Abstract
In the dairy industry, the characterization of Streptococcus thermophilus phage types is very important for the selection and use of efficient starter cultures. The aim of this study was to develop a characterization system useful in phage control programs in dairy plants. A comparative study of phages of different origins was initially performed based on their morphology, DNA restriction profiles, DNA homology, structural proteins, packaging mechanisms, and lifestyles and on the presence of a highly conserved DNA fragment of the replication module. However, these traditional criteria were of limited industrial value, mainly because there appeared to be no correlation between these variables and host ranges. We therefore developed a PCR method to amplify VR2, a variable region of the antireceptor gene, which allowed rapid detection of S. thermophilus phages and classification of these phages. This method has a significant advantage over other grouping criteria since our results suggest that there is a correlation between typing profiles and host ranges. This association could be valuable for the dairy industry by allowing a rational starter rotation system to be established and by helping in the selection of more suitable starter culture resistance mechanisms. The method described here is also a useful tool for phage detection, since specific PCR amplification was possible when phage-contaminated milk was used as a template (detection limit, 105 PFU ml−1).
In the dairy environment, lytic phages are the leading cause of fermentation failure during the manufacture of cheese and fermented milk products. Consequently, the success of commercial lactic starter cultures depends primarily on the selection of non-phage-related strains able to withstand viral infections. Streptococcus thermophilus, a gram-positive thermophilic lactic acid bacterium, is a component of dairy starter cultures used for the manufacture of yogurt and other fermented milk products. As observed for other dairy lactic acid bacteria, this streptococcus is often susceptible to phage attack, which can result in slow lactic acid fermentation and the loss of product quality. Besides its use as a starter in the yogurt industry, this species is very important in the production of a large variety of cheeses (26), from which many S. thermophilus phages responsible for fermentation failure have recently been isolated (32). Since a large dairy plant can processes more than 5 × 105 liters of milk per day, phage problems can be very costly. The economic implications have led to intensive research into methods to control bacteriophage infections in the industry. The traditional strategies used to minimize the consequences of phage infection include rotation of non-phage-related strains (the principal precautionary measure employed), the use of phage-inhibiting media for culture propagation, and aseptic processing conditions (14). However, the effectiveness is sometimes limited by the dynamic evolutionary properties of phages (7, 22) and, in the case of S. thermophilus, by the scarcity of data regarding phage-host relationships (2, 19). The rotation of strains is therefore performed with no knowledge of the host ranges of troublesome phages. In fact, all classifications of S. thermophilus phages indicate either limited or no correlation with host range (1, 4, 17, 25, 29), and this restricts the effectiveness of any precautionary measures employed. Phages of different origins were examined in terms of their morphology, restriction patterns, packaging mechanisms, and lifestyles, the presence of a highly conserved DNA fragment of the replication module (5), and structural proteins. Additionally, typing profiles, which are potentially meaningful for phage control programs in dairy plants, were established on the basis of genetic data. Duplessis and Moineau (11) characterized the antireceptor gene (orf18) of the tail morphogenesis module in the cos-containing phages φDT1 and φMD4 and found that a variable region designated VR2 is responsible for host specificity. This region has been found in all S. thermophilus bacteriophages sequenced so far, and it is flanked by highly conservative sequences. Based on these data, a PCR method was developed that allows S. thermophilus phages to be detected, providing a sensitive system useful to the dairy industry. Additionally, the phages can be classified by use of the VR2 sequence, verifying the correlation with the host range.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
The 17 phages used in this work (Table 1) were isolated from six different cheese and yogurt plants in Argentina and Spain (as the result of an abnormal fermentation pattern). These phages were preserved in the PROLAIN (Programa de Lactología Industrial, UNL, Santa Fe, Argentina) and IPLA (Instituto de Productos Lácteos de Asturias) collections. Phages φSfi11 and φSfi21 were used as controls in DNA restriction, Southern blotting, and PCR assays. Sixteen commercial S. thermophilus strains isolated from commercial cultures used in the industrial manufacture of milk products were identified as phage-sensitive strains. Additional collection strains were used for the host range assay (Table 1). These strains were routinely grown at 42°C in M17 broth or agar (Oxoid, Basingstoke, Hampshire, England) supplemented with 0.5% lactose (LM17). Phage enumeration was performed using the double-layer plaque titration method (12) in LM17 supplemented with 10 mM CaCl2 (LM17-Ca) and containing 10 mM glycine to enhance plaque formation (18). The host range was determined by a plaque assay as described by Suárez et al. (32).
TABLE 1.
Bacterial strains, phages, and primers used in this study
| Strain, phage, or primer | Relevant characteristics | Reference and/or source |
|---|---|---|
| S. thermophilus strains | ||
| 5-C | Host for phage φ021-5 | (32) |
| M1-C | Host for phage φCP | (32) |
| M10-C | Host for phage φCQ210 | (32) |
| M11-C | Host for phage φCQ211 | (32) |
| Sth10 | Host for phage φFcSth10 | (32) |
| 799 | Host for phage φ799-M1 | (32) |
| Abc2 | Host for phage φAbc2 | (32) |
| cLy1 | Host for phage φLy1 | (32) |
| cLy7 | Host for phage φLy7 | (32) |
| 10.3 | Host for phage φP10.3 | This study |
| 13.2 | Host for phage φP13.2 | This study |
| 15-C | Host for phage φ0BJ | (32) |
| 3.1 | Host for phage φP3.1 | This study |
| cLy4 | Host for phage φLy4 | This study |
| MiC7 | Host for phage φMil | (32) |
| 4-C | Host for phage φipla106 | (32) |
| CNRZ1066 | Host for phage φipla110 | INRA Collection |
| LMD-9 | NCCB Collection | |
| LMG 18311 | LMG Collection | |
| Phages | ||
| φ021-5 | Cuartirolo cheese (Córdoba, Argentina), plant 1 (1995) | (32) |
| φCP | Pategrás cheese (Buenos Aires, Argentina), plant 2 (1998) | (32) |
| φCQ210 | Por Salut cheese (Buenos Aires, Argentina), plant 2 (1998) | (32) |
| φCQ211 | Por Salut cheese (Buenos Aires, Argentina), plant 2 (1998) | (32) |
| φFcSth10 | Cuartirolo cheese (Santa Fe, Argentina), plant 3 (1998) | (32) |
| φ799-M1 | Cremoso cheese (Buenos Aires, Argentina), plant 4 (1999) | (32) |
| φAbc2 | Cremoso cheese (Buenos Aires, Argentina), plant 5 (2000) | (32) |
| φLy1 | Barra cheese (Santa Fe, Argentina), plant 3 (2000) | (32) |
| φLy7 | Barra cheese (Santa Fe, Argentina), plant 3 (2000) | (32) |
| φP10.3 | Cremoso cheese (Buenos Aires, Argentina), plant 4 (2000) | This study |
| φP13.2 | Cremoso cheese (Buenos Aires, Argentina), plant 4 (2000) | This study |
| φ0BJa | Cuartirolo cheese (Córdoba, Argentina), plant 1 (1995) | (32) |
| φP3.1a | Cremoso cheese (Buenos Aires, Argentina), plant 4 (2000) | This study |
| φLy4a | Barra cheese (Santa Fe, Argentina), plant 3 (2000) | This study |
| φMi1a | Cremoso cheese (Entre Rios, Argentina), plant 4 (1999) | (32) |
| φipla106a | Yoghurt (Asturias, Spain), plant 6 (2004) | This study |
| φipla110a | Yoghurt (Asturias, Spain), plant 6 (2004) | This study |
| φSfi11 | Lytic, pac, isolated from yoghurt | Nestlé collection (20) |
| φSfi21 | Temperate, cos, isolated from yoghurt | Nestlé collection (6) |
| Primers | ||
| A | 5′-GGAGTAGATATCAAGGCG-3′ | (5) |
| B | 5′-ATAGATAAGCTGGTAGGC-3′ | (5) |
| C | 5′-TGATAAGTGATTTCTGGG-3′ | (5) |
| D | 5′-TTGGTTAAGTTTCAAGGG-3′ | (5) |
| INT1 | 5′-GACATTTTAAAGCCTTTAACCATGC-3′ | This study |
| INT4 | 5′-CCACACTCTCAAACTTTGAGAATC-3′ | This study |
| HOST1 | 5′-GAATGATACTGCTGGCAGTATTTCGGTTGG-3′ | This study |
| HOST5 | 5′-CAGTCATGTAGCTATCGATGAAATTCCAACG-3′ | This study |
Used only in VR2 characterization experiments.
Phage multiplication and purification.
Overnight host bacterial cultures were inoculated (1%) into 50 ml of LM17-Ca broth. The cultures were infected with phage suspensions at a multiplicity of infection of 0.1 to 1 and incubated at 42°C until complete lysis occurred. Each lysate was centrifuged (10,000 × g, 30 min, 4°C), and the phage particles in the supernatant were precipitated with 4% polyethylene glycol 6000 in 0.2 M NaCl for 24 to 48 h at 4°C and centrifuged (17,000 × g, 120 min, 4°C). The pellet was resuspended in SM buffer (28) and, if DNA was extracted later, supplemented with 40 μg ml−1 RNase A (Roche, Basel, Switzerland) and 1 μg ml−1 DNase I (Roche) and incubated at 37°C for 30 min.
Electron microscopy.
Phage particles purified as previously described were deposited on Formvar-coated grids. They were then negatively stained with 2% uranyl acetate and observed using a JEOL 2000 Ex-II electron microscope at an acceleration voltage of 80 kV. Electron micrographs were obtained with AGFA (Mortsel, Belgium) scientific film plates.
DNA isolation and restriction analysis.
Phage DNA was obtained from 400 μl of a concentrated suspension of phage particles treated with 80 μl of lysis solution (0.25 M EDTA, pH 8.1; 0.5 M Tris-HCl, pH 9.6; 2.5% sodium dodecyl sulfate [SDS]) and incubated at 65°C for 30 min. One hundred microliters of 8 M potassium acetate was then added, and the mixture was incubated on ice for 15 min before centrifugation (16,100 × g, 10 min, 4°C). Phage DNA was precipitated from the supernatant with an equal volume of isopropanol, kept at room temperature for 5 min, and centrifuged again (16,100 × g, 10 min). The pellet was resuspended in 630 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) in the presence of 0.3 M sodium acetate and precipitated with isopropanol for 5 min, followed by centrifugation (16,100 × g, 10 min). The last precipitation step was then repeated. The precipitated DNA was washed once with absolute ethanol and twice with 70% ethanol, dried, and resuspended in TE buffer. Purified phage DNA was digested with restriction endonucleases ClaI, EcoRI, EcoRV, HaeIII, HindIII, PstI, and SalI (Takara, Otsu, Shiga, Japan) used according to the manufacturer's instructions. Restricted phage DNA was electrophoresed in a 0.8% agarose gel in TAE buffer (40 mM Tris-acetate, 1 mM EDTA) and visualized under UV light by ethidium bromide staining by using standard protocols (28).
Southern blot assays.
The EcoRV restriction fragments of phage DNAs were separated on a 0.8% agarose gel, blotted, and hybridized by using standard methods (28). The probes used for hybridization were the unrestricted DNAs of bacteriophages φSfi11, φSfi21, and φCP. Southern blotting was performed at 65°C, and the blots were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before autoradiography by using standard protocols (28, 30). The probes used for hybridization were radiolabeled with [α-32P]dATP by nick translation.
PFGE.
Undigested phage DNAs were heated at 56°C for 5 min to denature the cohesive termini, cooled, and rapidly loaded onto low-melting-temperature agarose (final concentration, 0.6%; Bio-Rad, Hercules, CA). Pulsed-field gel electrophoresis (PFGE) was performed using a CHEF-DRIII SYS220/240 system (Bio-Rad). The 1% gel was made with pulsed-field-certified agarose (Bio-Rad) in 0.5× TBE buffer (0.89 M Tris, 0.02 M EDTA, 0.89 M boric acid). The pulsed-field parameters were as follows: 0.5× TBE buffer; initial switch time, 0.1 s; final switch time, 8 s; 0.6 V/cm; run time, 15 h; and buffer temperature, 14°C. The gel was stained with ethidium bromide and then visualized on a UV transilluminator (312 nm). The genome size was determined with Quantity One software, version 4.2.1(Bio-Rad), using the Low Range PFG marker (New England Biolabs, Beverly, MA) as the standard.
SDS-PAGE.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed as described by Laemmli (16). Highly purified phage particles (approximately 1010 PFU ml−1) were suspended in 20 mM EDTA, pH 8.0, and boiled for 3 min to allow particle breakage and DNA release. The product was then treated with 4 μg ml−1 DNase I at 37°C for 30 min and boiled for 5 min with 1× SDS-PAGE loading buffer (12.5 mM Tris-HCl, pH 6.8, 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.005% bromophenol blue, pH 6.8). Proteins were separated using an SDS-polyacrylamide gel (13%), employing the mini-Protean II system (Bio-Rad). Electrophoresis was conducted in electrophoresis buffer (248 mM Tris-HCl, pH 8.8, 1.92 M glycine, 20 mM EDTA, 1% SDS) at 40 mA (maximum voltage, 250 V) until the tracking dye reached the bottom of the gel. Proteins were stained with Coomassie brilliant blue R-250.
Amplification of phage DNA by PCR.
PCRs were performed using puRe Taq Ready-To-Go PCR beads (Amersham-Biosciences, Buckinghamshire, England) with each primer at a concentration of 400 nM. The sequences of the oligonucleotides (Sigma-Genosys, Haverhill, United Kingdom) used as primers are shown in Table 1. When DNA was used as a template, 10 ng was diluted in 25 μl of MilliQ water and used directly in the reaction; 0.5-μl concentrated suspensions of viral particles or lysis plaques from LM17 soft agar were also employed as templates. To check the detection limit of the PCR method, sterile skim milk (Oxoid) was inoculated with serial dilutions of phage suspensions, and 1-μl samples were used directly in PCRs. φSfi11 and/or φSfi21 was used as a control in all PCRs. Additional experiments with DNAs from S. thermophilus hosts as the templates were performed to rule out the possibility of prophage DNA amplification. A negative control without a template was also included. DNA was amplified with an iCycler thermal cycler (Bio-Rad). The PCR conditions included an initial denaturation step (94°C for 3 min), followed by 35 amplification cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min (for replication module conserved fragments and VR2 amplification) or 2 min (for integrase amplification) and then a final extension step at 72°C for 7 min. Primers A, B, C, and D (5) were used to amplify the highly conserved DNA fragment of the replication module. To detect potentially temperate bacteriophages, a pair of primers (designated INT1 and INT4) (Table 1) was designed based on the integrase gene sequences of the temperate phages φO1205, φSfi21, and φTP-J34 (GenBank accession numbers U88974, AF013584, and AF020798, respectively). Amplification of the variable region (VR2) involved in host recognition (11) was performed with primers HOST1 and HOST5. The oligonucleotides were designed on the basis of the φDT1 orf18 gene and the homologous genes of φMD2, φSfi11, φSfi19, φSfi21, φ7201, and φO1205 (GenBank accession numbers AF085222, AF348736, AF158600, AF115102, AF115103, NC0002185, and U88974, respectively).
Nucleotide sequence analysis.
PCR products were purified using a GenElute PCR clean-up kit (Sigma-Genosys). The nucleotide sequences were determined with an ABI Prism 373 A Strech automated sequencer at the DNA Sequencing Service of the Centro de Investigaciones Biológicas (CIB, CSIC), Madrid, Spain. Sequence data were assembled and analyzed using a sequence analysis software package available from the EMBL Spanish node (CNB, CSIC, Spain). Alignment was performed using the Clustal W algorithm (33). A phylogenetic tree was constructed from the alignment using the MSVP 3.13d (1985-2002) software (Kovach Computing Services) and the neighbor-joining method (27).
RESULTS
Host range.
A host range study was carried out by testing 15 bacteriophages with 14 S. thermophilus strains from different bacterial collections (Table 2). The phages had different host ranges, and the number of hosts ranged from just one host strain (φ021-5, φCQ211, φ799-M1, φP3.1, and φP13.2) to four host strains. Phages φCQ210, φAbc2, φP10.3, and φ0BJ shared the broadest host range, since they infected the same four strains (S. thermophilus 15-C, M10-C, ST10.3, and LMD-9). φFcSth10 showed infectivity with three strains (S. thermophilus 4-C, M1-C, and Sth10), and phages φCP and φipla106 exhibited a host range related to that of φFcSth10, infecting S. thermophilus strains 4-C, M1-C, and ST13.2. φipla110 infected S. thermophilus M11-C and CNRZ 1066 and shared this host range with φSfi21. φSfi11 was not able to infect any of our available strains.
TABLE 2.
Host ranges of 15 S. thermophilus phages for 14 S. thermophilus strains
| Strain | Phage
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 021-5 | CP | CQ210 | CQ211 | FcSth10 | Abc2 | 799-M1 | P13.2 | P10.3 | P3.1a | OBJa | ipla106a | ipla110a | Sfi11 | Sfi21 | |
| 4-C | −b | +b | −b | −b | +b | − | − | − | − | − | −b | + | − | − | − |
| 5-C | +b | −b | −b | −b | −b | − | − | − | − | − | −b | − | − | − | − |
| 15-C | −b | −b | +b | −b | −b | + | − | − | + | − | +b | − | − | − | − |
| M1-C | −b | +b | −b | −b | +b | − | − | − | − | − | −b | + | − | − | − |
| M10-C | −b | −b | +b | −b | −b | + | − | − | + | − | +b | − | − | − | − |
| M11-C | −b | −b | −b | +b | −b | − | − | − | − | − | −b | − | + | − | + |
| Sth10 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| 799 | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| ST3.1 | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| ST10.3 | − | − | + | − | − | + | − | − | + | − | + | − | − | − | − |
| ST13.2 | − | + | − | − | − | − | − | + | − | − | − | + | − | − | − |
| CNRZ1066 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + |
| LMD-9 | − | − | + | − | − | + | − | − | + | − | + | − | − | − | − |
| LMG18311 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
Used only in VR2 characterization experiments.
Data from reference 32.
Electron microscopy.
The 11 phages that were characterized morphologically (φ021-5, φCP, φCQ210, φCQ211, φFcSth10, φAbc2, φLy1, φLy7, φ799-M1, φP10.3, and φP13.2) had the same morphology, including isometric heads (diameter, 40 to 70 nm) and long, flexible, noncontractile tails (length, 175 to 300 nm). They therefore belong to Bradley's group B (3) or the Siphoviridae family (morphotype B1) according to the International Committee on the Taxonomy of Viruses (21). For phages φ021-5 and φ799-M1, clusters of phage particles joined by the ends of their tails were observed (data not shown).
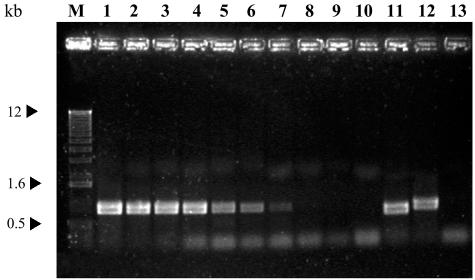
Comparison of phage DNA restriction endonuclease patterns.
The DNAs of the 11 S. thermophilus phages studied were subjected to restriction analysis with the enzymes ClaI, EcoRI, EcoRV, HaeIII, HindIII, PstI, and SalI. Figure 1A shows the restriction patterns after digestion with EcoRV. In general, the phages had different profiles; the exceptions were φLy1 and φLy7, which had identical restriction patterns. Similar fragments were seen in other comparisons. Thus, the EcoRV digests of some phages of different origins contained fragments that were the same sizes (phages φ021-5, φCP, and φP10.3; phages φLy1 or φLy7 and φP13.2) (Fig. 1A). Although φLy7 and φLy1 seem to be the same phage, they exhibited different efficiencies of plating on their host strains, cLy7 and cLy1. Restriction analysis revealed that the phage genomes contained submolar fragments. Treatment of DNA with T4 DNA ligase before restriction led to the loss of two bands and the appearance of a larger band of molar proportion in 10 of the phages. These results indicate that the genomes are packaged by a cos-type mechanism. Only in the remaining bacteriophage, φP13.2, did ligation prior to digestion fail to modify the restriction pattern. The absence of cohesive ends in the genome of this phage suggests that there is a pac-type packaging mechanism (data not shown).
FIG. 1.
Agarose gel electrophoresis of the EcoRV-generated DNA fragments of S. thermophilus phages (A) and the corresponding Southern blots hybridized with 32P-labeled φSfi11 DNA (B), φSfi21 DNA (C), and φCP DNA (D). Lane M, 1-kb DNA ladder (Invitrogen); lane 1, φ021-5; lane 2, φCP; lane 3, φCQ210; lane 4, φCQ211; lane 5, φFcSth10; lane 6, φAbc2; lane 7, φLy1; lane 8, φLy7; lane 9, φ799-M1; lane 10, φP10.3; lane 11, φP13.2; lane 12, φSfi11; lane 13, φSfi21.
Genome size determination.
The sizes of the phage genomes were determined by PFGE, and the results are as follows: φ021-5, 33.9 kb; φCP, 36.0 kb; φCQ210, 31.2 kb; φCQ211, 37.8 kb; φFcSth10, 39.7 kb; φAbc2, 32.8 kb; φLy1, 34.1 kb; φLy7, 34.5 kb; φ799-M1, 30.8 kb; φP10.3, 32.7 kb; and φP13.2, 42.7 kb.
Southern blot hybridization analysis.
The presence of homologous DNA sequences in the phage DNAs was determined by Southern blotting using genomic DNA from phage φSfi11 or φSfi21 as a probe. These phages were selected since they have very distinct features; φSfi11 is a lytic phage containing a pac site, whereas φSfi21 is a temperate, cos-containing phage. Moreover, these phages had an EcoRV restriction pattern that was different from the EcoRV restriction patterns of the 11 original phages used in this study (Fig. 1 A). These probes exhibited clear homology to DNA samples from 10 of the phages studied; more than 25% of the fragments of each phage hybridized with φSfi11, and more than 40% of the fragments of each phage hybridized with φSfi21 (Fig. 1B and C), independent of the packaging mode. φCP showed a weak hybridization signal with both probes, and for this reason, another Southern blot was prepared using its genomic DNA as a probe (Fig. 1D). In this case, all the phages exhibited DNA homology, mainly to bacteriophages φ021-5 and φP10.3, whose EcoRV restriction patterns contained common fragments.
Protein composition.
SDS-PAGE was used to determine the structural protein contents of phage particles in concentrated solutions. Two different profiles were observed. Ten phages were found to produce two major protein bands at molecular masses of approximately 32 and 26 kDa. Only phage φP13.2 contained three major proteins, which had estimated molecular masses of 41, 25, and 13 kDa (data not shown).
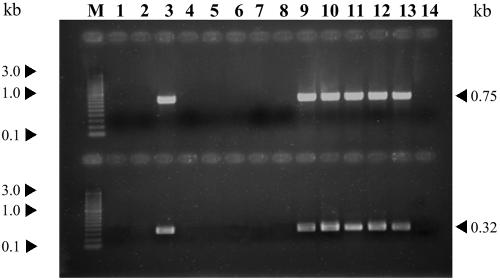
Presence of the conserved DNA fragment from the phage φSfi18 genome.
Brüssow et al. (5) described a DNA fragment in the replication module that belongs to the φSfi18 genome; this fragment appears to be very conserved both in cos- and pac-containing S. thermophilus phages. Using primers A and B and primers A and C, which were designed by Brüssow et al. (5), phages φCQ210, φ799-M1, φP10.3, and φP13.2 yielded the expected 748-bp and 320-bp PCR products, respectively (Fig. 2), but these phages gave a negative signal with primers A and D (1,207-bp amplification product). Only φ021-5, which could not be not amplified with primers A and B or primers A and C, gave positive PCR results when primers A and D were used (data not shown). φCP, φCQ211, φFcSth10, φAbc2, φLy1, and φLy7 could not be amplified with any of the three primer pairs (primers A and B, primers A and C, and primers A and D). The amplicons obtained with primers A and B were purified and sequenced. Comparison of their sequences showed that there was a high degree of similarity (>98%) with the conserved region of the replication module of bacteriophages φSfi18, φDT1, φO1205, φSfi11, φSfi19, and φSfi21 (GenBank accession numbers AF158601, AF085222, U88974, AF158600, AF115102, and AF004379, respectively).
FIG. 2.
Amplification products of the highly conserved DNA fragments from the replication module of S. thermophilus bacteriophages. Lane M, 100-bp PCR EZ load molecular ruler (Bio-Rad); lane 1, φ021-5; lane 2, φCP; lane 3, φCQ210; lane 4, φCQ211; lane 5, φFcSth10; lane 6, φAbc2; lane 7, φLy1; lane 8, φLy7; lane 9, φ799-M1; lane 10, φP10.3; lane 11, φP13.2; lane 12, φSfi11; lane 13, φSfi21; lane 14, negative control. Upper row, PCR products obtained with primers A and B; lower row, PCR products obtained with primers A and C.
Identification of temperate bacteriophages.
To determine whether the phages were temperate, and bearing in mind the strong conservation of integrases, primers INT1 and INT4 were designed to amplify an internal fragment of the genes coding for these enzymes. Bacteriophages φSfi21 (temperate) and φSfi11 (lytic) were employed as positive and negative PCR controls, respectively. Only φCQ211 gave a positive PCR signal (data not shown), and the sequence of this product exhibited a high degree of similarity (>98%) with the sequence of the integrase gene of bacteriophages φO1205, φSfi21, and φTP-J34 (GenBank accession numbers U88974, AF013584, and AF020798, respectively), suggesting that it could have a temperate nature.
Characterization of the antireceptor variable region.
On the basis of the DNA sequences of orf18 homologous genes from phages φDT1, φMD2, φSfi19, φSfi21, and φ7201, two PCR primers, HOST1 and HOST5, were designed to amplify VR2. A fragment whose size was variable (approximately 700 to 800 bp) was amplified for all the phages assayed. The sequences of the purified PCR products were compared with the VR2 sequences of all the S. thermophilus phages in the GenBank database (φDT1, φDT2, φDT4, φMD1, φMD2, φMD4, φQ5, φ7201, φO1205, φSfi11, φSfi18, φSfi19, and φSfi21; accession numbers AF085222, AF348739, AF348738, AF348737, AF348736, AF348735, AF348734, AF145054, U88974, AF158600, AF158601, AF115102, and AF115103, respectively). The alignment grouped the sequences into 19 typing profiles (>96% similarity) (Fig. 3). The most uniform groups were represented by phages φAbc2, φP10.3, φCQ210, and φ0BJ (99% to 100% similarity), phages φFcSth10 and φipla106 (99% similarity), and phages φLy7, φLy1, and φMi1 (>98% similarity). φipla110 was the only bacteriophage that was clustered with previously described phages (φSfi19, φSfi21, and φDT2) and had an identical VR2 sequence. The remaining phages constituted individual groups since their VR2 sequences exhibited less than 96% similarity.
FIG. 3.
Dendrogram (obtained by the neighbor-joining method) for the antireceptor VR2 sequences from all the S. thermophilus bacteriophages used in this work (boldface type) and the phages whose sequences are available in the GenBank database (italics). Boxes indicate highly homologous corresponding sequences (>96% similarity) in the phages.
Detection of S. thermophilus bacteriophages in milk.
To evaluate the usefulness of this VR2 PCR method to detect S. thermophilus bacteriophages directly in industrial samples, milk was artificially contaminated with all the phages used. One sample of milk contaminated with each phage was subjected to PCR with no prior treatment. All the phages were detected successfully (Fig. 4). The detection limit, 105 PFU ml−1 of milk, was determined with φFcSth10 (Fig. 5).
FIG. 4.
Amplification of the antireceptor VR2 variable region using bacteriophage-infected milk. Lane M, 100-bp PCR EZ load molecular ruler (Bio-Rad); lane 1, φ021-5; lane 2, φCP; lane 3, φCQ210; lane 4, φCQ211; lane 5, φFcSth10; lane 6, φAbc2; lane 7, φLy1; lane 8, φLy7; lane 9, φ799-M1; lane 10, φP10.3; lane 11, φP13.2; lane 12, φ0BJ; lane 13, φ021-4; lane 14, φP3.1; lane 15, φMi1; lane 16, φLy4; lane 17, φSfi21; lane 18, negative control.
FIG. 5.
Amplification of the antireceptor VR2 region using milk contaminated with different titers of S. thermophilus bacteriophage φFcSth10. Lane M, 1-kb DNA ladder (Invitrogen); lane 1, 1010 PFU ml−1 (M17); lanes 2 to 10, 1010 to 102 PFU ml−1 of milk; lane 11, φSfi11; lane 12, φSfi21; lane 13, negative control.
DISCUSSION
As observed for all previously characterized S. thermophilus phages, the phages used in this work belonged to the Siphoviridae family (corresponding to group B as defined by Bradley [3]) and showed a clear tendency to aggregate into clusters (6, 7, 12, 15). These phages have double-stranded DNA genomes whose sizes range from 30.7 to 42.7 kb, in accord with previous reports (1, 6, 7, 17, 22, 25). They are genetically related, as demonstrated by DNA-DNA hybridization experiments and restriction analysis, confirming that all S. thermophilus phages (lytic and temperate, cos and pac) exhibit DNA homology (1, 4, 6-8, 12, 17, 23-25). Only one likely temperate phage was found in the present study, via detection of its lysogeny module, and it may even be a virulent derivative of a temperate phage. This is not surprising since lysogeny appears to be rare in S. thermophilus (1, 4, 6, 8, 12, 17, 23). At the protein level, the phages could be classified into two groups, one group containing the phages with two major structural proteins and one group containing a single phage (φP13.2) with three major structural proteins. Le Marrec et al. (17) indicated that there is a strict correlation between the presence of a particular set of major structural proteins and the mechanism of DNA packaging. Therefore, phage φP13.2 probably uses a pac mechanism of DNA packaging, while the other phages use a cos-type mechanism. These results were confirmed by the absence and presence, respectively, of cohesive ends from the DNA restriction analysis. Furthermore, we observed that amplification of the DNA fragment from the replication module of φSfi18 was not useful for detecting bacteriophages from the Argentinean cheese plants; it was found in only five phages belonging to this collection. Brüssow et al. (5) verified that the majority of 81 S. thermophilus bacteriophages hybridized with this fragment of the φSfi18 replication module and exhibited positive PCR amplification with primers A and B. According to this classification criterion (31), the phages used in the present study that gave positive PCR results could be considered members of group I, since they contain a φO1205-like replication origin (very similar to φSfi18 and φSfi21 replication origins) (13). In such cases, extreme sequence conservation was observed, as reported by other authors (4, 5, 7, 9, 17). The much wider distribution of alternative DNA replication modules found in the Argentinean phages used in this work is noteworthy. Since starter companies are distributed worldwide, these results suggest that there is a different phage ecology from that in Europe, where most previously characterized S. thermophilus phages were obtained. Together, these results show that the traditional criteria used to classify S. thermophilus bacteriophages are of limited industrial value for phage types from dairy factories. The method proposed here, however, not only allows rapid detection of dairy industry S. thermophilus phages but also classifies them according to their VR2 region sequences. Duplessis and Moineau (11) characterized the orf18 gene of six phages. For φDT1 and φMD4 (different host ranges) it was clearly demonstrated that host specificity resulted from VR2, since the chimeric φDT1 phages constructed by recombination with orf18 of φMD4 acquired the host range of φMD4. φDT4, φMD1, and φQ5, which have the same infectivity profile, have identical VR2 sequences (as expected). The main point that should be emphasized is that, in general, bacteriophages with high levels of VR2 sequence similarity, independent of other characteristics, have similar host ranges, and consequently, a correlation between this criterion and this genetically based classification system is suggested by our findings. The validity of this method is supported by the distribution of VR2 sequences in phages whose sequences are in the GenBank database. For example, φSfi19 (lytic) and φSfi21 (temperate), which have identical VR2 sequences, have overlapping host ranges (6, 10) even though they were classified (6) into different lytic groups (groups I and III, respectively) based only on their different lifestyles. φSfi11, which belongs to lytic group II (6), shows little (32%) VR2 similarity with these phages. Therefore, the proposed typing system has a technologically significant advantage compared to other grouping criteria: information about the host range of detected phages can be obtained easily, and this could help in the design of rotational solutions to phage attack in thermophilic starter cultures. However, there are some exceptions. φCP and φipla106 have different VR2 sequences but the same host range. Likewise, the host range of φ7201 is a subset of the host range of φQ5 (17), and the VR2 sequences of these phages exhibit only 32% similarity. On the other hand, phages φFcSth10 and φipla106 exhibit 99% VR2 similarity and have two overlapping hosts but different host ranges (Table 2). These results seem to indicate that additional phage factors are involved in host specificity, as previously suggested for φDT2 and φMD2, whose VR2 sequences have a high degree of similarity (96%) while the phages have nonoverlapping host ranges (11). Nevertheless, considering that these cases are exceptions and the narrow host range of these phages, in practice, a resistant strain could always be unambiguously associated with a given VR2 sequence. Table 3 shows some practical examples of strains recommended for industrial use based on the VR2 sequence. In the most favorable case, different VR2 sequences and the same host range, all the strains having the host range can be discarded easily when a phage showing any of the associated VR2 sequences is detected (Table 3, cases 2 and 3); therefore, this case should not represent a problem for application in the dairy industry. The other case, the same VR2 sequence and different host ranges, should not be a problem either. When any phage carrying a VR2 sequence is detected, all the strains associated with that VR2 sequence must be discarded as starters (Table 3, cases 2 and 4).
TABLE 3.
Practical examples of recommended S. thermophilus strains for industrial use based on the VR2 type sequence
| Case | VR2 type sequencea | Sensitive strainsa | Discarded strains | Recommended strainsb |
|---|---|---|---|---|
| 1 | φ0BJ, φCQ210, φP10.3, φAbc2 | 15-C, ST10.3, M10-C, LMD-9 | 15-C, ST10.3, M10-C, LMD-9 | 4-C, 5-C, M1-C, M11-C, Sth10, 799, ST3.1, ST13.2, CNRZ1066, LMG18311 |
| 2 | φFcSth10 | 4-C, M1-C, Sth10 | 4-C, M1-C, Sth10, ST13.2 | 5-C, 15-C, M10-C, M11-C, 799, ST3.1, ST10.3, CNRZ1066, LMD-9, LMG18311 |
| φipla106 | 4-C, M1-C, ST13.2 | |||
| 3 | φCP | 4-C, M1-C, ST13.2 | 4-C, M1-C, ST13.2 | 5-C, 15-C, M10-C, M11-C, 799, ST3.1, ST10.3, CNRZ1066, LMD-9, LMG18311 |
| 4c | φMD2 | SMQ301, SMQ495, SMQ509, SMQ513, SMQ514 | SMQ301, SMQ495, SMQ509, SMQ513, SMQ514, M11-C, CNRZ1066, Sfi1, Sfi1.c16, Sfi2, Sfi3, Sfi4, Sfi7, Sfi10, Sfi15, Sfi18, Sfi19, Sfi21, Sfi22, Sfi32, Sfi33, S3, W3, YS3 | SMQ173, SMQ302, SMQ303, SMQ494, SMQ496, SMQ498, SMQ511, 4-C, 5-C, 15-C, MI-C, M10-C, 799, Sth10, ST3.1, ST10.3, ST13.2, LMD-9, LMG18311, Sfi11 |
| φipla110 | M11-C, CNRZ1066 | |||
| φSfi21 | M11-C, CNRZ1066, Sfi1, Sfi7, Sfi10, Sfi21, W3, YS3 | |||
| φSfi19 | Sfi1, Sfi1.c16, Sfi2, Sfi3, Sfi4, Sfi7, Sfi10, Sfi15, Sfi18, Sfi19, Sfi22, Sfi32, Sfi33, S3, W3 |
S. thermophilus bacteriophages and strains used in this study are indicated by boldface type.
S. thermophilus strains infected by only one or two phages are most highly recommended (underlined).
Not all the S. thermophilus strains from different studies were assayed with the four phages.
Furthermore, this method is a useful tool for detection of phages in industrial samples. A PCR signal was obtained for all the phages tested in milk (detection limit, 105 PFU per ml of milk). An additional benefit is that the proposed PCR analysis can be carried out directly with a phage suspension (in culture medium or milk) and lysis plaques. Neither phage particle concentration (purification by precipitation, CsCl gradient, filtration, etc.), DNA extraction, nor any other procedure is required to enrich the samples, which is very promising for routine applications. A PCR assay for the detection of S. thermophilus bacteriophages, based on amplification of the conserved replication module region, has been described previously (6). Although this test has a lower detection limit (103 PFU ml−1), it was not able to detect 6 of the 11 phages assayed in the present work. Moreover, since the target module is very conserved, the sequence of the amplified DNA does not allow phage typing.
Phage monitoring is critically important in dairy product manufacture, and the industry needs reliable methods for rapid phage detection. Future work will therefore evaluate whether S. thermophilus phages can be detected by multiplex PCR (i.e., simultaneously with bacteriophages of other lactic acid bacteria, such as Lactobacillus or Lactococcus, used as starters in industrial processes).
Acknowledgments
We thank Jorge A. Reinheimer and Juan E. Suárez for their encouragement during the initial phases of this work and Carlos Álvarez Villa of the Servicio de Microscopía Electrónica y Microanálisis (Oviedo University, Spain) for technical support. We are grateful to Nestec (Nestec Ltd., Nestlé Research Center, Lausanne, Switzerland), which provided phages φSfi11 and φSfi21, and to Harold Brüssow for his critical revision of the manuscript.
This research was supported by project BIO 2002-01458 from MEC, Spain (cofinanced by PLAN FEDER from the European Union).
REFERENCES
- 1.Benbadis, L., M. Faelen, P. Slos, A. Fazel, and A. Mercenier. 1990. Characterization and comparison of virulent bacteriophages of Streptococcus thermophilus isolated from yoghurt. Biochimie 72:855-862. [DOI] [PubMed] [Google Scholar]
- 2.Binetti, A. G., A. Quiberoni, and J. A. Reinheimer. 2002. Phage adsorption to Streptococcus thermophilus. Influence of environmental factors and characterization of cell-receptors. Food Res. Int. 35:73-83. [Google Scholar]
- 3.Bradley, D. E. 1967. Ultrastructure of bacteriophages and bacteriocins. Bacteriol. Rev. 31:230-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüssow, H., and A. Bruttin. 1995. Characterization of a temperate Streptococcus thermophilus bacteriophage and its genetic relationship with lytic phages. Virology 212:632-640. [DOI] [PubMed] [Google Scholar]
- 5.Brüssow, H., A. Probst, M. Frémont, and J. Sidoti. 1994. Distinct Streptococcus thermophilus bacteriophages share an extremely conserved DNA fragment. Virology 200:854-857. [DOI] [PubMed] [Google Scholar]
- 6.Brüssow, H., M. Frémont, A. Bruttin, J. Sidoti, A. Constable, and V. Fryder. 1994. Detection and classification of Streptococcus thermophilus bacteriophages isolated from industrial milk fermentation. Appl. Environ. Microbiol. 60:4537-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brüssow, H., A. Bruttin, F. Desière, S. Lucchini, and S. Foley. 1998. Molecular ecology and evolution of Streptococcus thermophilus bacteriophages. A review. Virus Genes 16:95-109. [DOI] [PubMed] [Google Scholar]
- 8.Bruttin, A., F. Desière, S. Lucchini, S. Foley, and H. Brüssow. 1997. Characterization of the lysogeny DNA module from the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology 233:136-148. [DOI] [PubMed] [Google Scholar]
- 9.Desière, F., S. Lucchini, A. Bruttin, M. C. Zwahlen, and H. Brüssow. 1997. A highly conserved DNA replication module from Streptococcus thermophilus phages is similar in sequence and topology to a module from Lactococcus lactis phages. Virology 234:372-382. [DOI] [PubMed] [Google Scholar]
- 10.Desière, F., S. Lucchini, and H. Brüssow. 1998. Evolution of Streptococcus thermophilus bacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology 241:345-356. [DOI] [PubMed] [Google Scholar]
- 11.Duplessis, M., and S. Moineau. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 41:325-336. [DOI] [PubMed] [Google Scholar]
- 12.Fayard, B., M. Haeflinger, and J. P. Accolas. 1993. Interaction of temperate bacteriophages of Streptococcus salivarius subsp. thermophilus with lysogenic indicators affect phage DNA restriction patterns and host ranges. J. Dairy Res. 60:385-399. [Google Scholar]
- 13.Foley, S., S. Lucchini, M. C. Zwahlen, and H. Brüssow. 1998. A short noncoding viral DNA element showing characteristics of a replication origin confers bacteriophage resistance to Streptococcus thermophilus. Virology 250:377-387. [DOI] [PubMed] [Google Scholar]
- 14.Forde, A., and G. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 15.Kivi, S., T. Peltomäki, K. Luomala, and S. S. Sarimo. 1987. Some properties for Streptococcus thermohilus bacteriophages. Folia Microbiol. 32:101-106. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Le Marrec, C., D. van Sinderen, L. Walsh, E. Stanley, E. Vlegels, S. Moineau, P. Heinze, G. Fitzgerald, and B. Fayard. 1997. Two groups of bacteriophages infecting Streptococcus thermophilus can be distinguished on the basis of mode of packaging and genetic determinants for major structural proteins. Appl. Environ. Microbiol. 63:3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillehaug, D. 1997. An improved plaque assay for poor plaque producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85-90. [DOI] [PubMed] [Google Scholar]
- 19.Lucchini, S., J. Sidoti, and H. Brüssow. 2000. Broad-range bacteriophage resistance in Streptococcus thermophilus by insertional mutagenesis. Virology 275:267-277. [DOI] [PubMed] [Google Scholar]
- 20.Lucchini, S., F. Desière, and H. Brüssow. 1998. The structural gene module in Streptococcus thermophilus bacteriophage phi Sfi11 shows a hierarchy of relatedness to Siphoviridae from a wide range of bacterial hosts. Virology 246:3-73. [DOI] [PubMed] [Google Scholar]
- 21.Matthews, R. E. F. 1982. Classification and nomenclature of viruses. Fourth report of the international committee on taxonomy of viruses. Intervirology 17:1-200. [DOI] [PubMed] [Google Scholar]
- 22.Moineau, S. 1999. Applications of phage resistance in lactic acid bacteria. Antonie Leeuwenhoek 76:377-382. [PubMed] [Google Scholar]
- 23.Neve, H., U. Krusch, and M. Teuber. 1989. Classification of virulent bacteriophage of Streptococcus salivarius subsp. thermophilus isolated from yoghurt and Swiss-type cheese. Appl. Microbiol. Biotechnol. 30:624-629. [Google Scholar]
- 24.Prevots, F., P. Relano, M. Mata, and P. Ritzenthaler. 1989. Close relationship of virulent bacteriophages of Streptococcus salivarius subsp. thermophilus at both the protein and the DNA level. J. Gen. Microbiol. 135:3337-3344. [Google Scholar]
- 25.Quiberoni, A., L. Auad, A. G. Binetti, V. B. Suárez, J. A. Reinheimer, and R. R. Raya. 2003. Comparative analysis of Streptococcus thermophilus bacteriophages isolated from a yogurt industrial plant. Food Microbiol. 20:461-469. [Google Scholar]
- 26.Reinheimer, J., A. Binetti, A. Quiberoni, N. Bailo, A. Rubiolo, and G. Giraffa. 1997. Natural milk cultures for Argentinean cheese production. J. Food Prot. 60:59-63. [DOI] [PubMed] [Google Scholar]
- 27.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Sebastiani, H., and H. Jäger. 1992. Bacteriophages of Streptococcus salivarius subsp. thermophilus: characterization of hereditary relationships and determination of virus-host interaction. Milchwissenschaft 48:25-29. [Google Scholar]
- 30.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 31.Stanley, E., L. Walsh, van A. der Zwet, G. F. Fitzgerald, and D. van Sinderen. 2000. Identification of four loci isolated from two Streptococcus thermophilus phage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol. Lett. 182:271-277. [DOI] [PubMed] [Google Scholar]
- 32.Suárez, V. B., A. Quiberoni, A. G. Binetti, and J. A. Reinheimer. 2002. Thermophilic lactic acid bacteria phages isolated from Argentinian dairy industries. J. Food Prot. 65:1597-1604. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]