Abstract
The human gastrointestinal microbiota produces antagonistic activities against gastrointestinal bacterial pathogens. We undertook a study to investigate the mechanism(s) by which a Lactobacillus acidophilus strain of human microbiota origin antagonizes the gram-negative enteroinvasive pathogen Salmonella enterica serovar Typhimurium. We showed that the cell-free culture supernatant of L. acidophilus strain LB (LB-CFCS) induced the following effects in S. enterica SL1344: (i) a decrease in intracellular ATP that paralleled bacterial death, (ii) the release of lipopolysaccharide, (iii) permeabilization of the bacterial membrane, and (iv) an increase in the sensitivity of Salmonella to the lytic action of sodium dodecyl sulfate. Finally, we showed using two mutant strains of Salmonella, PhoP MS7953s and PmrA JKS1170, that the two-component regulatory systems PhoP-PhoQ and PmrA-PmrB that regulate the mechanisms of resistance to antibacterial agents in Salmonella did not influence the anti-Salmonella effect of LB-CFCS.
One of the defense mechanisms by which a host species combats gastrointestinal microbial pathogens is a first line of chemical defense involving the production of antimicrobial peptides (AMPs) by the epithelial cells lining the gut epithelium (19). Together with this chemical system of defense of the host cells, one of the basic physiological functions of the resident intestinal microbiota is that it acts as a microbial barrier against microbial pathogens (25). There is increasing evidence that the antibacterial activities of the lactobacilli that are part of the human gastrointestinal microbiota (48) involve numerous mechanisms of action, including the production of H2O2, metabolites, and antimicrobial substances, including bacteriocins and nonbacteriocin molecules (44). Some Lactobacillus strains, including Lactobacillus johnsonii La1 (5, 36), L. rhamnosus GG (31, 46), L. rhamnosus DR20 (20), L. rhamnosus GR-1, and L. fermentum RC-14 (34), have been reported to produce antimicrobial activities. Little is known about the antibacterial mechanism(s) of action of nonbacteriocin molecules produced by Lactobacillus strains originating from the human intestinal microbiota. We decided to investigate the mechanism(s) by which Lactobacillus strains kill Salmonella enterica serovar Typhimurium (S. enterica SL1344). As a test strain, we chose L. acidophilus strain LB, a strain of human microbiota origin that has antagonistic activities against gram-negative enterovirulent pathogens (7-9, 33). In the cell-free culture supernatant (LB-CFCS), this strain produces a non-lactic-acid, nonbacteriocin molecule(s) with a low molecular mass which is heat stable and partially resistant to proteolytic enzymes and which exerts a rapid and dramatic killing activity against S. enterica SL1344 (7). Moreover, LB-CFCS treatment results in the killing of S. enterica SL1344 cells located within infected cultured human intestinal cells (9). We provide evidence suggesting that the mechanism of action involving bacterial membrane damage is lethal to Salmonella. This conclusion is based on data showing that exposing S. enterica SL1344 to LB-CFCS promotes (i) the depletion of intracellular ATP, (ii) an increase in membrane permeabilization, (iii) the release of lipopolysaccharide (LPS) from the bacterial membrane, and (iv) sensitization of the bacterial membrane towards the lytic action of detergent.
MATERIALS AND METHODS
Reagents.
Bis-benzamidine (Hoechst 33258), lysozyme, Triton X-100, sodium dodecyl sulfate (SDS), RNase A, DNase I, proteinase K, and polymyxin were purchased from Sigma-Aldrich Chimie SARL (L'Isle d'Abeau Chesnes, France). The C18G peptide (ALYKKLLKKLLKSAKKLG) was synthesized by Nanosphere Biotechnologies (Paris, France)
Bacterial strains.
Salmonella enterica serovar Typhimurium strain SL1344 was a gift of B. A. D. Stocker (Stanford, California). Wild-type S. enterica serovar Typhimurium strain 14028s, PhoP constitutive and PmrA-positive strains, and mutant PhoP and PmrA strains were a gift of E. A. Groisman (Howard Hughes Medical Institute, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, Mo.) (Table 1). Bacteria were cultured in Luria broth at 37°C and used until they reached the early logarithmic phase of growth.
TABLE 1.
Strains of S. enterica serovar Typhimurium used for this study
L. acidophilus strain LB, isolated from a human stool (Axcan Pharmaceutical Ltd., Houdan, France), was grown in De Man, Rogosa, Sharpe (MRS) broth (Difco Laboratories, Detroit, MI) for 18 h at 37°C. LB-CFCS was obtained by centrifuging at 10,000 × g for 30 min at 4°C. Centrifuged LB-CFCS was passed through a sterile 0.22-μm Millex GS filter unit (Millipore, Molsheim, France). A concentrated suspension (concentrated twofold) of the LB-CFCS was obtained by freeze-drying. Two controls were used in our experiments. Since the various LB-CFCSs displayed pH values ranging from 4.3 to 4.5, MRS broth adjusted to pH 4.5 with HCl (MRS-HCl) was used as a first control. Since lactic acid is known to permeabilize gram-negative bacteria (2) and since the twofold concentrate of LB-CFCS obtained after freeze-drying and used in the experiments contained 120 mM lactic acid, dl-lactic acid (MRS-LA) (final concentration, 120 mM) was used as a second control. The lactic acid content in the reconstituted, twofold-concentrated LB-CFCS was checked.
Determination of killing activity.
In order to investigate the mechanism of action of the non-lactic-acid, nonbacteriocin molecule(s) present in the LB-CFCS, we used an in vitro method that makes it possible to distinguish between the lactic acid- and non-lactic-acid-dependent anti-Salmonella activities of Lactobacillus strains (14). S. enterica SL1344 bacteria were centrifuged at 5,500 × g for 5 minutes at 4°C, washed once with phosphate-buffered saline (PBS), and then suspended in Dulbecco's modified Eagle's medium (DMEM). The bacteria were counted, and a volume containing 2 × 108 CFU/ml was used to determine the activity of LB-CFCS (twofold concentrate), MRS, MRS-HCl, or MRS-LA (120 mM). Colony count assays were performed by incubating 250 μl of this suspension with 250 μl of LB-CFCS or the control medium and 500 μl of DMEM at 37°C. After exposure for 4 h, aliquots were removed, serially diluted, and plated on tryptic soy agar to determine the bacterial colony counts. The pH of the incubation medium in the presence of LB-CFCS, MRS, MRS-HCl, or MRS-LA was 5.0 ± 0.2.
The killing activities of LB-CFCS (twofold concentrate) versus two concentrations of S. enterica SL1344 (105 and 108 CFU/ml [final concentration]) were compared to the killing activities of C18G (20 μg/ml) and polymyxin (10 μg/ml). In addition, the killing activities were determined both in the presence of DMEM as described above and in the presence of PBS-peptone, since C18G is known to be more active in the presence of PBS-peptone (3).
Determination of intracellular ATP.
To find out whether any damage had been caused to the cytoplasmic membranes, the intracellular ATP was determined using an ATP assay kit (Perkin-Elmer Life Sciences, Paris, France) with a white microtiter plate. The ATPLite monitoring system is based on firefly luciferase and was designed for mammalian cells. Technical modifications were necessary to enable us to measure the intracellular ATP of bacteria. S. enterica SL1344 bacteria (final concentration, 109 CFU/ml) were incubated with MRS, MRS-HCl, MRS-LA, or LB-CFCS (twofold concentrate) as described above for the determination of the killing activity. After centrifugation (5 min, 5,000 × g), the bacteria were washed twice and suspended in a similar volume of PBS. The bacteria were lysed as previously described (6). Briefly, the bacterial suspension was added to glass beads (0.10- to 0.11-mm diameter), and the mixture was shaken vigorously six times for periods of 30 s at 4°C. The supernatant was centrifuged (40 min at 14,000 × g, 4°C), and then the ATP content was assayed. Aliquots (100 μl) of bacterial supernatant were directly pipetted into the wells, and the lysis buffer and substrate solution were added according to the manufacturer's instructions. The luminescence was measured in a Genios luminometer (Tecan, Trappes, France). The ATP content (μM) was calculated using a standard curve plotted from a standard ATP solution.
Fluorescent labeling with bis-benzamidine (Hoechst 33258).
To find out whether the LB-CFCS treatment had caused membrane damage, we used an adaptation of the techniques described by Wouters et al. (53). Bacteria were incubated after being exposed to the DNA-binding probe Hoechst 33258, small amounts of which are able to pass through intact membranes. When the probe enters cells after damage of the membranes, binding to DNA increases the fluorescence of Hoechst 33258. After incubating S. enterica SL1344 bacteria (final concentration, 5 × 108 CFU/ml) for 3 h with DMEM, MRS, MRS-HCl, MRS-LA, or LB-CFCS (twofold concentrate), the bacteria were washed twice and suspended in a similar volume of PBS. Hoechst 33258 was added to a final concentration of 2 μg/ml and left to stand for 30 min before being washed three times. Passage across the membrane was measured using a black microtiter fluoroplate (Labsystems) and a Genios spectrofluorimeter (Tecan, Trappes, France). Aliquots (108 cells in 200 μl) of a suspension in PBS were directly pipetted into wells, and the fluorescence was monitored in three wells per sample (excitation, 360 nm, with a half-bandwidth of 35; emission, 465 nm, with a half-bandwidth of 25).
Release of LPS.
To measure the release of LPS, S. enterica SL1344 bacteria were radiolabeled with [14C]galactose as previously described (50). S. enterica SL1344 was grown as described above in the presence of [14C]galactose (10 nmol/ml) (CFA 435; Amersham Biosciences, Orsay, France). After two washes with PBS, the bacteria were suspended in DMEM. The bacteria (250 μl, 4 × 109 CFU/ml) were incubated with MRS, MRS-HCl, MRS-LA, or LB-CFCS (twofold concentrate) (250 μl) and 500 μl of DMEM for 1, 3, or 6 h at 37°C. Bacteria and supernatants were separated by centrifugation at 5,000 × g at 4°C for 30 min. Radioactivity was determined in the incubating medium (bacteria and supernatant) and in the supernatant. The amount of LPS release was expressed as a percentage of the total radioactivity (% LPS released).
The presence of LPS was determined in the supernatants. To do this, LPS was extracted from the supernatants by the hot phenol-water protocol of Moran et al. (37). LPS preparations were purified by treatment with RNase A, DNase I, and proteinase K and by ultracentrifugation at 100,000 × g at 4°C for 18 h. The purified LPS preparations were examined by SDS-polyacrylamide gel electrophoresis. After the gels had been fixed, LPS was detected by the modified silver staining technique described by Fomsgaard et al. (17), and the radioactivity in the gel was examined using a Typhoon scanner (Amersham Biosciences, Orsay, Ulis, France). The LPS profiles obtained resemble the profile described by Moran et al. (37), and labeled LPS was the main labeled product present in the supernatants.
Bacteriolysis assay.
Sensitization of S. enterica SL1344 by LB-CFCS to the lytic action of detergents (SDS and Triton X-100) and lysozyme was investigated in microplates as described by Helander et al. (26), with some modifications. After incubating the bacteria for 1h with DMEM, MRS, MRS-HCl, MRS-LA, or LB-CFCS (twofold concentrate) as described above, the bacteria were washed twice and then suspended in a similar volume of 10 mM HEPES containing 50 mM NaCl, pH 7.2. From this suspension, aliquots (108 cells in 100 μl) were pipetted into microtiter wells in the presence of lysozyme, detergents, or buffer only. Cell lysis was determined using a spectrophotometer at 405 nm. The value for a control with no added lysozyme or detergents was taken as the 100% reading; a lower percentage indicated that lysis had occurred.
Statistics.
Data are expressed as means ± standard deviations (SD) of at least three separate duplicate experiments. Student's t test was used to determine whether the killing activity, intracellular ATP release, membrane permeabilization, LPS release, or sensitization to the SDS lytic agent in the MRS-HCl, MRS-LA, or LB-CFCS group was significantly different from that in the control group.
RESULTS
LB-CFCS-induced intracellular ATP depletion in S. enterica SL1344.
Intracellular ATP was measured in wild-type S. enterica SL1344 with and without twofold-concentrated LB-CFCS (Fig. 1). In the presence of MRS acidified to pH 4.5 with HCl (MRS-HCl), there was no change in intracellular ATP compared with MRS. Compared to bacteria exposed to MRS, S. enterica SL1344 exposed to the twofold-concentrated LB-CFCS displayed a gradual decrease in intracellular ATP as a function of the time of contact. In parallel, a gradual decrease in viable S. enterica SL1344 developed as a function of the time of contact in the presence of LB-CFCS (at T0, 8.00 ± 0.05 log CFU/ml; at 2 h of contact, 6.10 ± 0.07 log CFU/ml; at 4 h of contact, 3.95 ± 0.06 log CFU/ml) compared with growth in DMEM (at 2 h of contact, 8.47 ± 0.05 log CFU/ml; at 4 h of contact, 8.70 ± 0.06 log CFU/ml) and MRS-HCl (at 2 h of contact, 8.04 ± 0.04 log CFU/ml; at 4 h of contact, 7.11 ± 0.05 log CFU/ml). After incubating S. enterica SL1344 with MRS-LA for 1 h or 2 h, the intracellular ATP was the same as that observed with MRS or MRS-HCl (Fig. 1), and the bacterial viability remained unchanged after 2 h of contact (7.70 ± 0.04 log CFU/ml). In contrast, a small decrease in intracellular ATP was observed after 3 h of contact without a significant change in bacterial viability (7.10 ± 0.07 log CFU/ml).
FIG. 1.
Intracellular ATP content of S. enterica SL1344 after exposure to LB-CFCS, MRS, MRS-HCl, or MRS-LA. S. enterica SL1344 bacteria (2 × 108 CFU/ml) were incubated in the presence of LB-CFCS (twofold concentrate), MRS, MRS-HCl, or MRS-LA (pH of incubation medium, 5.0 ± 0.2). At predetermined intervals, aliquots were removed for determination of the intracellular ATP content. Data are expressed as means ± SD of three separate duplicate experiments. Statistical analysis was performed with a Student t test. *, P < 0.01.
LB-CFCS-induced membrane permeabilization in S. enterica SL1344.
The decrease in intracellular ATP observed above indicates that the membranes of S. enterica SL1344 bacteria had been permeabilized. Membrane permeabilization by LB-CFCS (twofold concentrate) was determined by measuring the fluorescence due to the DNA-binding probe Hoechst 33258, of which a small percentage normally passes through intact membranes (Table 2). As first controls, MRS and MRS-HCl were used. The bacterial Hoechst 33258 fluorescence in MRS- and MRS-HCl-treated bacteria was the same as that for bacteria in DMEM. These results indicate that the membrane integrity of S. enterica SL1344 bacteria is preserved after extended incubation and washing in the presence of MRS and MRS-HCl. Consistent with the fact that lactic acid permeabilized the bacterial membrane (2), a 3.2-fold increase in bacterial Hoechst 33258 fluorescence was observed in the presence of MRS-LA versus the DMEM-treated control. It was noticed that MRS-LA induced less membrane permeabilization. In contrast, the Hoechst 33258 fluorescence of S. enterica SL1344 was 7.5-fold greater in the presence of the twofold concentrate of LB-CFCS than that of the DMEM-treated control.
TABLE 2.
Membrane permeabilization of S. enterica SL1344 by exposure to LB-CFCS
| Test agent | Relative fluorescence intensity | Ratio between assay and controls
|
|
|---|---|---|---|
| DMEM | MRS-LA | ||
| DMEM | 4,931 (1,130) | 1 | |
| MRS | 5,055 (950) | 1.02 | |
| MRS-HCl | 7,408 (846) | 1.5 | |
| MRS-LA (120 mM) | 15,786 (2,210)a | 3.2 | |
| LB-CFCS (twofold concentrate) | 37,120 (1,904)a,b | 7.5 | 2.35 |
The values for LB-CFCS and MRS-LA were significantly different from those for DMEM, MRS, and MRS-HCl (P < 0.005).
The value for LB-CFCS was significantly different from that for MRS-LA (P < 0.005).
LB-CFCS-induced LPS release from S. enterica SL1344.
To measure the release of LPS, S. enterica SL1344 bacteria were radiolabeled by growing the organisms in the presence of [14C]galactose. As reported in Table 3, in the presence of MRS or MRS-HCl very little LPS was released from S. enterica SL1344 into the incubating medium. Increases of 3.5-, 3.14-, and 3.57-fold in LPS release were observed after 1 h, 3 h, and 6 h of contact, respectively, with twofold-concentrated LB-CFCS. A control experiment conducted with MRS-LA showed that no LPS release was observed after 1 h or 3 h of contact and that 6 h of contact was required to obtain a twofold increase in the release of LPS from S. enterica SL1344. As described above for intracellular ATP, MRS-LA induced less of an effect than LB-CFCS.
TABLE 3.
LB-CFCS induces the release of LPS into the medium by S. enterica SL1344
| Test agent | % Radioactivity (mean [SD]) released into medium after indicated contact timea
|
||
|---|---|---|---|
| 1 h | 3 h | 6 h | |
| MRS | 2 (0.43) | 7 (0.33) | 7 (0.39) |
| MRS-HCl | 2 (0.35) | 7 (0.56) | 7 (0.41) |
| MRS-LA (120 mM) | 3 (0.38) | 8 (0.43) | 13 (1.03) |
| LB-CFCS (2-fold concentrate) | 7 (0.47)b | 22 (3.96)b | 25 (1.3)b |
Results are means of three separate duplicate experiments.
The values for LB-CFCS were significantly different from those for MRS, MRS-HCl, and MRS-LA (P < 0.01).
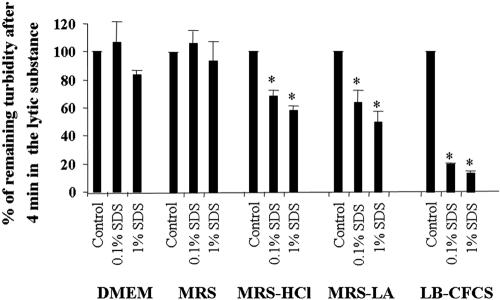
LB-CFCS sensitizes S. enterica SL1344 to the lytic action of SDS.
The data described above show that membrane permeability was increased after exposing S. enterica SL1344 to LB-CFCS. We conducted further experiments to find out whether S. enterica SL1344 was also sensitized to detergent- or lysozyme-induced bacteriolysis. The lytic effect of the anionic detergent SDS is shown in Fig. 2. SDS had no effect on S. enterica SL1344 after exposure for 1 h to DMEM or MRS. Exposure to twofold-concentrated LB-CFCS considerably sensitized S. enterica SL1344 to SDS. MRS-HCl and MRS-LA produced little sensitization to SDS. The sensitization of S. enterica SL1344 induced by MRS-LA was similar to that produced by MRS-HCl. The twofold concentrate of LB-CFCS had more effect than either MRS, MRS-HCl, or MRS-LA. There was no sensitization of S. enterica SL1344 in the presence of the nonionic detergent Triton X-100 and of lysozyme for MRS, MRS-LA, MRS-HCl, or LB-CFCS (not shown). It has previously been reported that Mg2+ inhibits the action of many membrane-permeabilizing agents (26, 28, 29). We therefore investigated the effect of Mg2+ on the permeabilization effect of LB-CFCS and found that in the presence of excess Mg2+ (10 mM), the LB-CFCS-induced sensitization of S. enterica SL1344 towards SDS is not inhibited (not shown).
FIG. 2.
Increase in bacteriolysis of S. enterica SL1344 by SDS in the presence of LB-CFCS. S. enterica SL1344 bacteria (2 × 108 CFU/ml) were pretreated for 1 h with or without LB-CFCS (twofold concentrate), MRS, MRS-HCl, or MRS-LA (pH of incubation medium, 5.0 ± 0.2). Cell lysis was monitored spectrophotometrically at 405 nm. The value for the cell control with no added SDS was taken as 100%. A lower percentage indicates that lysis has occurred. Data are expressed as means ± SD of three separate duplicate experiments. Statistical analysis was performed with a Student t test. *, P < 0.01.
Comparison of the killing activities of LB-CFCS and antimicrobial peptides against S. enterica SL1344.
The killing and membrane permeabilization activities of LB-CFCS reported above resemble the antibacterial activities produced by α-helical and β-sheet antimicrobial peptides (19). We therefore compared the killing activity of LB-CFCS against S. enterica SL1344 to that of C18G and polymyxin. C18G is a synthetic α-helical peptide derived from human platelet factor IV (11). Polymyxin is a modified amino acid peptide with a single fatty acid (51). Considering that C18G is more active against low levels of Salmonella in the presence of peptone (3), we examined the activities of C18G, polymyxin, and LB-CFCS with a low-level inoculum (105 CFU/ml) and a high-level inoculum (108 CFU/ml) of S. enterica SL1344 in the presence of DMEM or peptone. As shown in Table 4, C18G at a concentration of 20 μg/ml was inactive against S. enterica SL1344 at a concentration of 108 CFU/ml in the presence of DMEM or peptone. In contrast, C18G in the presence of peptone was active when the concentration of S. enterica SL1344 was 105 CFU/ml, but was inactive in the presence of DMEM. Polymyxin at a concentration of 10 μg/ml was active at low- and high-level inoculums of S. enterica SL1344 in the presence of DMEM or peptone. LB-CFCS produced a dramatic decrease in the viability of S. enterica SL1344 in low- and high-level inoculums in the presence of DMEM or peptone. It was noted that LB-CFCS produced the same killing activity as 10-μg/ml polymyxin in the presence of a low-level inoculum of Salmonella and was more active in the presence of a high-level inoculum. Moreover, LB-CFCS was active when C18G was inactive against S. enterica SL1344 at a high-level inoculum and in the presence of peptone.
TABLE 4.
Comparison of killing activities of LB-CFCS, C18G, and polymyxin against S. enterica SL1344
| Test agenta | No. of viable bacteria (log CFU/ml ± SD) after 4 h of contact with indicated concn of S. enterica SL1344b
|
|
|---|---|---|
| 105 CFU/ml | 108 CFU/ml | |
| Control DMEM | 7.03 ± 0.11 | 9.03 ± 0.09 |
| Control PBS-peptone | 7.07 ± 0.37 | 8.38 ± 0.06 |
| LB-CFCS in DMEM | 3.51 ± 0.06d | 2.21 ± 0.12d |
| LB-CFCS in PBS-peptone | 3.71 ± 0.26d | 3.03 ± 0.28d |
| C18G in DMEM | 5.94 ± 1.17c | 8.96 ± 0.13c |
| C18G in PBS-peptone | 3.80 ± 0.20d | 8.55 ± 0.12c |
| Polymyxin in DMEM | 3.02 ± 0.30d | 5.28 ± 0.06d |
| Polymyxin in PBS-peptone | 2.94 ± 0.38d | 5.05 ± 0.18d |
LB-CFCS, twofold concentrate; C18G, 20 μg/ml; polymyxin, 10 μg/ml.
Results are means of three separate duplicate experiments.
Value not significantly different from control.
Value significantly different (P < 0.01) from control.
The two-component regulatory systems PhoP-PhoQ and PmrA-PmrB in S. enterica serovar Typhimurium 14028s have no influence on the killing effect of LB-CFCS.
Resistance to AMPs secreted by intestinal epithelium Paneth cells has been reported, and its mechanism has been elucidated. Two two-component regulatory systems, PhoP-PhoQ and PmrA-PmrB, regulate the mechanisms of resistance of Salmonella to AMPs, and mutations in these regulatory systems render the mutated strains more sensitive to AMPs (13, 21). We investigated whether a mutation in PhoP or PmrA in the S. enterica serovar Typhimurium 14028s strain renders the mutant strains more sensitive to LB-CFCS treatment. As shown in Fig. 3, the wild-type 14028s strain was sensitive to LB-CFCS treatment, and after 4 h of contact, there was a 5-log decrease in viability. The 14028s strain exposed to MRS-LA displayed a slight decrease in viability. Consistent with previous reports (22, 45), we observed that a mutation in phoP (strain MS1953) or pmrA (strain EG7139) rendered the strains more sensitive to polymyxin than strains 55130 (phoP constitutive) and JKS1170 (pmrA positive), respectively. In contrast, no increase in sensitivity compared with the wild-type 14028s strain was observed when the mutated strains MS1953 and EG7139 were exposed to LB-CFCS, whereas strains 55130 and JKS1170 did display an increase in sensitivity (2.97- to 5.76-log decrease in viability).
FIG. 3.
Killing activity of LB-CFCS against wild-type S. enterica serovar Typhimurium strain 14028s and strains with mutations in the regulatory system phoP or pmrA. Strain 55130 (phoP constitutive), strain JKS1170 (pmrA positive), strain MS1953s (mutant in phoP), and strain EG7139 (mutant in pmrA) were used for this experiment. S. enterica 14028s or the mutants (2 × 108 CFU/ml) were exposed for 4 h to the action of the control (DMEM), LB-CFCS (twofold concentrate), MRS-LA, or polymyxin (10 μg/ml), and the numbers of viable bacteria were determined (log CFU/ml). Data are expressed as means ± SD of three separate duplicate experiments. Statistical analysis to compare 14028s with the phoP mutant strain MS1953, the phoP constitutive strain 55130, the pmrA mutant strain EG7139, and the pmrA-positive strain JSK1170 alone was performed with a Student t test. *, P < 0.01.
DISCUSSION
The present results and previous data (7, 9) indicate that a Lactobacillus strain of human intestinal microbiota origin displays killing activity within the range defined as bactericidal activity for an antibiotic against a microorganism, i.e., the killing activity needed to kill >99.9% of a test microorganism after incubation for a fixed length of time under controlled conditions (38). The data reported here offer new insights into the mechanisms underlying the antibacterial activity of the non-lactic-acid molecule(s) produced by LB-CFCS. The data reported here provide the first evidence that the non-lactic-acid molecule(s) present in the LB-CFCS can kill an enterovirulent pathogen, S. enterica SL1344, by damaging the bacterial membrane. Indeed, we observed a loss in intracellular ATP in Salmonella exposed to LB-CFCS which correlates with a dramatic decrease in bacteria viability. Interestingly, a similar mechanism has also been recently reported for antimicrobial molecules. Indeed, microcins produced by Escherichia coli produce an increase in cell membrane permeability accompanied by intracellular ATP depletion, resulting in the cell death of Listeria monocytogenes and diarrheagenic strains of E. coli (12, 43). Moreover, we provide evidence that a compound(s) present in LB-CFCS permeabilizes the membrane of S. enterica SL1344. Permeabilization of the bacterial membrane by antibacterial agents has been reported previously. For example, the ovotransferrin antimicrobial peptide OTAP-92, a cationic fragment of hen ovotransferrin, causes permeation of the E. coli membrane (32). The polycation polyethyleneimine permeabilizes the gram-negative membrane and increases the susceptibility of gram-negative bacteria to hydrophobic antibiotics (26, 27). Permeabilization of gram-negative membranes by antibacterial molecules is accompanied or not by the release of LPS. The release of LPS from S. enterica serovar Typhimurium has been reported after exposing the bacteria to polycations, protamine, and a 20-residue lysine polymer (lysine20) (50) as well as to LB-CFCS. The antimicrobial activity of lactoferrin and lactoferricin was accompanied by the release of LPS from the membrane of Salmonella (54). In contrast, the permeabilization effect of EDTA (1) and chitosan (29) on the bacterial membrane was not accompanied by LPS release. It is interesting that the level of release of LPS from the membrane of S. enterica SL1344 exposed to LB-CFCS is similar to that from the membrane of S. enterica serovar Typhi exposed to the β-lactam antibiotics ceftazidime and imipenem (52).
Recent reports have provided new insights into the activity of members of the intestinal microbiota against enteropathogens. Ramare et al. (41) have observed that when a human intestinal strain of Peptostreptococcus colonized the guts of gnotobiotic rats, it produced an antibacterial substance that was active against several gram-positive bacteria, including potentially pathogenic Clostridium spp. Similarly, a Ruminococcus gnavus strain was able to produce an antibacterial substance, called ruminococcin A, that is also active against various pathogenic clostridia (10). It has been established that E. coli participates in antibacterial defense by producing large proteins named colicins or microcins that insert into the inner membrane, forming pores in the cell membrane (4, 12, 42). Consistent with that, the microcin bactericidal spectrum of activity was found to be restricted to Enterobacteriaceae, specifically to E. coli (43) and Salmonella (40) species, and it has been observed that resident microbiota E. coli increased the survival of Salmonella-infected germfree mice (30). Interestingly, the non-lactic-acid molecules produced by resident Lactobacillus strains have a wide spectrum of microbicidal activities against a large variety of gram-negative and gram-positive bacteria (5, 7). Enteric bacterial pathogens have developed sophisticated mechanisms to resist AMPs (39) and microcins (18). For example, Salmonella induces remodeling of the bacterial envelope by enzymes that modify LPS (22, 24, 49). The two-component regulatory systems PhoP-PhoQ and PmrA-PmrB (13, 21) play a central role in resistance to AMPs, including polymyxin, the α-helical antimicrobial C18G peptide, and the β-sheet antimicrobial peptide protegrin (3, 23, 35). In contrast to AMPs, our findings show that S. enterica 14028s with mutations in phoP and pmrA showed no increase in sensitivity to the killing activity of LB-CFCS.
In conclusion, it has been previously reported that Lactobacillus strains producing non-lactic-acid molecules with in vitro anti-Salmonella activities have the capacity to increase the survival of Salmonella-infected germfree mice and to decrease the level of viable bacteria in feces of Salmonella-infected conventional mice (5, 7, 31). In the intestine, Paneth cells discharge effective concentrations of microbicidal AMPs into the small intestinal crypts, thus providing the first line of defense against pathogens and contributing to the innate immunity in the human small bowel (19). It is tempting to suggest that some strains of Lactobacillus that inhabit the intestinal microbiota may discharge an antimicrobial substance(s) into ecological niches within the intestine and thus also contribute to the first line of the chemical defense against enteric pathogens. However, the non-lactic-acid antibacterial molecule(s) that supports the killing activity of these Lactobacillus strains against gram-negative pathogens has yet to be identified. A challenge for the near future is to determine its structure(s) and to devise the tools necessary to detect its presence in the intestine.
Acknowledgments
This work was supported by a research contract between the Institut National de la Santé et de la Recherche Médicale (INSERM) and Axcan Pharma Ltd. (Houdan, France).
We thank E. Groisman (Howard Hughes Medical Institute, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, Mo.) for the generous gift of Salmonella mutants.
REFERENCES
- 1.Alakomi, H. L., M. Saarela, and I. M. Helander. 2003. Effect of EDTA on Salmonella enterica serovar Typhimurium involves a component not assignable to lipopolysaccharide release. Microbiology 149:2015-2021. [DOI] [PubMed] [Google Scholar]
- 2.Alakomi, H. L., E. Skytta, M. Saarela, T. Mattila-Sandholm, K. Latva-Kala, and I. M. Helander. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. [DOI] [PubMed] [Google Scholar]
- 4.Bellomio, A., R. G. Oliveira, B. Maggio, and R. D. Morero. 2005. Penetration and interactions of the antimicrobial peptide, microcin J25, into uncharged phospholipid monolayers. J. Colloid Interface Sci. 285:118-124. [DOI] [PubMed] [Google Scholar]
- 5.Bernet-Camard, M. F., V. Lievin, D. Brassart, J. R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binet, M. R., and O. M. Bouvet. 1998. Transport of glucose by a phosphoenolpyruvate:mannose phosphotransferase system in Pasteurella multocida. Res. Microbiol. 149:83-94. [DOI] [PubMed] [Google Scholar]
- 7.Coconnier, M. H., V. Lievin, M. F. Bernet-Camard, S. Hudault, and A. L. Servin. 1997. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 41:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coconnier, M. H., V. Lievin, E. Hemery, and A. L. Servin. 1998. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 64:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coconnier, M. H., V. Lievin, M. Lorrot, and A. L. Servin. 2000. Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl. Environ. Microbiol. 66:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabard, J., C. Bridonneau, C. Phillipe, P. Anglade, D. Molle, M. Nardi, M. Ladire, H. Girardin, F. Marcille, A. Gomez, and M. Fons. 2001. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 67:4111-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darveau, R. P., J. Blake, C. L. Seachord, W. L. Cosand, M. D. Cunningham, L. Cassiano-Clough, and G. Maloney. 1992. Peptides related to the carboxyl terminus of human platelet factor IV with antibacterial activity. J. Clin. Investig. 90:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Destoumieux-Garzon, D., X. Thomas, M. Santamaria, C. Goulard, M. Barthelemy, B. Boscher, Y. Bessin, G. Molle, A. M. Pons, L. Letellier, J. Peduzzi, and S. Rebuffat. 2003. Microcin E492 antibacterial activity: evidence for a TonB-dependent inner membrane permeabilization on Escherichia coli. Mol. Microbiol. 49:1031-1041. [DOI] [PubMed] [Google Scholar]
- 13.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 14.Fayol-Messaoudi, D., C. N. Berger, M.-H. Coconnier-Polter, V. Lievin-Le Moal, and A. L. Servin. pH, lactic acid, and non-lactic acid activities against Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 71:6008-6013. [DOI] [PMC free article] [PubMed]
- 15.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 16.Finlay, B. B., and S. Falkow. 1990. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162:1096-1106. [DOI] [PubMed] [Google Scholar]
- 17.Fomsgaard, A., M. A. Freudenberg, and C. Galanos. 1990. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 28:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frana, T. S., S. A. Carlson, D. C. Rauser, B. D. Jones, B. J. Fergen, and R. W. Griffith. 2004. Effects of microcin 24-producing Escherichia coli on shedding and multiple-antimicrobial resistance of Salmonella enterica serotype Typhimurium in pigs. Am. J. Vet. Res. 65:1616-1620. [DOI] [PubMed] [Google Scholar]
- 19.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 20.Gopal, P. K., J. Prasad, J. Smart, and H. S. Gill. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216. [DOI] [PubMed] [Google Scholar]
- 21.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 23.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 25.Hao, W. L., and Y. K. Lee. 2004. Microflora of the gastrointestinal tract: a review. Methods Mol. Biol. 268:491-502. [DOI] [PubMed] [Google Scholar]
- 26.Helander, I. M., H. L. Alakomi, K. Latva-Kala, and P. Koski. 1997. Polyethyleneimine is an effective permeabilizer of gram-negative bacteria. Microbiology 143:3193-3199. [DOI] [PubMed] [Google Scholar]
- 27.Helander, I. M., K. Latva-Kala, and K. Lounatmaa. 1998. Permeabilizing action of polyethyleneimine on Salmonella typhimurium involves disruption of the outer membrane and interactions with lipopolysaccharide. Microbiology 144:385-390. [DOI] [PubMed] [Google Scholar]
- 28.Helander, I. M., and T. Mattila-Sandholm. 2000. Fluorometric assessment of gram-negative bacterial permeabilization. J. Appl. Microbiol. 88:213-219. [DOI] [PubMed] [Google Scholar]
- 29.Helander, I. M., E. L. Nurmiaho-Lassila, R. Ahvenainen, J. Rhoades, and S. Roller. 2001. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 71:235-244. [DOI] [PubMed] [Google Scholar]
- 30.Hudault, S., J. Guignot, and A. L. Servin. 2001. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 49:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudault, S., V. Lievin, M. F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim, H. R., Y. Sugimoto, and T. Aoki. 2000. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta 1523:196-205. [DOI] [PubMed] [Google Scholar]
- 33.Lievin-Le Moal, V., R. Amsellem, A. L. Servin, and M. H. Coconnier. 2002. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 50:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGroarty, J. A., and G. Reid. 1988. Detection of a Lactobacillus substance that inhibits Escherichia coli. Can. J. Microbiol. 34:974-978. [DOI] [PubMed] [Google Scholar]
- 35.McPhee, J. B., S. Lewenza, and R. E. Hancock. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50:205-217. [DOI] [PubMed] [Google Scholar]
- 36.Michetti, P., G. Dorta, P. H. Wiesel, D. Brassart, E. Verdu, M. Herranz, C. Felley, N. Porta, M. Rouvet, A. L. Blum, and I. Corthesy-Theulaz. 1999. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion 60:203-209. [DOI] [PubMed] [Google Scholar]
- 37.Moran, A. P., I. M. Helander, and T. U. Kosunen. 1992. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J. Bacteriol. 174:1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NCCLS. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline. NCCLS document M26-A, p. 1-29. NCCLS, Wayne, Pa.
- 39.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 40.Portrait, V., S. Gendron-Gaillard, G. Cottenceau, and A. M. Pons. 1999. Inhibition of pathogenic Salmonella enteritidis growth mediated by Escherichia coli microcin J25 producing strains. Can. J. Microbiol. 45:988-994. [DOI] [PubMed] [Google Scholar]
- 41.Ramare, F., J. Nicoli, J. Dabard, T. Corring, M. Ladire, A. M. Gueugneau, and P. Raibaud. 1993. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl. Environ. Microbiol. 59:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rintoul, M. R., B. F. de Arcuri, R. A. Salomon, R. N. Farias, and R. D. Morero. 2001. The antibacterial action of microcin J25: evidence for disruption of cytoplasmic membrane energization in Salmonella newport. FEMS Microbiol. Lett. 204:265-270. [DOI] [PubMed] [Google Scholar]
- 43.Sable, S., A. M. Pons, S. Gendron-Gaillard, and G. Cottenceau. 2000. Antibacterial activity evaluation of microcin J25 against diarrheagenic Escherichia coli. Appl. Environ. Microbiol. 66:4595-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 45.Shi, Y., M. J. Cromie, F. F. Hsu, J. Turk, and E. A. Groisman. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53:229-241. [DOI] [PubMed] [Google Scholar]
- 46.Silva, M., N. Jacobus, C. Deneke, and S. L. Gorbach. 1987. Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother. 31:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannock, G. W. 2002. The bifidobacterial and Lactobacillus microflora of humans. Clin. Rev. Allergy Immunol. 22:231-253. [DOI] [PubMed] [Google Scholar]
- 49.Trent, M. S., W. Pabich, C. R. Raetz, and S. I. Miller. 2001. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083-9092. [DOI] [PubMed] [Google Scholar]
- 50.Vaara, M., and T. Vaara. 1983. Polycations as outer membrane-disorganizing agents. Antimicrob. Agents Chemother. 24:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaara, M., T. Vaara, M. Jensen, I. Helander, M. Nurminen, E. T. Rietschel, and P. H. Makela. 1981. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 129:145-149. [DOI] [PubMed] [Google Scholar]
- 52.van Langevelde, P., K. M. Kwappenberg, P. H. Groeneveld, H. Mattie, and J. T. van Dissel. 1998. Antibiotic-induced lipopolysaccharide (LPS) release from Salmonella typhi: delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob. Agents Chemother. 42:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wouters, P. C., A. P. Bos, and J. Ueckert. 2001. Membrane permeabilization in relation to inactivation kinetics of Lactobacillus species due to pulsed electric fields. Appl. Environ. Microbiol. 67:3092-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]