at 200 and 2,000 μg/liter; and to Esfenvalerate at 180 and 1,800 μg/liter. Because it was necessary to use a solvent (acetone or DMSO) to get the contaminants into solution, a solvent control was incorporated in the experimental design, and animals were also exposed to the concentration used to dissolve the contaminant without the pesticide. During the experiment, tadpoles were fed ad libitum a diet of ground rabbit chow. I changed container water every other day, and the chemical treatments were reapplied after water changes.
After 4 weeks, all tadpoles were moved into 75-ml containers and exposed to either 50 Ribeiroia sp. cercariae or 50 Telorchis sp. cercariae (20, 23). Tadpoles were moved into a small volume of water to eliminate the influence of tadpole behavior on exposure rates. Thus, any differences in the number of cercariae successfully encysted would be a function of internal characteristics (e.g., immune response) of the host tadpole. Cercariae were harvested from snails that were collected from the wild (23). Trematode cercariae of Ribeiroia sp. were harvested from snail P. campanulata, and cercariae of Telorchis sp. were harvested from snail Pseudosuccinea columella. Snails were suspended in 5 ml of water placed in individual 20-ml centrifuge tubes and exposed to intense fluorescent light on 14-hr light/10-hr dark cycle for 48 hours. The water from each tube was removed and examined under a dissecting scope for the presence of cercariae. Cercariae were collected with a pipette and immediately transferred to tadpole test containers.
Tadpoles were exposed to cercariae for 4 hours and then returned to their original containers under control conditions. No cercariae were found in the 75-ml containers after the exposure period, indicating that all cercariae had penetrated the tadpoles. One week after exposure, tadpoles were killed, and animals were preserved for later enumeration of metacercarial cysts. One week is a realistic time frame for cyst formation and is when developing limb buds might be sensitive to damage from cysts.
Immediately before the tadpoles were killed, a blood sample was collected, and the leukocytes within a count of 5,000 RBCs were counted and identified. Blood samples from individual tadpoles were placed on standard microscope slides and spread with a coverslip. Blood smears were stained with a standard Wright's stain, and white blood cells were identified by using a compound light microscope at ×100.
Statistical Analysis.
Proportion of limb deformities, mass at metamorphosis, and survival to metamorphosis of animals used in the field experiment were analyzed by using multivariate ANOVA (MANOVA). Before analysis, the percent of individuals surviving to metamorphosis and the percent of individuals exhibiting limb deformities were angular transformed. Both site and exposure to trematodes were treated as categorical fixed factors. It was not possible to perform a MANOVA including the interaction between factors, because a large number of zero abnormality values rendered the matrix singular. Therefore, I performed a MANOVA by using a reduced model including site and trematode exposure main effects. I also examined the main effects of site and trematode exposure as well as their interaction by using an ANOVA restricted to each response variable separately.
The proportion of cercariae successfully encysting and the number of eosinophils/5,000 RBCs were analyzed by using MANOVA. The three pesticides (Atrazine, Malathion, and Esfenvalerate) and trematode species (Telorchis and Riberiroia) were treated as levels of each factor (pesticide exposure and trematode infection). Before analysis, the percent cercariae successfully encysting was angular transformed.
Results
The results suggest that the occurrence of limb deformities at natural oviposition sites is directly related to trematode infection of developing amphibians (Table 1; Fig. 1). However, my analyses revealed strong main effects of exposure to trematode infection and exposure to agricultural runoff (Table 1, MANOVA: trematode exposure, F3,22 = 86.278, P = <0.001; agricultural runoff F15,61 = 6.784, P = <0.001). In addition, there was a strong interaction between these factors for two (limb abnormalities and mass) of the three responses variables. Wood frogs in ponds adjacent to agricultural fields developed a significant number of limb deformities (Fig. 1). Wood frogs from agricultural runoff ponds exposed to cercariae were 37% smaller than their counterparts in the same ponds not exposed to cercariae (ANOVA: mass F5,24 = 3.47, P = 0.031). Moreover, in the agricultural runoff ponds, 28.6% of the wood frogs exposed to trematode infection developed limb deformities compared with 0% among tadpoles shielded from trematode infection (ANOVA: prevalence of abnormalities, F5,24 = 20.43, P = <0.001).
Table 1.
Types and relative frequencies of hind-limb deformities from field experiments conducted with wood frogs (Rana sylvatica)
| Abnormality | Site
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| (5/23) | (8/25) | (6/23) | (1/26) | (1/24) | (2/23) | |
| Ectrodactyly (missing digit) | 2.3 | 2.3 | 12.8 | 0 | 0 | 25 |
| Polydactyly (extra digit) | 5.5 | 0 | 0 | 0 | 0 | 0 |
| Apody (missing foot) | 12.4 | 9.3 | 0 | 0 | 0 | 0 |
| Polypody (extra foot) | 0 | 0 | 0 | 0 | 0 | 25 |
| Ectromely (missing limb) | 24.6 | 28.3 | 37.8 | 0 | 50 | 0 |
| Polymely (extra limb) | ||||||
| Symmetrical | 16.7 | 9.7 | 11.9 | 0 | 0 | 25 |
| Asymmetrical | 38.5 | 42.7 | 37.5 | 100 | 50 | 25 |
| Micromely (short limb) | 0 | 7.7 | 0 | 0 | 0 | 0 |
The value shown for each type of abnormality is the percentage of the total number of abnormalities observed for all animals that survived to metamorphosis. Number in parentheses indicates the number of animals exhibiting limb deformities/the total number of animals reaching metamorphosis. The total number of abnormalities may not equal the number of abnormal animals, as several animals exhibited more than one type of abnormality. Measurements are reported only from animals developing in enclosures that exposed them to trematode infection, because these are the only animals that exhibited deformities. Sites 1–3 are agricultural runoff sites, sites 4–6 are sites with no detectable contaminants.
In contrast to patterns seen in ponds exposed to agricultural runoff, I found the impact of trematode exposure was reduced in ponds not receiving agricultural runoff. Wood frogs from enclosures exposed to cercariae were 22% smaller than their counterparts in the same ponds not exposed to cercariae, but only 4% of them developed limb deformities. All abnormalities observed in frogs exposed to cercariae involved hindlimbs, and metacercariae were consistently found in proximity to the area containing the deformity. The majority of these deformities involved polymelia (multiple limb segments) and, to a lesser extent, amelia (no limb) and polydactyly (extra digit) (Table 1).
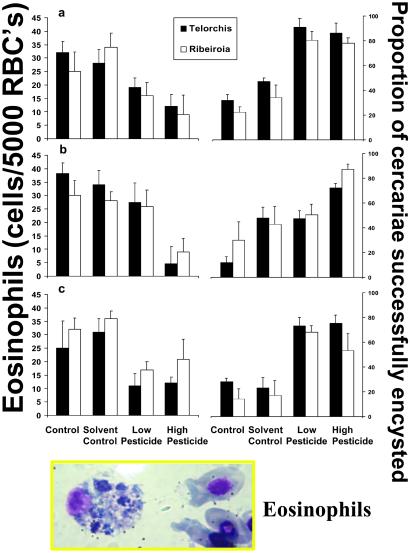
Exposure to low concentrations of contaminants had dramatic effects on the immune response and rates of cercarial encystment. For each of the three contaminants, exposure increased the proportion of both Ribeiroia sp. and Telorchis sp. that successfully encysted (Fig. 2). Likewise, exposure also altered wood frogs' immune response, as measured by circulating leukocytes. Here I will focus on the responses of eosinophils, for simplicity and because of the role they play in macroparasite infections (25). As expected, exposure to pesticides resulted in a decrease in the number of eosinophils found circulating in the blood (Fig. 2). At all but the lowest exposure to Malathion, all three pesticides had a similar effect on eosinophil numbers and proportion of cercariae that encysted (MANOVA, pesticide exposure, F4,190 = 3.697, P = 0.006; Pesticide x treatment, F12,190 = 1.153, P = 0.320). Similarly pesticide exposure had a comparable effect on the animals' responses to the two different parasites (MANOVA, pesticide x trematode interaction, F4,190 = 0.159, P = 0.711).
Figure 2.
Summary of the effects of pesticide exposure (a) Atrazine; (b) Malathion; and (c) Esfenvalerate, and exposure to trematode infection (Telorchis and Riberiroia) on the number of eosinophils/5,000 RBCs (Left) and the proportion of cercariae (out of exposure to either 50 Telorchis or Riberiroia cercariae) that successfully encysted (Right). Each bar represents the mean + 1 SE of responses from six replicate animals. Individual animals were used for both measures of eosinophil counts and to assess number of metacercariae. (Inset) Typical wood frog eosinophil with RBCs for reference.
Discussion
My findings support the hypothesis that parasite infection explains the development of limb deformities observed in frog populations in nature (19, 20, 26). By preventing access of cercariae to the developing amphibians, I was able to prevent developmental abnormalities. These results provide definitive evidence that links deformities under natural conditions to trematode infection. The occurrence of trematode-mediated limb deformities, however, depended on the context of the interaction. Stress in the form of pesticide exposure decreased the host tadpoles' ability to resist infection, resulting in higher parasite loads and higher risk of limb deformities. Laboratory experiments revealed association between pesticide exposure and increased infection. The decreased immunocompetency associated with pesticide exposure could explain the observed differences in limb deformities at field sites given the link between increased cyst formation and increased limb deformities observed in other studies (21). However, although chemical contaminants can contribute to the occurrence of deformities, I found that it was necessary for developing amphibians to be exposed to trematode infection for limb deformities to occur. Parasite removal experiments conducted at natural breeding sites, like those conducted in this study, should be used as a first step to assess the etiology of limb deformities. Development of limb deformities in treatments exposed only to trematodes would guide researchers to focus on the role of trematode infection and factors that modify this host–pathogen interaction. However, occurrences of limb deformities in all treatments would then warrant more sophisticated chemical analyses and studies designed to determine which contaminants are responsible for deformities.
The types of deformities I observed encompass those seen in other studies reporting increases in abnormalities from around North America (12–14, 16–21). The strong context dependence observed in this study may help explain the disparities among the observations of deformities under natural conditions and those experimentally induced under laboratory conditions that have been reported in numerous studies (13). Moreover, it was clear that tadpoles possess the ability to prevent the formation of, or to shed, metacercarial cysts. Given evidence for the role of metacercariae in limb deformities, it will be important to determine whether metacercariae can be shed after they induce limb deformities. Such a pattern could explain the occurrence of deformities in wild collected frogs that are not associated with metacercariae (12–14).
The occurrence of amphibian limb deformities is not a new phenomenon; in fact, amphibian limb deformities can be found in reports dating back to the early 1700s, suggesting that the processes that are responsible for deformities have been present for centuries (27). Increases in the observation of limb deformities could be the result of increased attention paid to monitoring amphibian populations in recent years. Alternatively, increases in developmental abnormalities could be due to either alterations in the prevalence of the parasite or changes in the host ability to resist infection (28). There is a broad perception among epidemiologists, parasitologists, and health professionals that the incidence of several trematode diseases is greatly affected by human-mediated changes in freshwater environments (29, 30). As humans develop the landscape, bodies of freshwater are often modified. There are several studies reporting correlations between human modifications of freshwaters and associated changes in the incidence of human disease (31, 32). In a number of these cases, scientists have been able to link disease outbreaks with increases in snail abundance. Thus, human development may inadvertently alter the environment in ways that increase the prevalence of vector-borne diseases, including snail-borne trematodes. For example, the use of pesticides often associated with agricultural activity may stress hosts, thereby making them more susceptible to infection. Pesticide levels used in my laboratory experiments reflected EPA maximum contaminant levels for drinking water and thus likely reflect realistic exposure levels (33). Moreover, exposure to Atrazine, the herbicide used in this study, is responsible for induced hermaphroditism and demasculinized larynges of laboratory populations of male African clawed frogs, Xenopus laevis, at levels well below the EPA drinking water standard (34). Many pesticides are commonly found in amphibian breeding habitats at these levels and thus may present a real threat to amphibian populations (33, 34). Moreover, accelerated eutrophication associated with agricultural practices has been shown to increase snail abundance and incidence of parasitic infection. However, at this point little is known about how habitat modification can alter the prevalence of Ribeiroia or how these changes may influence developing amphibians to defend against infection (26).
These results highlight the importance of environmental stress on the pattern of disease outbreaks in amphibian populations. The recurring theme of epidemic disease associated with many amphibian declines (3, 7, 9, 10) and with declines of a wide range of other organisms as well (35) may be explained by the impacts of environmental stress on lethal diseases (15). Clearly, the interaction of human-induced habitat modifications and disease dynamics in aquatic systems deserves further study.
Acknowledgments
Thanks to Cheri Kiesecker, Mike Rubbo, Ryan Peterson, Gia Vigiano, Sara Storrs, Julian Avery, S. Doo, and Norville Rogers for providing technical assistance; and Velma Dinkley, Daphne Blake, and Fred Jones for helpful discussions regarding experimental design. Mike Rubbo, Lisa Belden, KeAloha Friedenburg, and Dave Skelly provided helpful suggestions on earlier versions of this manuscript. Funding was provided by National Institutes of Health/National Science Foundation Ecology of Infectious Diseases Grant (1R01ES11067–01).
Abbreviations
- EPA
Environmental Protection Agency
- MANOVA
multivariate ANOVA
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wake D B. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein A R, Wake D B, Sousa W P. Cons Biol. 1994;8:60–71. [Google Scholar]
- 3.Wake D B. Trends Ecol Evol. 1998;13:379–380. doi: 10.1016/s0169-5347(98)01428-1. [DOI] [PubMed] [Google Scholar]
- 4.Alford R A, Richards S J. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- 5.Houlahan J E, Findlay C S, Schmidt B R, Meyer A H, Kuzmin S L. Nature (London) 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- 6.Alford R A, Dixon P M, Pechmann J H K. Nature (London) 2001;412:499–500. doi: 10.1038/35087658. [DOI] [PubMed] [Google Scholar]
- 7.Kiesecker J M, Blaustein A R, Belden L K. Nature (London) 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 8.Pounds J A, Fogden M P L, Campbell J H. Nature (London) 1999;398:611–615. [Google Scholar]
- 9.Berger L C, Speare R, Daszak P, Green D E, Cunningham A A, Goggin C L, Slocombe R, Ragan M A, Hyatt A D, McDonald K R, et al. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiesecker J M, Blaustein A R. Proc Natl Acad Sci USA. 1995;92:11049–11052. doi: 10.1073/pnas.92.24.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaustein A R, Hoffman P D, Hokit D G, Kiesecker J M, Walls S C, Hays J B. Proc Natl Acad Sci USA. 1994;91:11049–11052. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhart J G, Ankley G, Bell H, Carpenter H, Fort D, Gardiner D, Gardner H, Hale R, Helgen J C, Jepson P, et al. Environ Health Perspect. 2000;108:83–90. doi: 10.1289/ehp.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocum D L. Teratology. 2000;62:147–150. doi: 10.1002/1096-9926(200009)62:3<147::AID-TERA2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C W. Environ Sci Technol. 1997;31:A324–A326. doi: 10.1021/es9723693. [DOI] [PubMed] [Google Scholar]
- 15.Epstein P R. Science. 1999;285:347–348. doi: 10.1126/science.285.5426.347. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner D M, Hoppe D M. J Exp Zool. 1999;284:207–216. doi: 10.1002/(sici)1097-010x(19990701)284:2<207::aid-jez10>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Ouellet M, Bonin J, Rodrigue J, DesGranges J L, Lair S. J Wild Dis. 1997;33:95–104. doi: 10.7589/0090-3558-33.1.95. [DOI] [PubMed] [Google Scholar]
- 18.Sessions S K, Ruth S B. J Exp Zool. 1990;254:38–47. doi: 10.1002/jez.1402540107. [DOI] [PubMed] [Google Scholar]
- 19.Sessions S K, Franssen R A, Horner V L. Science. 1999;284:800–802. doi: 10.1126/science.284.5415.800. [DOI] [PubMed] [Google Scholar]
- 20.Johnson P T J, Lunde K B, Ritchie E G, Launer A E. Science. 1999;284:802–804. doi: 10.1126/science.284.5415.802. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P T J, Lunde K B, Ritchie E G, Launer A E. Herpetologica. 2001;57:336–352. [Google Scholar]
- 22.Gosner K L. Herpetologica. 1960;16:183–190. [Google Scholar]
- 23.Kiesecker J M, Skelly D K. Ecology. 2001;82:1956–1963. [Google Scholar]
- 24.Aspelin A L. Pesticide Industry Sales and Usage. Washington, DC: Environmental Protection Agency; 1997. [Google Scholar]
- 25.Edwards S W. Biochemistry and Physiology of the Neutrophil. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]
- 26.Johnson P T J, Lunde K B, Thurman E M, Ritchie E G, Wray S N, Sutherland D R, Kapfer J M, Frest T J, Bowerman J, Blaustein A R. Ecol Monog. 2002;72:151–168. [Google Scholar]
- 27.Ouellet M. In: Ecotoxicology of Amphibians and Reptiles. Sparling D W, Linder G, Bishop C A, editors. Pensacola, FL: SETAC; 2000. pp. 617–661. [Google Scholar]
- 28.Anderson R M, May R M. Infectious Diseases of Humans: Dynamics and Control. Oxford, U.K.: Oxford Univ. Press; 1991. [Google Scholar]
- 29.Lardans V, Dissous C. Parasitol Today. 1998;14:413–417. doi: 10.1016/s0169-4758(98)01320-9. [DOI] [PubMed] [Google Scholar]
- 30.Alemayehu T, Ye-ebiyo Y, Ghebreyesus T A, Witten K H, Bosman A, Teklehaimanot A. Parassitologia. 1998;40:259–267. [PubMed] [Google Scholar]
- 31.Molyneux D H. Int J Parasitol. 1998;28:927–993. doi: 10.1016/s0020-7519(98)00067-8. [DOI] [PubMed] [Google Scholar]
- 32.Spielman A. Prevent Med. 1994;23:693–699. doi: 10.1006/pmed.1994.1116. [DOI] [PubMed] [Google Scholar]
- 33.Sparling D W, Linder G, Bishop C A, editors. Ecotoxicology of Amphibians and Reptiles. Pensacola, FL: SETAC; 2000. [Google Scholar]
- 34.Hayes T B, Collins A, Lee M, Mendoza M, Noriega N, Stuart A A, Vonk A. Proc Natl Acad Sci USA. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daszak P, Cunningham A A, Hyatt A D. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]