Abstract
Lignans are dietary diphenolic compounds which require activation by intestinal bacteria to exert possible beneficial health effects. The intestinal ecosystem plays a crucial role in lignan metabolism, but the organisms involved are poorly described. To characterize the bacterial communities responsible for secoisolariciresinol (SECO) activation, i.e., the communities that produce the enterolignans enterodiol (ED) and enterolactone (EL), a study with 24 human subjects was undertaken. SECO activation was detected in all tested fecal samples. The intestinal bacteria involved in ED production were part of the dominant microbiota (6 × 108 CFU g−1), as revealed by most-probable-number enumerations. Conversely, organisms that catalyzed the formation of EL occurred at a mean concentration of approximately 3 × 105 CFU g−1. Women tended to have higher concentrations of both ED- and EL-producing organisms than men. Significantly larger amounts of EL were produced by fecal dilutions from individuals with moderate to high concentrations of EL-producing bacteria. Two organisms able to demethylate and dehydroxylate SECO were isolated from human feces. Based on 16S rRNA gene sequence analyses, they were named Peptostreptococcus productus SECO-Mt75m3 and Eggerthella lenta SECO-Mt75m2. A new 16S rRNA-targeted oligonucleotide probe specific for P. productus and related species was designed and further used in fluorescent in situ hybridization experiments, along with five additional group-specific probes. Significantly higher proportions of P. productus and related species (P = 0.012), as well as bacteria belonging to the Atopobium group (P = 0.035), were typical of individuals with moderate to high concentrations of EL-producing communities.
Phytoestrogens are estrogen-like compounds that are found in plants and essentially comprise two families, the flavonoids and the lignans (14). Secoisolariciresinol (SECO) is one of the most abundant dietary lignans. It occurs predominantly in a glycosylated form in food products (33). While flaxseed and rye are the major sources of SECO, a wide variety of other food items also contain significant concentrations, illustrating the relevance of SECO as a component of standard Western diets (35, 53).
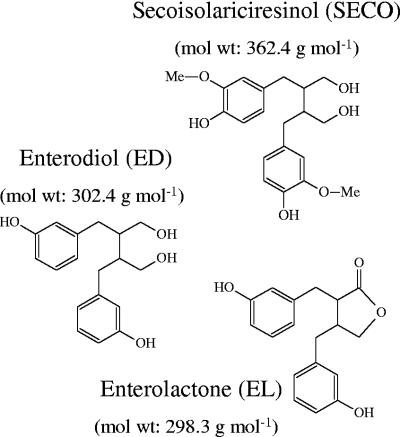
Dietary lignans, particularly SECO, are of interest because they have been proposed to play a role in the prevention of breast and colon cancer (52), atherosclerosis (42), and diabetes (39, 41). However, plant lignans per se are devoid of any biological properties. Only the enterolignans enterodiol (ED) and enterolactone (EL), produced from SECO by intestinal bacteria, have interesting biological properties such as estrogen agonism and antagonism (36, 48, 54) as well as antioxidative and enzyme-inhibiting activities (40, 55). Transformation reactions catalyzed by intestinal bacteria include the demethylation, dehydroxylation, and dehydrogenation of SECO (4, 5, 56) (Fig. 1).
FIG. 1.
Structure of SECO and the enterolignans.
Although intestinal bacteria are crucial for any health effects of dietary lignans (6, 50), the bacterial activation of SECO and the underlying mechanisms are still poorly understood. An early study on the in vitro metabolism of SECO by human fecal microbiota suggested that enterolignans were produced under both anaerobic and aerobic conditions by organisms that occurred at concentrations of approximately 103 to 104 cells per gram of wet feces (5). Recently, Wang and coworkers isolated two strict anaerobes, Peptostreptococcus sp. strain SDG-1 and Eubacterium sp. strain SDG-2, which catalyzed the demethylation and dehydroxylation of SECO, respectively (56). Thus, more work is required to better understand the intestinal production of enterolignans. Some of the main issues are as follows. Under which conditions does the activation of SECO take place in vitro? What is the occurrence of the organisms that carry out at least one of the aforementioned reactions? To what extent do they contribute to enterolignan production? To which bacterial groups may these organisms belong? Can they be isolated?
Therefore, the purpose of the present study was to better understand the microbial ecology of SECO activation in the human intestinal tract, i.e., to describe the consortium of organisms that produce the enterolignans ED and EL. We enumerated ED- and EL-producing fecal communities by the most probable number (MPN) method and looked for possible correlations with enterolignan production and dominant bacterial groups. In addition, we focused on the isolation of pure bacterial strains able to activate SECO.
MATERIALS AND METHODS
Chemicals.
Standards for ED and EL were purchased from VTT Technical Research Centre of Finland (Espoo, Finland). SECO was purchased from Sigma-Aldrich (Taufkirchen, Germany). These compounds were racemic mixtures.
Human volunteers.
Twenty-four healthy German and French adults (23 to 59 years old) gave their informed consent to take part in the MPN study. They did not take antibiotics for 3 months prior to the study. Seven additional individuals were tested to determine the prevalence of SECO activation, i.e., the proportion of individuals capable of producing enterolignans.
Collection and processing of fecal samples.
Fecal samples were collected in plastic boxes, kept under anoxic conditions using an AnaeroGen Compact (Oxoid, Hampshire, England), and stored at 4°C for a maximum of 4 h before processing. For MPN enumeration, dilutions ranging from 10−1 to 10−10 were prepared at room temperature in an anaerobic tent (Coy Laboratory Products), using sterile phosphate-buffer saline (PBS-a; 8.5 g liter−1 NaCl, 0.3 g liter−1 KH2PO4, 0.6 g liter−1 Na2HPO4, pH 7.0) containing 0.1 g liter−1 peptone and 0.25 g liter−1 cysteine-HCl (PC/PBS-a). Briefly, 1 g of fresh fecal aliquots was added to 9 ml PC/PBS-a, mixed well with sterile plastic loops, and vortexed to obtain homogenized 10−1 fecal dilutions. Samples were left to stand for 5 min, and volumes of 1 ml were transferred to 9 ml PC/PBS-a (10−2 fecal dilutions). Successive transfers were performed using sterile tips to obtain the desired range of dilutions. Before each transfer, samples were mixed by repeated inversions of the tubes. For each sample, two 1-g aliquots of fresh feces were lyophilized to estimate the fecal water content. For fluorescent in situ hybridization (FISH), 1-g fecal aliquots were fixed with paraformaldehyde (PFA). Briefly, the aliquots were suspended in 9 ml of PBS-b (7.54 g liter−1 NaCl, 2.5 g liter−1 Na2HPO4, 0.47 g liter−1 NaH2PO4, pH 7.2), and the suspensions were homogenized for 5 min with a magnetic stirrer. Volumes of 0.2 ml were added to 0.6 ml of a 4% PFA solution (Electron Microscopy Sciences) in PBS-b and fixed overnight at 4°C. Aliquots were stored at −80°C until analysis.
Culture conditions for incubation of SECO with fecal dilutions.
The liquid medium Mt-6 contained the following reagents per liter: 3 g yeast extract, 3 g peptone from casein, 2.5 g sodium acetate trihydrate, 2.5 g sodium formate, 0.5 g cysteine-HCl monohydrate, 100 ml salt 1 solution, 50 ml rumen fluid, 2 ml salt 2 solution, 1 ml vitamin solution (13), 1 ml resazurin solution (1 mg ml−1), and 0.1 ml trace element solution. Salt 1 solution was 48 mM NaHCO3, 17 mM NaCl, 12 mM NH4Cl, 2.2 mM KH2PO4, 1.7 mM K2HPO4, and 1.2 mM MgSO4 in H2O. Salt 2 solution was 540 μM MnSO4, 475 μM CaCl2, 360 μM FeSO4, 260 μM ZnCl2, 225 μM (NH4)2SO4, and 160 μM CoCl2 in H2O. Trace element solution was 12.5 μM CuSO4, 8 μM NiCl2, and 8 μM MoNa2O4 in H2O. For collection of the rumen fluid, an oral stomach tube (19) was connected to a suction pump, and the rumen fluid was collected from a healthy cow in a sterile culture bottle. Aliquots were centrifuged (100,000 × g for 30 min) at room temperature, sterilely filtered (0.22 μm), and kept at 4°C. SECO dissolved in methanol was added to a final concentration of 1 mM. The final concentration of methanol was <2% (vol/vol). The pH was adjusted to 7.5, and the medium was gassed with 80% N2 plus 20% CO2 (vol/vol) and autoclaved at 121°C for 15 min. After autoclaving, glucose and fructose were added from N2/CO2-gassed sterile stock solutions to a final concentration of 10 mM each.
MPN experiments.
The sterile SECO-containing broth Mt-6 was dispensed into the 1.2 ml-deep wells of a 96-well plate (250 μl per well) in an anaerobic tent. Fecal dilutions (100 μl) were each inoculated in triplicate into wells. Controls consisted of fecal bacteria in medium without SECO and Mt-6 without bacteria. The controls were done in triplicate. Plates were incubated at 37°C under N2/CO2 in an anaerobic jar pressurized at 1.5 × 105 Pa and placed on a rotary shaker (150 rpm). After 48 h of growth, plates were centrifuged at 4,000 × g for 15 min, and the supernatants were analyzed by liquid chromatography.
High-performance liquid chromatography (HPLC).
Separation was carried out with an RP-18 column (Lichrocart Lichrospher 100; 250 mm by 4 mm by 5 μm; Merck, Darmstadt, Germany) protected with a guard RP-18 column (4 mm by 4 mm by 5 μm) and maintained at 37°C. The eluents were as follows: A, 85% H2O plus 15% methanol (vol/vol), adjusted to pH 3 with 98% formic acid; and B, methanol. The gradient was 20 to 100% B within 8 min, 100% B for 1 min, and back to 20% B for 5 min. The dwell volume of the system was 4.4 ml, the flow rate was 1 ml min−1, and the injection volume was 20 μl. Lignans were detected at 275 nm using a UV diode array detector. Chromeleon software, version 6.40 (Dionex, Idstein, Germany), and the Millenium32 Chromatography Manager (Waters, Milford, Massachusetts) were used for data acquisition and analysis of German and French samples, respectively. To quantify SECO, ED, and EL, calibration curves were obtained with standards using the following concentrations: 1,500 μM, 1,000 μM, 500 μM, 250 μM, 100 μM, 50 μM, and 10 μM. The solvent was methanol, and each concentration was prepared in duplicate.
Procedure for MPN enumeration.
A well was considered positive if the corresponding dilution of the given fecal sample produced enterolignans. This was the case if (i) we observed a significant decrease in the concentration of SECO, (ii) we detected peaks at the retention times of the enterolignans (8.3 and 8.7 min for ED and EL, respectively), (iii) these peaks corresponded to concentrations of ED or EL above 10 μM, and (iv) they displayed the specific spectra obtained with standards. MPN results were calculated using a table based on three replicates (11). They were adjusted according to fecal water contents and dilution factors and expressed either as CFU per gram of dried feces (CFU g−1) or as logarithmic values thereof.
EL and ED production levels.
From the MPN data for the German samples (n = 13), chromatograms obtained with the 10−1 fecal dilutions were used to estimate the production of EL and ED. The parameter λ was defined as the ratio between the final concentration of EL and the initial concentration of SECO. The parameter δ was defined as the ratio between the final concentration of both enterolignans (ED plus EL) and the initial concentration of SECO. Since each sample was analyzed in triplicate, λ and δ were expressed as mean values.
Aerobic incubations.
Six German samples (MPN 2, 6, 13, 14, 15, and 17) were tested for aerobic conversion of SECO. A volume of 900 μl of Mt-6 was inoculated with 100 μl of the 10−1 fecal dilutions. Samples were incubated at 37°C in Eppendorf tubes equipped with membrane lids (Eppendorf LidBAC; Eppendorf, Hamburg, Germany) and placed on a rotary shaker (150 rpm). Supernatants were analyzed by HPLC before incubation and after 48 h. Controls consisted of Mt-6 without fecal bacteria, fecal bacteria in medium without SECO, and fecal bacteria in Mt-6 incubated under anoxic conditions.
Isolation of SECO-activating bacteria.
All steps were carried out using strictly anaerobic techniques (7, 9). Serial dilutions of a fecal sample from a healthy male adult (MPN 14) were prepared as described above and plated onto Mt-6 supplemented with 14 g liter−1 agar (Serva, Heidelberg, Germany). After 48 h of growth at 37°C, a mixed culture of bacteria obtained from the initial 10−5 fecal dilution produced ED. After several transfers in Mt-6, serial dilutions of the mixed culture were plated onto the selective medium Mt-75, which contained the following reagents (per liter): 10 g agar, 2 g NaHCO3, 1.25 g sodium acetate trihydrate, 1.25 g sodium formate, 0.5 g cysteine-HCl monohydrate, 0.1 g yeast extract, 100 ml of 10-fold concentrated basal solution (13), 20 ml trace element solution (13), 2.5 ml rumen fluid, 1 ml vitamin solution (13), and 1 ml resazurin solution (1 mg ml−1). SECO was added as described previously. The pH was adjusted to 7.2, and the medium was autoclaved at 121°C for 15 min. The medium was allowed to cool down to 45°C, and sterilely filtered bromoethanesulfonate solved in H2O was added to a final concentration of 4 mM to prevent the growth of methanogens. Once poured into petri dishes and allowed to dry under sterile conditions, the medium was transferred into an anaerobic tent and allowed to equilibrate for 48 h before inoculation. After 95 h of growth at 37°C under 80% H2 plus 20% CO2 (vol/vol) in an anaerobic jar pressurized at 1.5 × 105 Pa, pure cultures were obtained from the 10−4 dilution of the mixed culture and screened for conversion of SECO. To ensure purity, active organisms were streaked two times on peptone-yeast-glucose (PYG) agar (medium 104; DSMZ, Braunschweig, Germany) before further analyses. The purity was controlled by comparisons of colony morphology and Gram stains. These were confirmed by means of the KOH test (20). The cultures were grown under aerobic conditions on PYG agar to look for aerobic contaminants.
Molecular identification of active isolates.
DNAs were extracted with an Invisorb Genomic DNA kit III (Invitek, Berlin-Buch, Germany) following the manufacturer's instructions. Primers 27f (5′ AGA GTT TGA TCC TGG CTC AG) and 1492r (5′ TAC CTT GTT ACG ACT T) (27) were used to amplify the bacterial 16S rRNA gene. PCR mixes (50 μl) contained 50 mM KCl, 20 mM Tris-HCl, 1 mM MgCl2, a 0.25 mM concentration of each deoxynucleoside triphosphate, a 1 μM concentration of each primer, 2.5 units Taq DNA polymerase (Invitrogen, Karlsruhe, Germany), and 1 μl of a 10−1 dilution of template DNA. The PCR program was as follows: 94°C for 5 min, 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and finally 72°C for 10 min. PCR products were purified with a High Pure PCR product purification kit (Roche, Indianapolis, Ind.) following the manufacturer's instructions. The products were analyzed by electrophoresis on a 1.5% agarose gel (wt/vol) in Tris-borate-EDTA buffer (Roth, Karlsruhe, Germany). The DNA concentration was estimated using a Low DNA mass ladder (Invitrogen, Carlsbad, Calif.). For sequencing, we used either primer 27f, 338f (5′ GCT GCC TCC CGT AGG AGT) (3), 338r (5′ ACT CCT ACG GGA GGC AGC), or 1492r. Sequencing reactions were performed in duplicate with a DYEnamic ET Dye Terminator cycle sequencing kit (Amersham Biosciences, Buckinghamshire, England) following the manufacturer's instructions. Sequencing products were analyzed with the MegaBACE 1000 system (Molecular Dynamics, Sunnyvale, Calif.). Sequences were assembled and manually adjusted using the ContigExpress function of the Vector NTI suite 9.0.0 (Invitrogen, Carlsbad, Calif.). They were subsequently aligned with highly similar sequences (92% similarity or more) obtained with the BLAST function of the National Center for Biotechnology Information (NCBI) server (2). Percentages of similarity were calculated from unambiguously aligned sequences using the sequence identity matrix function of Bioedit software, version 5.0.9 (22).
Growth and fixation of pure cultures.
Bacteria were streaked three times on PYG agar, and purity was controlled as described for the isolates. Pure cultures were grown in 9 ml PYG broth and harvested in the exponential growth phase to maximize the rRNA content. Cells were centrifuged at 8,000 × g for 3 min and resuspended in 1 ml PBS-b. A volume of 0.2 ml was added to 0.6 ml of 4% PFA solution and fixed for 3 h at 4°C. Aliquots were stored at −80°C until analysis.
Design and optimization of species-specific 16S rRNA oligonucleotide probe.
Sequences of organisms closely related to Peptostreptococcus productus were obtained from the GenBank database. They were aligned using Bioedit software and screened for P. productus-specific regions. Probes targeting these regions were controlled using the probe match and BLAST functions of the Ribosomal Database Project (10) and the NCBI, respectively. They were further tested by FISH on reference strains to establish their in vitro specificity. Relative probe fluorescence was determined as described previously (44).
FISH and flow cytometry (FC) analyses.
The specific probes used in this study are listed in Table 1. The EUB-338-5′ probe (GCT GCC TCC CGT AGG AGT), conserved within the bacterial domain (3), and the NON-EUB-5′ probe (ACA TCC TAC GGG AGG C) were used as positive and negative controls, respectively. The labeling quality of probes was controlled as described previously (18). Fixed bacterial suspensions were hybridized as described previously (43). For fecal samples, 200 μl of the PFA-fixed suspension was added to 800 μl of PBS-b. For pure cultures, 200 to 800 μl of PFA-fixed suspension was used, depending on the bacterial concentration before fixation. Data acquisition was performed as described previously (43). Cell granularity was measured with the side scatter channel and assigned as the primary acquisition parameter. The voltage setting of the corresponding photomultiplier tube was 458 V, with a threshold of 253 V. The multiplying factor of the photodiode used for the detection of forward scatter signals was 102. Voltage settings for FL1 and FL4 fluorescence were 649 and 800 V, respectively. All amplifiers were set to logarithmic mode.
TABLE 1.
16S rRNA probes used in FISH experiments for enumeration of fecal bacteria
| Target | Probe name | Sequence (5′-3′) | Reference |
|---|---|---|---|
| C. coccoides-E. rectale cluster | S-*-Erec-0482-a-A-19 | GCT TCT TAG TCA RGT ACC G | 16 |
| C. leptum subgroup | S-*-Clept-0866-a-A-18 | GGT GGA TWA CTT ATT GTG | 32 |
| Clept-0866 competitor 1 | GGT GGA AWA CTT ATT GTG | 32 | |
| Clept-0866 competitor 2 | GGT GGA TWA CTT ATT GCG | 32 | |
| Bacteroides and relatives | S-*-Bacto-303-a-A-17 | CCA ATG TGG GGG ACC TT | 34 |
| Atopobium group | S-*-Ato-0291-a-A-17 | GGT CGG TCT CTC AAC CC | 23 |
| Bifidobacterium spp. | S-G-Bif-0164-a-A-18 | CAT CCG GCA TTA CCA CCC | 30 |
| P. productus and C. coccoides | S-*-ProCo-1264-a-A-24 | TTG GGA TTC GCT CAA CAT CGC TG | This study |
Enumeration of fecal bacterial groups.
Proportions of bacterial groups or species were obtained by the combined use of fluorescein isothiocyanate (FITC)-labeled EUB-338 and Cy5-labeled group- or species-specific probes. Dot plots of the FITC-labeled NON-EUB and EUB-338 controls were used to determine the percentage of hybridization, i.e., the proportion of acquired events with specific bacterial green fluorescence. Thereby, a specific gate that includes the total number of bacteria was defined for each fecal sample. This gate was subsequently applied to the analysis of every FITC-Cy5 double-labeled aliquot of the corresponding sample to exclude autofluorescence events. Four samples were excluded from statistical analyses because the percentages of hybridization were below 35%, i.e., the proportions of autofluorescence events were too high to ensure reliable counting. Bacterial proportions were determined as the ratio of specific red fluorescence events to bacterial green fluorescence events. These percentages were corrected by subtraction of the background fluorescence obtained by concomitant hybridization with Cy5-labeled NON-EUB and FITC-labeled EUB-338 (i.e., the percentage of red autofluorescence within bacterial green fluorescence events). Samples were analyzed in duplicate, and the percentages expressed are the means. Analyses were repeated if the coefficient of variation was above 10%.
Statistical analyses.
Data are expressed as means ± standard deviations (SD). SD were determined as follows: SD = [Σ(xi − mean)2/n]1/2, where xi is the given value of sample i and n is the total number of samples. For the MPN data, analyses were performed on logarithmic values. The Shapiro-Wilk test was used to ensure normal distributions. Variances were checked for their uniformity by means of F tests. Unless specified, P values were obtained by two-tailed homoscedastic Student tests. Chi-square analyses were performed to compare the bacterial composition results with data from previous studies. Pearson's correlation coefficients were calculated using version 11.5 of SPSS software (SPSS Inc., Chicago, Ill.).
RESULTS
Quantification of anaerobic SECO-activating bacterial communities.
Enterolignans were detected after anoxic incubation of SECO with 10-fold dilutions of fecal samples from all individuals (n = 31). EL production was not detectable in one male subject (MPN 16) (Table 2). Conversion of SECO was not observed under aerobic conditions (n = 6).
TABLE 2.
MPN values, enterolignan production, bacterial compositions, and personal parameters for the whole cohort of individuals (n = 24)b
| Subject | Enterolignan-producing communities log10 (CFU g−1 dried feces)
|
Enterolignan production (%)a
|
Dominant fecal microbiota composition (% of total bacteria)
|
Sex | Age (yr) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELmpn | EDmpn | λ | δ | Erec | ProCo | Clept | Bac | Ato | Bif | |||
| 1 | 5.5 | 8.6 | 13.7 | 42.9 | Excludedc | F | 23 | |||||
| 2 | 6.7 | 9.9 | 21.8 | 56.4 | 21.2 | 0.8 | 33.2 | 6.2 | 0.7 | 0.0 | F | 59 |
| 3 | 6.7 | 6.7 | 25.3 | 93.1 | 34.0 | 0.6 | 28.0 | 10.4 | 0.4 | 1.8 | F | 36 |
| 4 | 2.7 | 11.6 | 0.0 | 59.6 | 33.8 | 0.1 | 21.9 | 14.7 | 0.0 | 0.9 | F | 32 |
| 5 | 6.5 | 11.1 | 23.7 | 51.6 | 3.5 | 0.7 | 33.4 | 1.4 | 0.2 | 5.4 | F | 23 |
| 6 | 6.5 | 10.0 | 17.7 | 53.1 | 20.4 | 1.1 | 27.0 | 7.0 | 4.4 | 0.0 | F | 24 |
| 7 | 6.1 | 11.5 | 22.6 | 49.2 | Excludedc | F | 28 | |||||
| 8 | 3.9 | 7.7 | 10.7 | 60.9 | 27.5 | 0.7 | 22.9 | 15.3 | 0.5 | 2.8 | F | 48 |
| 9 | 7.9 | 7.9 | ND | ND | 23.1 | 0.3 | 4.6 | 46.9 | 11.0 | 2.1 | F | 54 |
| 10 | 7.7 | 8.8 | ND | ND | 35.4 | 0.8 | 13.3 | 13.6 | 13.5 | 0.0 | F | 26 |
| 11 | 6.5 | 9.8 | ND | ND | 29.4 | 2.1 | 18.4 | 4.9 | 6.3 | 3.6 | F | 32 |
| 12 | 8.0 | 8.9 | ND | ND | 37.6 | 0.0 | 20.0 | 9.5 | 14.3 | 0.0 | F | 32 |
| 13 | 3.1 | 4.1 | 2.8 | 56.9 | 39.9 | 0.1 | 23.1 | 13.8 | 3.6 | 1.5 | M | 49 |
| 14 | 8.2 | 9.8 | 7.1 | 62.4 | 43.2 | 0.3 | 25.1 | 8.0 | 5.2 | 1.0 | M | 25 |
| 15 | 3.6 | 4.7 | 4.0 | 101.6 | 28.8 | 0.0 | 17.8 | 29.7 | 1.1 | 1.7 | M | 50 |
| 16 | 0.0 | 11.0 | 0.0 | 57.1 | 24.4 | 0.0 | 13.8 | 35.8 | 0.7 | 0.5 | M | 25 |
| 17 | 3.0 | 7.4 | 4.7 | 33.4 | 15.1 | 0.0 | 17.2 | 27.0 | 3.3 | 2.4 | M | 31 |
| 18 | 7.3 | 8.7 | ND | ND | 32.0 | 0.3 | 31.8 | 18.8 | 5.1 | 4.2 | M | 27 |
| 19 | 5.7 | 11.2 | ND | ND | 39.3 | 0.5 | 28.5 | 5.0 | 6.2 | 1.4 | M | 44 |
| 20 | 6.8 | 8.9 | ND | ND | 34.3 | 0.9 | 13.4 | 21.4 | 7.4 | 0.5 | M | 34 |
| 21 | 7.9 | 8.7 | ND | ND | 32.1 | 1.4 | 11.1 | 10.4 | 4.6 | 0.0 | M | 28 |
| 22 | 4.5 | 6.5 | ND | ND | Excludedc | M | 40 | |||||
| 23 | 4.2 | 9.2 | ND | ND | 28.1 | 0.0 | 21.8 | 8.0 | 7.6 | 3.0 | M | 33 |
| 24 | 2.1 | 7.7 | ND | ND | Excludedc | M | 34 | |||||
| Mean | 5.5 | 8.8 | 11.9 | 59.9 | 29.2 | 0.5 | 21.3 | 15.4 | 4.8 | 1.6 | 35 | |
| SD | 2.2 | 1.9 | 9.1 | 17.7 | 9.1 | 0.5 | 7.6 | 11.3 | 4.2 | 1.5 | 8.5 | |
| CV (%) | 40 | 22 | 77 | 30 | 31 | 101 | 36 | 74 | 88 | 92 | 24 | |
The parameters λ and δ characterize EL and enterolignan (ED plus EL) production, respectively, after 48 h of growth in Mt-6 medium. They are expressed as percentages of the initial SECO concentration.
Abbreviations: Ato, Atopobium group; Bac, Bacteroides and relatives; Bif, Bifidobacterium spp.; Clept, Clostridium leptum group; CV, coefficient of variation; ELmpn and EDmpn, concentration of enterolactone- or enterodiol-producing bacteria; Erec, Eubacterium rectale-Clostridium coccoides cluster; ND, not determined; ProCo, P. productus and C. coccoides.
<35% hybridization.
ED-producing bacteria occurred at a mean concentration of 6 × 108 CFU g−1 (n = 24). Organisms responsible for EL production occurred at significantly lower concentrations, with a mean value of 3 × 105 CFU g−1 (P < 0.001). Women had higher concentrations of both ED- and EL-producing organisms than did men. These gender-related differences were significant (P = 0.043 by a one-tailed Student test) for EL-producing organisms, with mean concentrations of 2 × 106 and 5 × 104 CFU g−1 for women (n = 12) and men (n = 12), respectively. From one subject with high concentrations of ED- and EL-producing communities (MPN 14), additional samples were collected after 6 months and 1 year. An analysis of the corresponding MPN enumerations gave coefficients of variation of 11% and 1% for EL and ED, respectively.
Data on enterolignan production are listed in Table 2. For the German cohort of the MPN study (n = 13), EL and enterolignan production accounted for 11.9% ± 9.1% and 59.9% ± 17.7% of the initial concentration of SECO, respectively. At 48.0% ± 19.8%, ED was produced in larger amounts than EL. There was a significant correlation between the occurrence of EL-producing communities and EL production, as follows: r (ELmpn, λ) = 0.749 (P = 0.003). Significantly larger amounts of enterolactone were produced by fecal samples from individuals with moderate to high concentrations of EL-producing bacteria (n = 7, six women and one man) than by samples from individuals with low concentrations of EL-producing bacteria (n = 6, two women and four men) (Table 3).
TABLE 3.
MPN counts and EL production (n = 13)
| Subject (gender)a | ELmpn | λb (%) |
|---|---|---|
| Individuals with low concns of EL producers | ||
| 4 (W) | 2.7 | 0.0 |
| 8 (W) | 3.9 | 10.7 |
| 13 (M) | 3.1 | 2.8 |
| 15 (M) | 3.6 | 4.0 |
| 16 (M) | 0.0 | 0.0 |
| 17 (M) | 3.0 | 4.7 |
| Mean | 2.7c | 3.7d |
| SD | 1.3 | 3.6 |
| CV (%) | 46 | 98 |
| Individuals with moderate to high concns of EL producers | ||
| 1 (W) | 5.5 | 13.7 |
| 2 (W) | 6.7 | 21.8 |
| 3 (W) | 6.7 | 25.3 |
| 5 (W) | 6.5 | 23.7 |
| 6 (W) | 6.5 | 17.7 |
| 7 (W) | 6.1 | 22.6 |
| 14 (M) | 8.2 | 7.1 |
| Mean | 6.6c | 18.8d |
| SD | 0.8 | 6.0 |
| CV (%) | 12 | 32 |
M, man; W, woman.
λ is defined as the ratio between the final concentration of EL and the initial concentration of SECO.
c,dValues with the same letters were significantly different (P < 0.001).
Marked interindividual differences were observed with regard to concentrations of SECO-converting organisms and enterolignan production.
Identification of SECO-activating bacteria.
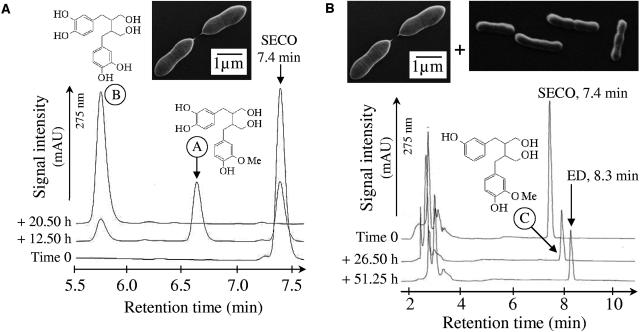
After the isolation of fecal bacteria on SECO-containing medium, we obtained a gram-positive coccobacillus which demethylated SECO under anoxic conditions (Fig. 2A). The unambiguously aligned 16S rRNA gene sequence of this organism (1,343 nucleic acids) (GenBank accession number AY937379) displayed 97.4% similarity with that of P. productus ATCC 27340T (L76595). Anoxic coincubations of the P. productus isolate with a likewise isolated pure culture of a gram-positive rod led to the production of ED from SECO (Fig. 2B). The unambiguously aligned 16S rRNA gene sequence of the latter organism (1,394 nucleic acids) (AY937380) displayed 98.2% similarity with Eggerthella lenta ATCC 25559T (AF292375) (12). The identity of this second isolate was confirmed by PCR amplification of an E. lenta-specific region using primers LEN-F2 and LEN-R2 (27) (data not shown). The E. lenta isolates alone were not able to convert SECO (data not shown).
FIG. 2.
(A) Demethylation of SECO by the isolated strain P. productus SECO-Mt75m3. Incubation took place at 37°C in Mt-6 broth. Samples were collected at the times indicated on the chromatograms. Supernatants were analyzed by HPLC as described previously. The demethylation of SECO led to the formation of compound B (5.8 min) via compound A (6.7 min). The molecular weight of A was 348 g mol−1, as determined by mass spectrometry. This weight corresponds to the molecular weight of SECO with one methyl group removed. Compound B was identified as 2,3-bis(3,4-dihydroxybenzyl)butene-1,4-diol by comparison with the retention time and spectrum of the standard. It lacks the two methyl groups of SECO. (B) Formation of ED from SECO by coculture of the isolated strains P. productus SECO-Mt75m3 and E. lenta SECO-Mt75m2. Incubation took place at 37°C in Mt-75 broth. The molecular weight of C (7.9 min) was 332 g mol−1, as determined by mass spectrometry. This weight corresponds to the molecular weight of SECO with one methyl group and one hydroxyl group removed.
Design and optimization of the species-specific probe.
An alignment of 16S rRNA sequences from 35 organisms phylogenetically related to P. productus did not allow the identification of regions suitable for the specific detection of this species. A specific region for both P. productus and Clostridium coccoides was found at position 1264 according to Escherichia coli numbering. Homologous regions revealed by probe match and BLAST analyses confirmed the in silico specificity of the corresponding probe. The probe was named S-*-ProCo-1264-a-A-24 (1). Table 4 shows the sequences of the probe and the target and nontarget organisms. Species with up to two mismatches and closely related species that are members of the human intestinal microbiota were subsequently tested using FISH-FC. Table 5 illustrates the in vitro specificity of the probe. On average, positive strains hybridized with ProCo-1264 (n = 4) provided good fluorescent signals with a relative probe fluorescence of 84.3% ± 16.6%. The P. productus isolates could be successfully detected by the newly designed probe (data not shown).
TABLE 4.
Sequences of the designed 16S rRNA specific probe and of targeted and nontargeted organisms
| Probe (OPD nomenclature)a or organism | Sequence (5′-3′) | Organism present in human feces |
|---|---|---|
| S-*-ProCo-1264-a-A-23 | TTGGGATTCGCTCAACATCGCTG | |
| Target | CAGCGATGTTGAGCGAATCCCAA | |
| Ruminococcus callidus ATCC 27760 | .........G....A........ | + |
| Clostridium clostridiiforme ATCC 25537 | ...T.....G....A........ | + |
| Ruminococcus flavefaciens ATCC 49949 | ...T.....G.........T... | |
| Peptostreptococcus ivorii DSM 10023 | ..........A...........T | |
| Eubacterium fissicatena DSM 3598 | .C.......G.........T... | |
| Eubacterium angustum ATCC 43737 | G.........C.G.......... |
OPD, Oligonucleotide Probe Database (1).
TABLE 5.
Specificity of probe ProCo-1264
| Strain | Eub-338 signal | ProCo-1264 datab
|
|
|---|---|---|---|
| RPFa(% Eub-338 signal) | % Hybridization (mean ± SD) | ||
| P. productus DSM 2950 | + | 79.9 ± 2.2 | 92.0 ± 0.3 |
| P. productus DSM 3507 | + | 81.3 ± 0.7 | 91.9 ± 0.8 |
| P. productus ATCC 27340 | + | 110.9 ± 2.5 | 90.4 ± 0.3 |
| C. coccoides ATCC 29236 | + | 65.0 ± 2.9 | 74.3 ± 6.6 |
| C. coccoides strain 3110 (28) | + | 16.2 ± 1.6 | 0 |
| R. callidus ATCC 27760 | + | 13.2 ± 1.3 | 0 |
| Ruminococcus hansenii DSM 20583 | + | 3.7 ± 0.0 | 0 |
| Ruminococcus obeum ATCC 29174 | + | 2.7 ± 0.2 | 0 |
RPF, relative probe fluorescence.
Data are means ± SD.
Fecal bacterial composition.
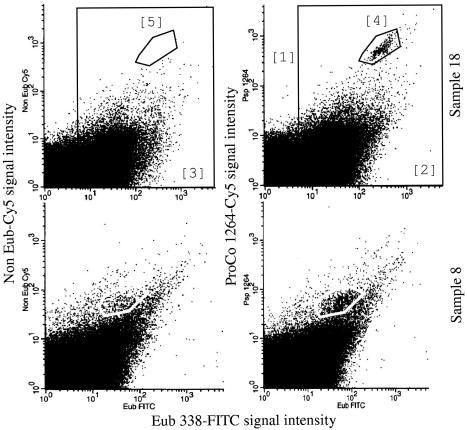
Figure 3 illustrates FISH-FC dot plots obtained with the specific probe ProCo-1264. For all subjects (n = 20), dot plot analyses gave a mean of 0.5% ± 0.5% for P. productus and C. coccoides. Percentages ranged from 0 to 2.1% of total bacteria and from 0 to 20% (2.7% ± 4.4%) of members of the Eubacterium rectale-Clostridium coccoides cluster. The prevalence of the two organisms was 75%, i.e., specific fluorescent signals were detected for 15 of 20 subjects. Table 2 shows the mean bacterial composition for all subjects (n = 20). The proportions of the Clostridium coccoides-Eubacterium rectale cluster, the Clostridium leptum subgroup, Bacteroides and relatives, the Atopobium group, and Bifidobacterium spp. were not statistically different from data obtained for 91 adults from five European countries (31). There were also no significant differences in bacterial composition between women and men. Both showed large interindividual differences.
FIG. 3.
FISH-FC dot plots for MPN samples 8 and 18 hybridized with ProCo-1264. Fluorescence intensities are displayed as logarithmic values. Regions: 1, autofluorescence events; 2 and 3, gated regions that include total bacterial events; 4, ProCo-1264-specific events; 5, specific control region. Bacterial percentages for P. productus and C. coccoides were calculated as follows: 100 × [(counts in region 4/counts in region 2) − (counts in region 5/counts in region 3)].
Indicators of enterolignan production.
We found positive correlations between concentrations of EL-producing organisms and bacterial compositions. When compared with subjects with a low concentration of EL-producing organisms (2.9 ± 1.3 cells g−1; n = 7), subjects with moderate to high concentrations of EL-producing organisms (7.1 ± 0.7 cells g−1; n = 13) had higher proportions of bacteria that belong to the Atopobium group (6.1% ± 1.4% versus 2.4% ± 2.5%; P = 0.035) and the species P. productus and C. coccoides (0.8% ± 0.5% versus 0.1% ± 0.2%; P = 0.012). Coefficients of correlation were as follows: r (EL, ProCo-1264) = 0.449 and r (EL, Ato) = 0.542 (P < 0.05).
DISCUSSION
Bacterial activation is required for dietary lignans to exert biological activities. Therefore, we investigated the occurrence and activity of SECO-activating bacterial communities in human feces.
We showed that the activation of SECO is widespread among humans. Indeed, the prevalence of enterolignan production was 100%. Thus, beneficial health effects associated with SECO activation would be relevant to the entire population and not restricted to a certain proportion of individuals as in the case of equol (45).
The human intestinal tract harbors approximately 1012 CFU per gram of content. At >108 CFU per gram of dried feces, microbes responsible for ED production occurred at relatively high population levels. Thus, organisms that demethylate and dehydroxylate SECO seem to be members of the dominant anaerobic intestinal microbiota. Conversely, organisms responsible for SECO and/or ED dehydrogenation make up a subdominant anaerobic population (approximately 105 CFU g−1). An early study of the in vitro metabolism of SECO by human fecal bacteria described the conversion of SECO to ED and EL under aerobic conditions (5). However, the authors also reported that feces from an individual treated with metronidazole could no longer convert SECO to ED, indicating the need for anaerobes for SECO activation. We could not detect enterolignan formation after aerobic incubation of SECO with fecal dilutions from six individuals. Another previous study reported results similar to ours (56). Although we cannot exclude that aerobic or facultative anaerobic microorganisms may be able to demethylate and dehydroxylate SECO, the data presented here challenge the presumption of an aerobic activation of SECO.
Our data show an association between the occurrence and activity of EL-producing communities, i.e., EL is produced in larger amounts by individuals with higher concentrations of EL-producing organisms. Our in vitro experiments also showed that ED was produced from SECO in larger amounts than EL. This is in agreement with previous in vivo data on human plasma and urine concentrations of lignans after flaxseed ingestion (37) and rat urine concentrations of lignans after SECO supplementation (47). In these studies, ED was the main metabolite produced from the ingested precursors. We found no association between MPNs and the activity of ED-producing communities. Since the concentrations of ED-producing communities were fairly homogeneous within the MPN cohort, i.e., individuals with low or high concentrations of active organisms were not as distinguishable as in the case of EL, MPN enumerations based on three replicates may not warrant the accuracy required to reveal differences within such a set of data. It is also possible that ED-producing communities are more diverse than EL-producing communities. Thus, with an assortment of active organisms that exhibit different activating capabilities, it would be difficult to directly associate the total numbers and activities of ED-producing communities. As an example, the activities measured from a fecal sample with a low concentration of ED-producing organisms, where species with high activating capabilities prevail, and from a sample with a high concentration of ED-producing communities, where other species with low activating capacities predominate, may not greatly differ. In vitro experiments might also be limited to accurately study such diverse communities. These concerns highlight the need to gain access to the active bacterial communities at the species level.
Two organisms involved in SECO activation were isolated from feces. The two organisms were required to produce ED from SECO. Our in vitro experiments indicate that demethylation occurs before dehydroxylation, as suggested earlier (56). The same author reported the isolation of Peptostreptococcus sp. strain SDG-1 and Eubacterium sp. strain SDG-2, which are capable of SECO demethylation and dehydroxylation, respectively. The molecular analyses conducted for the present study showed that our isolated SECO-demethylating and -dehydroxylating strains belong to the species P. productus and E. lenta.
A new 16S rRNA probe specific for the species P. productus and C. coccoides was designed. The detection of P. productus-like bacteria in 15 of 20 subjects by in situ hybridization using the specific probe ProCo-1264 confirms the occurrence of SECO-activating organisms within the dominant fecal microbiota. This agrees with previous data on positive PCR detection of P. productus in 10−4 to 10−7 dilutions of human fecal samples (n = 12) (57). These results, along with the MPN enumerations, show that bacteria responsible for enterolignan production occur at concentrations higher than those reported earlier (5). MPN enumerations of acetogenic bacteria in feces from two non-methane-excreting individuals revealed concentrations of 7.2 × 107 and 3.1 × 108 acetogens g−1 wet feces (15). Since P. productus belongs to the functional group of acetogenic bacteria, many of which are capable of demethylating aromatic compounds (17, 24), it is tempting to speculate that such organisms are involved in SECO activation. This also supports the occurrence of SECO-activating organisms within dominant microbial communities of the human intestine.
The interindividual variations observed for MPNs and enterolignan production are in agreement with previous reports on lignan metabolism and the diversity of intestinal bacteria (29, 46, 47, 58). Despite these variations, women tended to have higher concentrations of enterolignan-producing organisms. However, this could not be explained by quantitative differences in dominant bacterial groups. A study of enterolactone concentrations in serum and urine after a rye bread diet did not show significant differences between women and men (26). Nonetheless, over 4 weeks, women had higher serum enterolactone concentrations (39.3 ± 4.4 nmol liter−1; n = 21) than did men (28.1 ± 3.8 nmol liter−1; n = 18), in spite of significantly higher intakes of test bread and fibers by the men. Thus, even if it is difficult to appraise the effect of high concentrations of SECO-activating organisms on the bioavailability of enterolignans, our results hint at a connection between lignans and endogenous hormone metabolism (8, 21, 38, 51). Besides, concentrations of enterolignan-producing communities were relatively stable over a year in a healthy male adult without major dietary changes during that time. Since previous studies in humans showed that dietary interventions influence the blood concentration of enterolignans (25), an appealing goal is to know to what extent functional foods or specific dietary supplementations would alter the SECO-activating bacterial communities beyond the influence of endogenous factors.
High concentrations of P. productus and C. coccoides positively correlated with high concentrations of EL-producing organisms in approximately two-thirds of the cohort. The reason why a similar correlation could not be observed with ED-producing organisms remains unclear, but the isolation of an active P. productus strain indubitably supports our findings. Differences in EL production were not only reflected by differences in P. productus and C. coccoides counts. High concentrations of the Atopobium group, which includes E. lenta, also characterized individuals with high concentrations of EL-producing organisms. In the future, it might be of interest to stratify human subjects with respect to these bacterial parameters to assess the possible health effects of dietary lignans, as proposed for isoflavones (49).
In conclusion, we showed that the widespread conversion of dietary lignans results from the catalytic activities of both dominant and subdominant anaerobic bacterial communities in the human intestinal tract. The fecal microbiota composition data and the isolation of active bacteria illustrate how bacterial species interact with compounds from their environment to produce metabolites with possible health implications. To allow for an in-depth view of the microbial ecology of lignan activation and a better understanding of the involved mechanisms, we strive to identify other lignan-activating organisms and to characterize their metabolic potentials.
Acknowledgments
We gratefully acknowledge the support of Annick Bernalier-Donadille from INRA in Clermont-Ferrand/Theix, who provided the strain P. productus DSM 3507; Christophe Lay and Chantal Bridonneau from INRA in Jouy-en-Josas for their help with bacterial fixation and anaerobic culture techniques; Wolfram Engst, Pawel Namsolleck, and Martin Osterhoff from the German Institute of Human Nutrition for their assistance with mass spectrometry, scanning electron microscopy, and DNA sequencing; Masao Hattori from the Toyama Medical and Pharmaceutical University for providing (+)-2,3-bis(3,4-dihydroxybenzyl)butene-1,4-diol; and the volunteers who took part in the study.
REFERENCES
- 1.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelson, M., J. Sjövall, B. E. Gustafsson, and K. D. R. Setchell. 1982. Origin of lignans in mammals and identification of a precursor from plants. Nature 298:59-60. [DOI] [PubMed] [Google Scholar]
- 5.Borriello, S. P., K. D. Setchell, M. Axelson, and A. M. Lawson. 1985. Production and metabolism of lignans by the human faecal flora. J. Appl. Bacteriol. 58:37-43. [DOI] [PubMed] [Google Scholar]
- 6.Bowey, E., H. Adlercreutz, and I. Rowland. 2003. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem. Toxicol. 41:631-636. [DOI] [PubMed] [Google Scholar]
- 7.Breznak, J. A., and R. N. Costilow. 1994. Physicochemical factors in growth, p. 137-154. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 8.Brooks, J. D., W. E. Ward, J. E. Lewis, J. Hilditch, L. Nickell, E. Wong, and L. U. Thompson. 2004. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am. J. Clin. Nutr. 79:318-325. [DOI] [PubMed] [Google Scholar]
- 9.Bryant, M. P. 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324-1328. [DOI] [PubMed] [Google Scholar]
- 10.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Man, J. C. 1985. MPN tables, corrected. Eur. J. Appl. Microbiol. Biotechnol. 17:301-305. [Google Scholar]
- 12.Dewhirst, F. E., B. J. Paster, N. Tzellas, B. Coleman, J. Downes, D. A. Spratt, and W. G. Wade. 2001. Characterization of novel human oral isolates and cloned 16S rDNA sequences that fall in the family Coriobacteriaceae: description of Olsenella gen. nov., reclassification of Lactobacillus uli as Olsenella uli comb. nov. and description of Olsenella profusa sp. Int. J. Syst. Evol. Microbiol. 51:1797-1804. [DOI] [PubMed] [Google Scholar]
- 13.Diekert, G. 1992. The acetogenic bacteria, p. 517-533. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, Berlin, Germany.
- 14.Dixon, R. A. 2004. Phytoestrogens. Annu. Rev. Plant Biol. 55:225-261. [DOI] [PubMed] [Google Scholar]
- 15.Doré, J., B. Morvan, F. Rieu-Lesme, I. Goderel, P. Gouet, and P. Pochart. 1995. Most probable number enumeration of H2-utilizing acetogenic bacteria from the digestive tract of animals and man. FEMS Microbiol. Lett. 130:7-12. [DOI] [PubMed] [Google Scholar]
- 16.Franks, A. H., H. J. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazer, A. 1994. O-demethylation and other transformation of aromatic compounds by acetogenic bacteria, p. 445-483. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, N.Y.
- 18.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geishauser, T. 1993. An instrument for the collection and transfer of ruminal fluid and for the administration of water soluble drugs in adult cattle. Bovine Pract. 27:38-42. [Google Scholar]
- 20.Gregerson, T. 1978. Rapid method for distinction of gram negative from gram positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5:123-127. [Google Scholar]
- 21.Haggans, C. J., E. J. Travelli, W. Thomas, M. C. Martini, and J. L. Slavin. 2000. The effect of flaxseed and wheat bran consumption on urinary estrogen metabolites in premenopausal women. Cancer Epidemiol. Biomarkers Prev. 9:719-725. [PubMed] [Google Scholar]
- 22.Hall, T. A. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 23.Harmsen, H. J., G. R. Gibson, P. Elfferich, G. C. Raangs, A. C. Wildeboer-Veloo, A. Argaiz, M. B. Roberfroid, and G. W. Welling. 2000. Comparison of viable cell counts and fluorescence in situ hybridization using specific rRNA-based probes for the quantification of human fecal bacteria. FEMS Microbiol. Lett. 183:125-129. [DOI] [PubMed] [Google Scholar]
- 24.Hur, H. G., and F. Rafii. 2000. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol. Lett. 192:21-25. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, D. R., Jr., M. A. Pereira, K. Stumpf, J. J. Pins, and H. Adlercreutz. 2002. Whole grain food intake elevates serum enterolactone. Br. J. Nutr. 88:111-116. [DOI] [PubMed] [Google Scholar]
- 26.Juntunen, K. S., W. M. Mazur, K. H. Liukkonen, M. Uehara, K. S. Poutanen, H. C. Adlercreutz, and H. M. Mykkanen. 2000. Consumption of wholemeal rye bread increases serum concentrations and urinary excretion of enterolactone compared with consumption of white wheat bread in healthy Finnish men and women. Br. J. Nutr. 84:839-846. [PubMed] [Google Scholar]
- 27.Kageyama, A., Y. Benno, and T. Nakase. 1999. Phylogenetic evidence for the transfer of Eubacterium lentum to the genus Eggerthella as Eggerthella lenta gen. nov., comb. nov. Int. J. Syst. Bacteriol. 49:557-565. [DOI] [PubMed] [Google Scholar]
- 28.Kamlage, B., B. Gruhl, and M. Blaut. 1997. Isolation and characterization of two new homoacetogenic hydrogen-utilizing bacteria from the human intestinal tract that are closely related to Clostridium coccoides. Appl. Environ. Microbiol. 63:1732-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkkinen, A., K. Stumpf, P. Pietinen, L. M. Valsta, H. Tapanainen, and H. Adlercreutz. 2001. Determinants of serum enterolactone concentration. Am. J. Clin. Nutr. 73:1094-1100. [DOI] [PubMed] [Google Scholar]
- 30.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lay, C., L. Rigottier-Gois, K. Holmstrøm, M. Rajilic, E. E. Vaughan, W. M. De Vos, M. D. Collins, R. Thiel, P. Namsolleck, M. Blaut, and J. Doré. 2005. Colonic microbiota signatures across five northern European countries. Appl. Environ. Microbiol. 71:4153-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lay, C., M. Sutren, V. Rochet, K. Saunier, J. Doré, and L. Rigottier-Gois. 2005. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 7:933-946. [DOI] [PubMed] [Google Scholar]
- 33.Liggins, J., R. Grimwood, and S. A. Bingham. 2000. Extraction and quantification of lignan phytoestrogens in food and human samples. Anal. Biochem. 287:102-109. [DOI] [PubMed] [Google Scholar]
- 34.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 35.Mazur, W. 1998. Phytoestrogen content in foods. Baillieres Clin. Endocrinol. Metab. 12:729-742. [DOI] [PubMed] [Google Scholar]
- 36.Mueller, S. O., S. Simon, K. Chae, M. Metzler, and K. S. Korach. 2004. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor (ER)alpha and ERbeta in human cells. Toxicol. Sci. 81:530-531. [DOI] [PubMed] [Google Scholar]
- 37.Nesbitt, P. D., Y. Lam, and L. U. Thompson. 1999. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 69:549-555. [DOI] [PubMed] [Google Scholar]
- 38.Orcheson, L. J., S. E. Rickard, M. M. Seidl, and L. U. Thompson. 1998. Flaxseed and its mammalian lignan precursor cause a lengthening or cessation of estrous cycling in rats. Cancer Lett. 125:69-76. [DOI] [PubMed] [Google Scholar]
- 39.Prasad, K. 2001. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 138:32-39. [DOI] [PubMed] [Google Scholar]
- 40.Prasad, K. 2000. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int. J. Angiol. 9:220-225. [DOI] [PubMed] [Google Scholar]
- 41.Prasad, K., S. V. Mantha, A. D. Muir, and N. D. Westcott. 2000. Protective effect of secoisolariciresinol diglucoside against streptozotocin-induced diabetes and its mechanism. Mol. Cell Biochem. 206:141-149. [DOI] [PubMed] [Google Scholar]
- 42.Prasad, K. 1999. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation 99:1355-1362. [DOI] [PubMed] [Google Scholar]
- 43.Rigottier-Gois, L., A. G. Le Bourhis, G. Gramet, V. Rochet, and J. Dore. 2003. Fluorescent hybridisation combined with flow cytometry and hybridisation of total RNA to analyse the composition of microbial communities in human faeces using 16S rRNA probes. FEMS Microbiol. Ecol. 43:237-245. [DOI] [PubMed] [Google Scholar]
- 44.Rigottier-Gois, L., V. Rochet, N. Garrec, A. Suau, and J. Dore. 2003. Enumeration of Bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16S rRNA probes. Syst. Appl. Microbiol. 26:110-118. [DOI] [PubMed] [Google Scholar]
- 45.Rowland, I., H. Wiseman, T. Sanders, H. Adlercreutz, and E. Bowey. 1999. Metabolism of oestrogens and phytoestrogens: role of the gut microflora. Biochem. Soc. Trans. 27:304-308. [DOI] [PubMed] [Google Scholar]
- 46.Rowland, I. R., H. Wiseman, T. A. Sanders, H. Adlercreutz, and E. A. Bowey. 2000. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 36:27-32. [DOI] [PubMed] [Google Scholar]
- 47.Saarinen, N. M., A. Smeds, S. I. Makela, J. Ammala, K. Hakala, J. M. Pihlava, E. L. Ryhanen, R. Sjoholm, and R. Santti. 2002. Structural determinants of plant lignans for the formation of enterolactone in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 777:311-319. [DOI] [PubMed] [Google Scholar]
- 48.Schottner, M., D. Gansser, and G. Spiteller. 1997. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med. 63:529-532. [DOI] [PubMed] [Google Scholar]
- 49.Setchell, K. D., N. M. Brown, and E. Lydeking-Olsen. 2002. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J. Nutr. 132:3577-3584. [DOI] [PubMed] [Google Scholar]
- 50.Setchell, K. D. R., A. M. Lawson, S. P. Borriello, R. Harkness, H. Gordon, D. M. Morgan, D. N. Kirk, H. Adlercreutz, L. C. Anderson, and M. Axelson. 1981. Lignan formation in man—microbial involvement and possible roles in relation to cancer. Lancet ii:4-7. [DOI] [PubMed] [Google Scholar]
- 51.Setchell, K. D. R., A. M. Lawson, M. Axelson, and H. Adlercreutz. 1979. The excretion of two new phenolic compounds during the human menstrual cycle and in pregnancy, p. 207-215. In H. Adlercreutz, R. D. Bulbrook, H. J. Van der Molen, A. Vermeulen, and F. Sciarra (ed.), Research on steroids, vol. IX. Excerpta Medica, Amsterdam, The Netherlands. [Google Scholar]
- 52.Thompson, L. U. 1998. Experimental studies on lignans and cancer. Baillieres Clin. Endocrinol. Metab. 12:691-705. [DOI] [PubMed] [Google Scholar]
- 53.Thompson, L. U., P. Robb, M. Serraino, and F. Cheung. 1991. Mammalian lignan production from various foods. Nutr. Cancer 16:43-52. [DOI] [PubMed] [Google Scholar]
- 54.Tou, J. C., and L. U. Thompson. 1999. Exposure to flaxseed or its lignan component during different developmental stages influences rat mammary gland structures. Carcinogenesis 20:1831-1835. [DOI] [PubMed] [Google Scholar]
- 55.Wang, C., T. Makela, T. Hase, H. Adlercreutz, and M. S. Kurzer. 1994. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid Biochem. Mol. Biol. 50:205-212. [DOI] [PubMed] [Google Scholar]
- 56.Wang, L. Q., M. R. Meselhy, Y. Li, G. W. Qin, and M. Hattori. 2000. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 48:1606-1610. [DOI] [PubMed] [Google Scholar]
- 57.Wang, R. F., W. W. Cao, and C. E. Cerniglia. 1996. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 62:1242-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]