Abstract
Aquaporins are members of the major intrinsic protein superfamily of integral membrane proteins which enable the transport of water, glycerol, and other solutes across membranes in various organisms. In microorganisms, the physiological role of aquaporins is not yet defined. We found a clear correlation between expression of the Candida albicans aquaporin-encoding gene AQY1 and freeze tolerance. A connection with the function for the aquaporin in the natural environment of C. albicans is, however, not obvious.
Aquaporins are members of the major intrinsic protein (MIP) superfamily of integral membrane proteins (4, 23). They mediate water transport across cell membranes in numerous species (7). Mammalian and plant aquaporins have important functions in water homeostasis and osmoregulation (1, 20), but the physiological function of microbial aquaporins remains a matter of debate (9, 16, 17, 18, 26, 27). Transport processes that involve the uptake or efflux of water mediated by water channel proteins are nevertheless believed to be of major importance for microorganisms, particularly under conditions where the passive diffusion of water through the lipid bilayer is insufficient.
In Saccharomyces cerevisiae, two aquaporin-encoding genes have been identified and characterized, AQY1 and AQY2 (6, 11, 21). Aquaporin expression is strongly correlated with freeze tolerance (28). Deletion of both aquaporin genes from a laboratory strain makes the cells more sensitive to freezing, and overexpression of the genes improves freeze tolerance (28). These findings are consistent with a role for plasma membrane water transport activity in determining freeze tolerance in S. cerevisiae.
A functional water channel is known in Candida albicans (12), but a aquaporin null strain does not have a pronounced phenotype (12). Whereas an S. cerevisiae aquaporin null strain displays a small but significant decrease in sensitivity to osmotic shock (6), deletion of C. albicans AQY1 resulted in a far less pronounced phenotype (12). The minor effects of aquaporin deletion on cell surface hydrophobicity, flocculation, cell aggregation, and invasive growth observed in S. cerevisiae (11) were not noticeable in C. albicans (12).
In this study, we determined whether the aquaporin-encoding gene AQY1 is a determinant of C. albicans freeze tolerance, as previously demonstrated for S. cerevisiae AQY1 and AQY2 (28). We verified the freeze-sensitive C. albicans AQY1 deletion phenotype, which is remedied by reintroduction of the gene. These findings strengthen the correlation between aquaporin function and freeze tolerance in microorganisms.
We compared the freeze tolerance of heterozygous AQY1 deletion strain JC0186 (aqy1Δ/AQY1) (12) and homozygous AQY1 deletion strain JC0188 (aqy1Δ/aqy1Δ) (12). Both strains were grown overnight in 5 ml YPD (1% wt/vol yeast extract, 2% wt/vol Bactopeptone, 2% glucose) or uracil-deficient minimal medium (6.67 g/liter yeast nitrogen base without amino acids [Bio101, Qbiogene, France], 0.77 g/liter complete supplement mixture minus uracil [Bio101], 2% glucose) at 30°C in an orbital shaker at 220 rpm. The cells were collected by centrifugation (2,000 × g, 1 min, room temperature) and resuspended in 1 ml of fresh ice-cold medium without glucose (optical density at 600 nm [OD600] = 20).
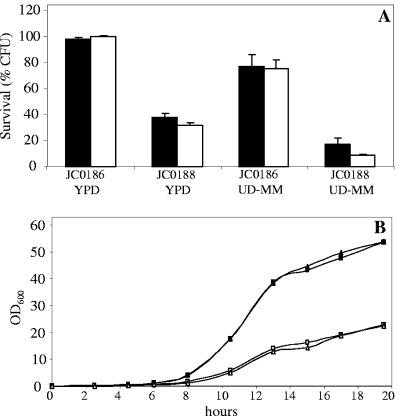
The cell suspensions were divided into eight equal portions. Four 40-μl aliquots were kept on ice and four additional aliquots were frozen in an ethanol bath at −30°C in 1.5-ml microcentrifuge tubes. After both one hour and one day, two control samples and two thawed test samples were diluted in ice-cold water, plated on YPD-plates containing 50 mg/liter uridine and grown for 2 days at 30°C. Percent survival was determined as the number of CFU of frozen samples relative to the control samples. Whether grown in rich or minimal medium, the C. albicans aquaporin null strain JC0188 was significantly less freeze tolerant than was strain JC0186, which has a functional AQY1 allele (Fig. 1A).
FIG. 1.
(A) Freeze tolerance of heterozygous (JC0186, aqy1Δ/AQY1) and homozygous (JC0188, aqy1Δ/aqy1Δ) C. albicans AQY1 deletion strains. Cells were grown overnight in YPD or uracil-deficient minimal medium (UD-MM) and were frozen for 1 hour (dark bars) or 1 day (light bars) at −30°C. Survival of frozen cells compared to nonfrozen cells (cooled on ice) is expressed as % CFU. Experiments were repeated at least three times, and mean values and standard deviations are depicted. (B) Growth of JC0186 (squares) and JC0188 (triangles) in YPD (solid symbols) or uracil-deficient minimal medium (open symbols). Experiments were repeated three times, and the standard error associated with each point was always <2% of the value of the point.
To rule out an indirect effect of aquaporin deletion on stress resistance due to a change in growth, C. albicans cells freshly grown on YPDuri plates (YPD supplemented with 50 mg/liter uridine and 1.5% agar) were inoculated in 3 ml of YPD or uracil-deficient minimal medium. These precultures were grown overnight and subsequently diluted to OD600 of 0.1 in 50 ml of the corresponding medium. These cultures were kept at 30°C on an orbital shaker (220 rpm) and every 2 to 3 hours OD600 was measured. No difference in growth characteristics was observed between the heterozygous and homozygous AQY1 deletion strain in both conditions (Fig. 1B).
To confirm the connection between the lower freeze tolerance and the deletion of AQY1, the gene was reintroduced in the AQY1 homozygous deletion strain. First, an ura- homozygous diploid deletion strain MS1 (aqy1Δ/aqy1Δ ura) was isolated by streaking out the Ura+ JC0188 strain on 5-fluoroorotic acid plates (uracil-deficient plates with 50 mg/liter uridine and 1 g/liter 5-fluoroorotic acid, Sigma, St. Louis, MO). Loss of the URA3 gene due to recombination with the flanking hisG repeats was confirmed by Southern analysis (results not shown).
The PCR-amplified C. albicans AQY1 open reading frame, generated by using BamHI-linearized plasmid pXβG-ev1/CaAQY1 (12) as the template, was cloned into pCaEx (13) downstream of the MET3 promoter by using standard cloning methods (25), resulting in plasmid pCaEx/CaAQY1. The correct orientation and the absence of mutations were verified by sequence analysis. BstBI-linearized pCaEx and pCaEx/CaAQY1 were transformed into MS1. Ura+ transformants were selected on uracil-deficient plates, resulting in strains MS2 (aqy1Δ/aqy1Δ+pCaEx) and MS3 (aqy1Δ/aqy1Δ+pCaEx/CaAQY1), respectively. Integration and orientation at the RP10 locus were verified by Southern analysis (results not shown).
Freeze tolerance of both the control strain (MS2, aqy1Δ/aqy1Δ+ pCaEx) and the C. albicans AQY1 reintroduced strain (MS3, aqy1Δ/aqy1Δ+pCaEx/CaAQY1) was tested as described above. To generate different expression levels of AQY1 in strains harboring this gene behind the regulatable MET3 promoter (13), the strains were grown overnight in uracil- and methionine-deficient minimal medium: 6.67 g/liter yeast nitrogen base without amino acids (Bio101), 0.73 g/liter complete supplement mixture minus uracil minus methionine (Bio101), and 2% glucose supplemented with cysteine and/or methionine. Partial repression was obtained by addition of 0.1 mM cysteine (expression reduced to about 50%) or 0.1 mM methionine (expression reduced to about 30%) and full repression by addition of 0.5 mM cysteine and 0.5 mM methionine (13). In the morning, the cultures were diluted 100-fold in 200 ml of the corresponding medium and grown for another 6 hours to ensure homogeneity of expression in the population of cells (13).
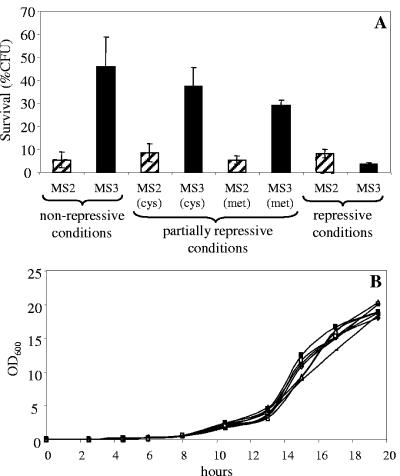
The anticipated C. albicans AQY1 expression levels (13) and freeze tolerance are correlated, and with increasingly repressive conditions for C. albicans AQY1 the freeze tolerance of the cells drops further (Fig. 2A). From the data it cannot be concluded that the complementation does not restore the high survival levels of the heterozygous diploid aqy1 deletion strain JC0186 completely (Fig. 1A) since in MS3 AQY1 is overexpressed in JC0188-background and from the MET3 promoter. Moreover, although both JC0186 and MS3-strains are Ura+, this has been accomplished at a different locus: in case of JC0186 at the AQY1 locus, in case of MS3 at the RP10 locus. No difference in growth characteristics was noticed between strains MS2 and MS3 (Fig. 2B). Taken together, these results demonstrate that the aquaporin-encoding gene AQY1 is a determinant of C. albicans freeze tolerance and are consistent with previous observations in S. cerevisiae (28).
FIG. 2.
(A) Freeze tolerance of C. albicans MS3 (aqy1Δ/aqy1Δ+pCaEx/CaAQY1) harboring a reintegrated copy of C. albicans AQY1 behind the regulatable MET3 promoter and C. albicans MS2 (aqy1Δ/aqy1Δ+pCaEx) having integrated the corresponding empty plasmid. Cells were grown overnight in nonrepressive (NR, uracil- and methionine-deficient minimal medium), partially repressive (PR, minimal medium containing 0.1 mM cysteine or 0.1 mM methionine) or fully repressive (R, minimal medium containing 0.5 mM cysteine and 0.5 mM methionine) conditions for the MET3 promoter, and were frozen for 1 day at −30°C. Survival of frozen cells compared to nonfrozen cells (cooled on ice) is expressed as % CFU. Experiments were repeated at least three times, and mean values and standard deviations are depicted. (B) Growth of MS3 (open symbols, short stripes) and MS2 (solid symbols, long stripes) in nonrepressive (triangles), partially repressive (0.1 mM cysteine [diamonds] or 0.1 mM methionine [squares]), and fully repressive (stripes) conditions for the MET3 promoter. Experiments were repeated three times, and the standard error associated with each point was always <2% of the value of the point.
Many studies of C. albicans are related to its pathogenicity and the search for antifungal drugs (15). In pathogenic bacteria aquaporin-like proteins are required for the expression of specific surface antigens that are necessary for virulence (8). In C. albicans the virulence of aquaporin deletion strains was unchanged in a murine model for systemic candidiasis (12). The unchanged pathogenicity of these C. albicans null mutants may result from unchanged cell surface hydrophobicity, flocculation, cell aggregation, invasive growth, and pseudohyphal formation; in S. cerevisiae, similar mutants are unchanged only in pseudohyphal formation. As several pathogenic microorganisms lack MIP channels altogether (8) and since aquaporins are ubiquitous in human cells (14), aquaporins are very unlikely to be useful antifungal targets.
The primary habitat of C. albicans is the skin, oral cavity, gastrointestinal tract, and vagina of warm-blooded animals, including humans, where this yeast usually lives as a commensal and only rarely as a pathogenic organism. Unlike most other Candida species, including those of medical importance, C. albicans is not commonly found in soil, plants, foods, and forages (5). C. albicans has been reported occasionally from clinical environments (19, 24), the air (10, 30), lemons (22), dust and litter from chicken stalls (29), beach sand (2), and seawater (3). There is no clear selective advantage in these environments for strains that are freeze stress tolerant, and there is no obvious reason for the retention of aquaporin function in the genome. Nonetheless, there is a clear correlation between the freeze resistance of this yeast and the presence of water channel-encoding genes. Alternatively, this commensal organism could be equipped to survive outside its presently known habitat where freeze tolerance might be an important selectable trait. Aquaporin-mediated improvement of yeast freeze tolerance is restricted to rapid freezing conditions (27), which could limit the physiological importance of aquaporin-mediated freeze resistance of yeast even further.
In this study we demonstrated that the aquaporin-encoding gene AQY1 is a determinant of freeze tolerance in C. albicans. Deletion of the gene causes a freeze-sensitive phenotype that can be remedied by reintroduction of the C. albicans AQY1 gene. This report is the first of a clear phenotype for a C. albicans AQY1 deletion strain, and strengthens the correlation between aquaporin function and freeze tolerance in microorganisms. It raises questions regarding the role that aquaporin-mediated water transport might play in freeze stress survival in natural microbial environments. A function for aquaporins in the known natural environments from which C. albicans has been recovered is, however, not obvious.
Acknowledgments
This work was supported by a fellowship from the Institute for Scientific and Technological Research (IWT) and the Katholieke Universiteit Leuven (PDM/02/114) to A.T. and by the Fund for Scientific Research—Flanders and the Research Fund of the Katholieke Universiteit Leuven (Concerted Research Actions) to P.V.D. and J.M.T., respectively.
We thank M. Swinnen for technical assistance, B. Prior and G. Kayingo for carefully reading the manuscript, and P. Sudbery for providing the pCaEx plasmid.
REFERENCES
- 1.Agre, P., and D. Kozono. 2003. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 555:72-78. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. H. 1979. In vitro survival of human pathogenic fungi in Hawaiian beach sand. Sabouraudia 17:13-22. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. H. 1979. In vitro survival of human pathogenic fungi in seawater. Sabouraudia 17:1-12. [PubMed] [Google Scholar]
- 4.Baker, M. E., and M. H. Saier. 1990. Common ancestor for bovine lens fiber major intrinsic protein, soy bean nodulin-26 protein, and E. coli glycerol facilitator. Cell 60:185-186. [DOI] [PubMed] [Google Scholar]
- 5.Blaschke-Hellmessen, R. 1999. Habitats for Candida in medical and hygienic respects. Mycoses 42:22-29. [In German.] [DOI] [PubMed] [Google Scholar]
- 6.Bonhivers, M., J. M. Carbrey, S. J. Gould, and P. Agre. 1998. Aquaporins in Saccharomyces-Genetic and functional distinctions between laboratory and wild-type strains. J. Biol. Chem. 273:27565-27572. [DOI] [PubMed] [Google Scholar]
- 7.Borgnia, M., S. Nielsen, A. Engel, and P. Agre. 1999. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 68:425-458. [DOI] [PubMed] [Google Scholar]
- 8.Calamita, G. 2000. The Escherichia coli aquaporin-Z water channel. Mol. Microbiol. 37:254-262. [DOI] [PubMed] [Google Scholar]
- 9.Calamita, G. 2000. Understanding microbial MIP channels. Trends Microbiol. 8:104-105. [DOI] [PubMed] [Google Scholar]
- 10.Calvo, M. A., J. Guarro, G. Suarez, and C. Ramirez. 1980. Airborne fungi in the air of Barcelona, Spain. V. The yeasts. Ann. Allergy 45:115-116. [PubMed] [Google Scholar]
- 11.Carbrey, J. M., M. Bonhivers, J. D. Boeke, and P. Agre. 2001. Aquaporins in Saccharomyces: characterization of a second functional water channel protein. Proc. Natl. Acad. Sci. USA 98:1000-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbrey, J. M., B. P. Cormack, and P. Agre. 2001. Aquaporin in Candida: characterization of a functional water channel protein. Yeast 18:1391-1396. [DOI] [PubMed] [Google Scholar]
- 13.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1990. The MET3 promotor: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792-798. [DOI] [PubMed] [Google Scholar]
- 14.Castle, N. A. 2005. Aquaporins as targets for drug discovery. Drug Discov. Today 10:485-493. [DOI] [PubMed] [Google Scholar]
- 15.De Backer, M. D., and P. Van Dijck. 2003. Progress in functional genomics approaches to antifungal drug target discovery. Trends Microbiol. 11:470-478. [DOI] [PubMed] [Google Scholar]
- 16.Hill, A. E., B. Shachar-Hill, and Y. Shachar-Hill. 2004. What are aquaporins for? J. Membrane Biol. 197:1-32. [DOI] [PubMed] [Google Scholar]
- 17.Hohmann, S., R. M. Bill, G. Kayingo, and B. A. Prior. 2000. Microbial MIP channels. Trends Microbiol. 8:33-38. [DOI] [PubMed] [Google Scholar]
- 18.Kayingo, G., R. M. Bill, G. Calamita, S. Hohmann, and B. A. Prior. 2001. Microbial water channels and glycerol facilitators, p. 335-380. In S. Hohmann, S. Nielsen, and P. Agre (ed.), Aquaporins. Academic Press, New York, N.Y.
- 19.Mahayni, R., J. A. Vazquez, and J. Zervos. 1995. Nosocomial candidiasis: epidemiology and drug resistance. Infect. Agents Dis. 4:248-253. [PubMed] [Google Scholar]
- 20.Maurel, C., H. Javot, V. Lauvergeat, P. Gerbeau, C. Tournaire, V. Santoni, and J. Heyes. 2002. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. 215:105-148. [DOI] [PubMed] [Google Scholar]
- 21.Meyrial, V., V. Laizé, R. Gobin, P. Ripoche, S. Hohmann, and F. Tacnet. 2001. Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur. J. Biochem. 268:334-343. [DOI] [PubMed] [Google Scholar]
- 22.Newton-John, H. F., K. Wise, and D. F. Looke. 1984. Role of the lemon in disseminated candidiasis of heroin abusers. Med. J. Aust. 140:780-781. [DOI] [PubMed] [Google Scholar]
- 23.Park, J. H., and M. H. Saier Jr. 1996. Phylogenetic characterization of the MIP family of transmembrane channel proteins. J. Membr. Biol. 153:171-180. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22:89-94. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sidoux-Walter, F., N. Pettersson, and S. Hohmann. 2004. The Saccharomyces cerevisiae aquaporin Aqy1 is involved in sporulation. Proc. Natl. Acad. Sci. USA 101:17422-17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanghe, A., P. Van Dijck, D. Colavizza, and J. M. Thevelein. 2004. Aquaporin-mediated improvement of yeast freeze tolerance is restricted to rapid freezing conditions. Appl. Environ. Microbiol. 70:3377-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanghe, A., P. Van Dijck, F. Dumortier, A. Teunissen, S. Hohmann, and J. M. Thevelein. 2002. Aquaporin expression correlates with freeze tolerance in yeast and overexpression improves freeze tolerance in industrial yeast. Appl. Environ. Microbiol. 68:5981-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vissiennon, T. 1999. Fungal flora in chicken stalls and its etiopathogenic importance for humans and animals. Berl. Munch. Tierarztl. Wochenschr. 112:104-107. [In German.] [PubMed]
- 30.Wolf, D. G., I. Polacheck, C. Block, C. L. Sprung, M. Muggia-Sullam, Y. G. Wolf, A. Oppenheim-Eden, A. Rivkind, and M. Shapiro. 2000. High rate of candidemia in patients sustaining injuries in a bomb blast at a marketplace: a possible environmental source. Clin. Infect. Dis. 31:712-716. [DOI] [PubMed] [Google Scholar]