Abstract
We examined cooxidation of three different dichloroethenes (1,1-DCE, 1,2-trans DCE, and 1,2-cis DCE) by butane monooxygenase (BMO) in the butane-utilizing bacterium “Pseudomonas butanovora.” Different organic acids were tested as exogenous reductant sources for this process. In addition, we determined if DCEs could serve as surrogate inducers of BMO gene expression. Lactic acid supported greater rates of oxidation of the three DCEs than the other organic acids tested. The impacts of lactic acid-supported DCE oxidation on BMO activity differed among the isomers. In intact cells, 50% of BMO activity was irreversibly lost after consumption of ∼20 nmol mg protein−1 of 1,1-DCE and 1,2-trans DCE in 0.5 and 5 min, respectively. In contrast, a comparable loss of activity required the oxidation of 120 nmol 1,2-cis DCE mg protein−1. Oxidation of similar amounts of each DCE isomer (∼20 nmol mg protein−1) produced different negative effects on lactic acid-dependent respiration. Despite 1,1-DCE being consumed 10 times faster than 1,2,-trans DCE, respiration declined at similar rates, suggesting that the product(s) of oxidation of 1,2-trans DCE was more toxic to respiration than 1,1-DCE. Lactate-grown “P. butanovora” did not express BMO activity but gained activity after exposure to butane, ethene, 1,2-cis DCE, or 1,2-trans DCE. The products of BMO activity, ethene oxide and 1-butanol, induced lacZ in a reporter strain containing lacZ fused to the BMO promoter, whereas butane, ethene, and 1,2-cis DCE did not. 1,2-trans DCE was unique among the BMO substrates tested in its ability to induce lacZ expression.
Chlorinated ethenes (CEs), such as perchloroethene (PCE) and trichloroethene (TCE), are common groundwater contaminants that have been linked to liver and kidney damage and are suspected carcinogens (1). Although the microbially driven process of reductive dechlorination can effectively reduce the concentrations of both PCE and TCE under anaerobic groundwater conditions (5, 23, 27, 28), partially dechlorinated products, such as dichloroethenes (DCEs), often persist and disperse in groundwater plumes (35). Recent evidence has emerged for the existence of bacteria that will grow aerobically on 1,2-cis DCE as a sole C source, yet their enrichment is difficult and growth is extremely slow (8). In contrast, it is well documented that hydrocarbon-oxidizing microorganisms are able to degrade DCEs via reductant-driven cooxidative mechanisms (4, 6, 9, 15, 17, 19, 38, 39). Unfortunately, the need for the natural hydrocarbon to serve both as enzyme inducer and source of reductant results in competition between the natural substrate and CEs, and cooxidation efficiency is compromised (6, 11, 21, 24, 25, 37).
In this connection, several studies have shown that a combination of TCE and nonhydrocarbon substrates simultaneously induce the genes for toluene oxygenases and provide reductant for TCE oxidation by some toluene-degrading bacterial strains (26, 30, 36, 41). Conceptually, this observation has some appeal for bioremediation because it implies that oxygenases could be induced by CEs in the absence of a growth-supporting substrate and that cooxidation might be driven by alternative electron donors without interference from hydrocarbon substrates. The potential of DCEs to act as inducers of hydrocarbon-oxidizing systems has rarely been examined, and the results are equivocal. McClay et al. (30) showed that 1,2-cis DCE was a poor inducer of toluene oxygenase activity in Pseudomonas mendocina KR1, and 1,2-trans DCE did not induce at all. Neither compound significantly induced toluene monooxygenase in Pseudomonas stutzeri OX1 (33). In contrast, both 1,2-cis and 1,2-trans DCEs induced suboptimal levels of alkene monooxygenase activity in Xanthobacter sp. strain Py2, whereas short-chain-length alkanes and chloroalkanes did not (10). We thought it would be interesting to determine if DCEs induced butane monooxygenase (BMO) in the butane-oxidizing bacterium “Pseudomonas butanovora.” Butane-oxidizing bacteria degrade a wide range of unsaturated and saturated aliphatic chlorinated hydrocarbons individually and in complex mixtures (15, 17, 24, 25). Furthermore, because short-chain-length organic acids, such as acetic, propionic, butyric, and lactic acids, are metabolized effectively by butane-utilizing bacteria and are likely to be produced as fermentative end products under conditions where DCEs are produced, we thought it would be worthwhile to examine the efficacy of fatty acids as electron donors for BMO-dependent DCE oxidation. As the project evolved, it became clear that the response of “P. butanovora” to different DCE isomers pointed to the potential usefulness of the compounds for interrogating BMO operon regulation that extends beyond the practical implications of the work to DCE bioremediation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
“P. butanovora” (ATCC 43655) is an aerobic gram-negative bacterium that grows on n-alkanes (C2 to C9). The 16S rRNA gene nucleotide sequence of “P. butanovora” indicates that this bacterium is phylogenetically affiliated with the Rhodocyclus group, and its closest relatives belong to the genus Thauera rather than to the genus Pseudomonas (2). No formal attempt has been made to publish an official classification paper at this time. “P. butanovora” was grown in 750-ml flasks sealed with butyl rubber stoppers and containing 200 ml of a mineral salts medium consisting of (per liter) 2.7 g (NH4)2HPO4, 4.3 g Na2HPO4, 4.2 g KH2PO4, 0.5 g MgSO4 · 7H2O, 0.06 g CaCl2 · 2H2O, and 1 ml trace element solution (16) adjusted to pH 7.1. When butane served as the growth substrate, the gas (99% Airgas; Randor, Pennsylvania) was sterilized by passage through a 0.2-μm-pore-size filter and supplied as an overpressure of 7% volume/total volume (about 10% headspace) of the gas. When organic acids served as the carbon source for growth, a concentration of 10 mM was routinely used. Cultures were routinely grown at 30°C with constant circular shaking (180 rpm) for 2 days. Once cultures reached an optical density at 600 nm of about 0.7 (stationary phase), cells were harvested by centrifugation (10 min at 6,000 rpm and 10°C) and washed three times in 50 mM NaK phosphate buffer (pH 7.1). Harvested cells, kept on ice as a concentrated cell suspension, remained active for >3 h. Fresh cells were grown and harvested as needed.
Ethene oxidation assay.
Ethene-dependent ethene oxide (EtO) production is a convenient measure of BMO activity (16, 34). Vials (10 ml) sealed with butyl rubber stoppers and aluminum crimp caps served as the reaction chambers for this assay. Each vial contained 1 ml of whole-cell suspension (0.35 mg of cellular protein) and the specified electron donor, and 20% (vol/vol) ethene gas was added as overpressure to start the assay. The vials were agitated (150 rpm) at 30°C in a covered water bath shaker. Ethene oxide formation was followed by gas chromatography with a Shimadzu GC-8A equipped with a flame ionization detector and a 120-cm Porapak Q column (Alltech Associates, Inc.). The column temperature was maintained at 120°C, and the injector and detector were maintained at 200°C as previously described (17). Protein concentrations in the cell suspensions were determined using the biuret assay (13) and were 0.35 mg protein per vial.
Cooxidation of DCEs.
The cooxidation of DCEs was followed by measuring their disappearance from the headspace by gas chromatography. Abiotic controls, which did not receive “P. butanovora,” and butane-grown “P. butanovora” pretreated with acetylene, a mechanism-based inhibitor of BMO (16), were used to ensure that the disappearance of DCEs was due to BMO activity. Vials (10 ml) containing phosphate buffer (900 μl) and the specified electron donor were prepared. Aliquots of DCE saturated solutions were injected into vials that had been sealed with Teflon-lined butyl rubber stoppers. DCEs were allowed to equilibrate between the gas and aqueous phases by shaking the vials at 150 rpm and 30°C for 30 min prior to the start of the assay. Assays were initiated by the injection of 100 μl (0.5 mg protein) of concentrated cell suspension.
Effects of DCE cooxidation on BMO activity.
Vials were prepared as described above. Accurate assessment of time-dependent inhibition of BMO required the prompt interruption of the cooxidation reaction. To accomplish this, butane was flushed into the headspace of the vial. Conceptually, this prevented the continued cooxidation of the DCE during the washing procedure by purging DCE out of the vial while providing the natural substrate to compete for the BMO active site. Two 60-ml syringes were used; the first syringe was kept empty with the plunger depressed, and the other syringe was filled with butane. Both syringes were inserted into the vial, and pulling back the plunger of the empty syringe pulled the butane from the second syringe through the vial. The cells were washed three times by centrifugation, resuspended in phosphate buffer, and tested for residual BMO activity using the ethene oxidation assay.
Inhibition of O2 uptake by DCEs.
Lactate-dependent O2 uptake was followed using a Clark style O2 electrode in a glass reaction vial (YSI model 5300 biological oxygen monitor; Yellow Springs Co., Yellow Springs, OH). The temperature of the reaction chamber was maintained at 30°C. Phosphate buffer (1.75 ml; 50 mM) was allowed to equilibrate with air for 5 min before the start of each assay, and the reaction chamber was washed 20 times with water and twice with phosphate buffer between assays to remove residual carbon sources and DCEs. The reaction chamber was capped, and additions were made in the following order: butane-grown “P. butanovora” cells (0.2 mg protein), sodium lactate (2 mM final concentration), and the appropriate cosubstrate (5 nmol in the electrode chamber). This order of addition allowed the sequential determination of resting-cell O2 consumption, lactate-dependent O2 consumption, and the effects of the cosubstrate on O2 consumption. Controls were treated in the same manner, except that cells were first treated with acetylene to inactivate BMO.
Induction of BMO by DCEs.
“P. butanovora” was grown in mineral salts medium containing 10 mM lactate, a repressor of BMO (34). Sealed 10-ml vials containing 900 μl of medium and various concentrations of the test compounds (10 to 100 μM) were allowed to reach equilibrium by shaking them at 30°C for 30 min. Aliquots of washed (to remove lactate) uninduced cells (100 μl) were injected into the vials and incubated for 4 h. Positive (butane) and negative buffer controls were included. After 4 h, cells were harvested by centrifugation and tested for BMO activity using the ethene oxidation assay described above.
Induction of the lacZ reporter strain.
A reporter strain of “P. butanovora” (bmoX::lacZ::kanr) was constructed with a bicistronic expression system in which kanamycin resistance is constitutive and the BMO promoter controls β-galactosidase (lacZ) expression. The lacZ transcriptional fusion provided multiple in-frame transcriptional stop codons immediately upstream of the BMO structural genes. The reporter strain was grown in the same growth medium as the wild-type cells, except that kanamycin sulfate (20 μg/ml) was added to the medium. Inductions were performed in 10-ml vials with 1 ml of the reporter strain culture diluted with growth medium to an optical density at 600 nm of 0.3. All vials were sealed with butyl rubber stoppers and aluminum crimp caps. Inductions were 2 or 4 h in length and were initiated by the addition of cells. β-Galactosidase activity was determined by adding 2 μl sodium dodecyl sulfate (10%), 2 μl β-mercaptoethanol, 4 μg orthonitrophenyl β-d-galactopyranoside (ONPG), and 2 μl chloroform. The vials were incubated at 36°C, and the reactions were stopped by the addition of 200 μl 5% (wt/vol) calcium carbonate. The amount of nitrophenyl product formed was determined colorimetrically and is reported in Miller units (31).
Analytical methods.
Analytical-high-performance liquid chromatography grade PCE, TCE, 1,2-trans DCE, 1,2-cis DCE, and 1,1-DCE were acquired from Sigma-Aldrich (St. Louis, Mo.). PCE, TCE, 1,2-cis DCE, 1,2-trans DCE, and 1,1-DCE saturated solutions were prepared by agitating two-phase solutions (aqueous/chlorinated ethene) for 24 h on an orbital shaker. The saturated aqueous phase was used to aliquot chlorinated ethenes into the reaction vials. Concentrations of chlorinated ethenes in the aqueous phase were assumed to follow their unitless Henry's constants (14).
RESULTS
BMO-dependent cooxidation of ethenes by different exogenous electron donors.
Organic acids supported ethene cooxidation by butane-grown “P. butanovora” at different rates, with lactate and acetate being superior to butyrate, while formate and propionate did not increase BMO activity above endogenous background levels (Table 1). 1,2-trans DCE was transformed the most slowly of the three DCEs tested, and the superiority of both lactate and acetate over the other electron donors was definitely evident. 1,1-DCE was transformed the most rapidly, and lactate clearly supported the highest rate of oxidation. Furthermore, the organic acids showed differences in their abilities to sustain linear rates of ethene oxidation. For example, when butyrate served as the exogenous electron donor, the linearity of ethene oxidation could not be maintained for greater than 5 min. In contrast, lactate-supported cooxidation remained linear for at least 20 min (data not shown). Lactate was chosen to be the exogenous electron donor for the remainder of the study.
TABLE 1.
Influences of exogenous sources of reductant on initial rate of ethene, 1,1-DCE, 1,2-cis DCE, and 1,2-trans DCE cooxidation in butane-grown “P. butanovora”
| Reductant source | Initial rate of oxidationa (nmol min−1 mg protein−1)
|
|||
|---|---|---|---|---|
| Ethene | 1,1-DCE | 1,2-cis DCE | 1,2-trans DCE | |
| Buffer | 3.6 (1) | 4.0 (1.0) | 0.9 (1.0) | 1.6 (1.0) |
| Butyrate | 13.2 (3.7) | 22.0 (5.5) | 6.8 (7.6) | 2.0 (1.3) |
| Propionate | 3.4 (0.9) | 10.0 (2.5) | 5.9 (6.6) | 0.4 (0.3) |
| Acetate | 17.0 (4.7) | 16.4 (4.1) | 8.5 (9.4) | 3.8 (2.8) |
| Formate | 0.7 (0.2) | 3.6 (0.9) | 1.4 (1.6) | 1.2 (0.7) |
| Lactate | 20.1 (5.6) | 28.4 (7.1) | 11 (12.2) | 4.5 (2.8) |
Values in parentheses represent the increase (n-fold) above the endogenous (buffer) rate.
Properties of DCE cooxidation by butane-grown “P. butanovora.”
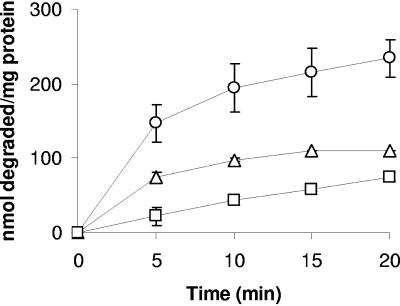
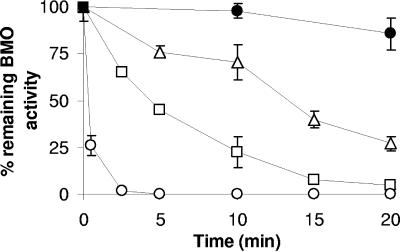
The kinetics of lactate-driven oxidation varied among the DCEs. The initial rates of oxidation of 1,1-DCE and 1,2-cis DCE were highest and also decreased rapidly with time, while the rate of 1,2-trans DCE oxidation was lower and remained constant for approximately 15 min (Fig. 1). Distinctly different time-dependent irreversible loss of BMO activity was observed upon exposure of butane-grown “P. butanovora” to each of the DCEs (Fig. 2). In the case of 1,1-DCE, one half of BMO activity was irreversibly lost within 0.5 min of exposure and after oxidation of approximately 25 nmol mg protein−1. In the case of 1,2-trans DCE, one half of BMO activity was lost after 5 min of incubation, during which a similar amount (20 nmol 1,2-trans DCE mg protein−1) was oxidized. The products of 1,2-trans DCE and 1,1-DCE must be equally toxic to BMO activity despite being produced at 10-fold-different rates. Decreases in cooxidation activity caused by damage to the BMO enzyme per se versus damage to cellular processes, such as reductant generating potential or loss of membrane integrity, could not be distinguished.
FIG. 1.
Time course of 1,2-cis DCE (▵), 1,2-trans DCE (□), and 1,1-DCE (○) degradation by butane-grown “P. butanovora.” The concentration of each DCE at the start of the assay was 25 μM aqueous phase. Lactate (5 mM) served as the exogenous electron donor. The data points represent the means of three replicates, and the error bars represent standard deviations.
FIG. 2.
Time course of the irreversible loss of BMO activity during the consumption of 1,2-cis DCE (▵), 1,2-trans DCE (□), and 1,1-DCE (○) by butane-grown “P. butanovora.” Incubations were performed as described in the legend to Fig. 1, except that 50 μM DCE was used to ensure that the substrate was not limiting. Cells that did not receive DCEs served as positive controls (•). The data points are the means of three replicates, and the error bars represent standard deviations.
In the case of 1,2-cis DCE, however, BMO activity declined to 50% of initial activity after transformation of a much larger amount (120 nmol mg protein−1) over 15 min.
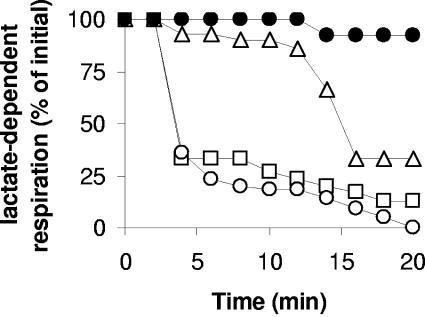
More evidence was obtained for different oxidation-induced cytotoxic effects among the DCEs by measuring lactic-acid-supported O2 consumption (Fig. 3). Cells were allowed to oxidize an amount of each DCE (25 nmol mg protein−1) that was significantly less than the maximum transformation capacities of these compounds (i.e., 200, 130, and 90 nmol mg protein−1 of 1,1-DCE, 1,2-cis DCE, and 1,2-trans DCE, respectively). After exposure to either 1,1-DCE or 1,2-trans DCE, lactic-acid-dependent O2 consumption followed similar kinetics of decline despite the fact that these amounts of 1,1-DCE and 1,2-trans DCE were consumed in <0.5 min and 5 min, respectively. In contrast, lactic-acid-dependent O2 consumption was much less sensitive to 1,2-cis DCE oxidation. Cells oxidized the aliquot of 1,2-cis DCE in about 2.5 min and retained the majority of their lactate-dependent O2 uptake for about 14 min before it declined abruptly (Fig. 3). These data indicate that (i) lactate-supported cellular respiration is generally less sensitive than BMO activity to the product(s) of 1,1-DCE oxidation; (ii) despite being produced at a lower rate, the product(s) of 1,2-trans DCE oxidation is a potent inhibitor of both cellular respiration and BMO activity; and (iii) given a long enough incubation, a small amount of the product(s) of 1,2-cis DCE oxidation is toxic to lactic-acid-driven cellular respiration, while BMO activity is less sensitive. In all three cases, cell viability had declined by >90% (as measured by dilution plating onto R2A agar) after 15 min of incubation, indicating that oxidation of an amount of each DCE much lower than their respective transformation capacities was sufficient to incapacitate cell division by the majority of cells (data not shown).
FIG. 3.
Time course of lactate-dependent O2 respiration by butane-grown “P. butanovora” during the consumption of 25 μM of 1,2-cis DCE (▵), 1,2-trans DCE (□), and 1,1-DCE (○). The values are expressed as percentages of the initial rate of respiration. Cells treated with acetylene to inactivate BMO served as positive controls (•).
Induction of BMO activity in wild-type “P. butanovora” and of LacZ expression in a reporter strain by CEs.
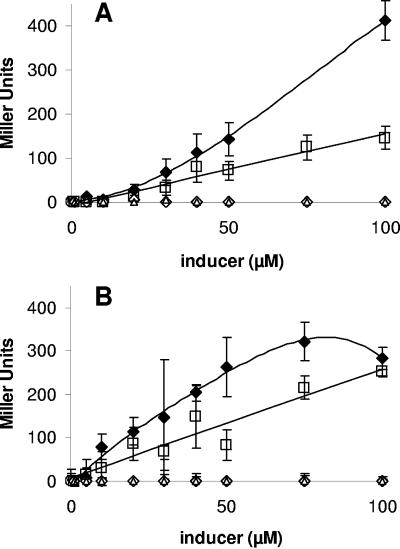
1,2-trans DCE and 1,2-cis DCE induced 47 and 25%, respectively, of the BMO activity of butane-exposed cells over a 4-h incubation (Table 2). In contrast, neither PCE, TCE, nor 1,1-DCE induced measurable expression of BMO activity. A minimum concentration (20 μM) of DCE was required to induce the BMO activity, which could not be induced further by increasing the DCE concentration. In the case of the lacZ reporter strain, neither butane, ethene, nor most chlorinated substrates, including 1,2-cis DCE, was able to induce lacZ expression. In contrast, the stable products of BMO activity, 1-butanol and ethene oxide, effectively induced lacZ expression (Table 2). 1,2-trans DCE was the only substrate that induced lacZ expression in the reporter strain. Increases in lacZ activity occurred more rapidly in response to 1-butanol than to 1,2-trans DCE (Fig. 4). However, over a 4-h incubation, 75 to 100 μM 1,2-trans DCE induced the same amount of lacZ activity in the reporter strain as did the same concentrations of 1-butanol.
TABLE 2.
Induction of BMO activity in wild-type “P. butanovora” and β-galactosidase activity in the lacZ-BMO fusion in response to various substrates and products of BMO
| Compound | Concn (μM) | BMO activity in wild type (% of butane control) | LacZ activity in reporter strain (Miller units)e |
|---|---|---|---|
| Butane | 50 | 100 | 0 |
| 1-Butanol | 50 | >10b | 263 ± 68 |
| Butyrate | 50 | 0 | 0 |
| Ethylene | 517a | 24c | 0 |
| Ethylene oxide | 8,000a | NDd | 89 ± 13 |
| PCE | 50 | 0 | 0 |
| TCE | 50 | 0 | 0 |
| 1,2-trans DCE | 50 | 47 | 84 ± 36 |
| 1,2-cis DCE | 50 | 25 | 0 |
| 1,1-DCE | 50 | 0 | 0 |
Gas added as 10% headspace.
1-Butanol is consumed during the 4-h incubation, and the extent of induction of BMO relative to butane could not be accurately determined in wild-type cells.
Ethene oxide accumulation was measured during a 4-h incubation of lactate-grown cells in the presence of ethene.
ND, not determined. Ethene oxide, in the absence of ethene, inhibits BMO activity in wild-type cells, and induction of BMO could not be accurately quantified in wild-type cells.
Results indicate the increase in β-galactosidase above the level of the buffer control. Background levels of β-galactosidase were typically about 50 Miller units.
FIG. 4.
Induction response of lacZ in the reporter strain of “P. butanovora” to increasing concentrations of 1-butanol (⧫), 1,2-cis DCE (▵), 1,2-trans DCE (□), and 1,1-DCE (○). β-Galactosidase (lacZ) activities were determined at time intervals of 2 h (A) and 4 h (B) as described in Materials and Methods. The data points are the means of three replicates, and the error bars represent standard deviations.
DISCUSSION
Many studies have been carried out over the past 15 years on the cooxidation of CEs by hydrocarbon-oxidizing bacteria, and the subject has been thoroughly investigated and reviewed (4, 12, 29, 35). Nonetheless, this study uncovered some novel findings, including the unexpected superiority of lactic acid metabolism to supply reductant to BMO, and it illustrated the potential of DCEs to uncover new insights into the regulation of the BMO operon.
The use of exogenous electron donors has allowed researchers to examine the substrate range and kinetic parameters of cooxidation while avoiding the competitive effects that occur when natural substrates are provided as electron donors (6, 7, 16, 17, 18). For example, formic acid has been used widely as an electron donor in whole-cell kinetic studies of CE cooxidation by methanotrophic bacteria (6, 7, 38, 39), and by analogy, initial studies carried out on butane-oxidizing bacteria have routinely used butyric acid as an electron donor (16, 17). Nonetheless, our data show that while various organic acids supported BMO activity in “P. butanovora,” differences exist in the maximum rates obtained. Lactic acid consistently supported greater rates of oxidation than did the other organic acids, and the choice of butyric acid as an electron donor might have compromised interpretation of previously published data from our own laboratory that indicated 1,2-trans DCE was not cooxidized by butane-grown “P. butanovora” (17).
Although it remains unclear why lactic acid is a superior reductant for driving cooxidation in “P. butanovora,” several possibilities should be considered. First, enzymes involved in generating reductant from butyric acid might be extremely sensitive to toxic products of the cooxidation process. In this context, it is interesting that pyruvate, a known antioxidant and the first product of lactic acid metabolism, offset some of the damage that occurred during TCE cooxidation in Burkholderia cepacia G4 (41). Second, it is possible that downstream products of alkane oxidation (alcohols, aldehydes, and fatty acids) are structurally similar enough to the natural substrates to make them inhibitors of BMO. Both a bacterial aromatic oxygenase and a peroxidase were irreversibly inactivated either during the catalytic oxidation of phenylpropionaldehyde and phenylbutyraldehyde (32) or by aliphatic fatty acids in general (20). If a similar phenomenon occurs in BMO, it would provide a mechanism to explain why the aliphatic fatty acids, in particular, did not sustain BMO activity.
Finally, it was interesting that relative to lactic acid, propionate did not support BMO-dependent oxidation activities in butane-grown cells as well as it did in propane-grown cells (D. M. Doughty, D. J. Arp, P. J. Bottomley, unpublished observations). Although alkane substrates, such as butane and propane, appear to be oxidized to their respective aliphatic acids by the same enzyme (3), different downstream pathways are probably involved in metabolism of these fatty acids and will influence their efficacy as electron donors for cooxidation by BMO. Further studies are under way to examine the roles of “downstream” propionate and butyrate metabolism in supporting and regulating butane- and propane-oxidizing activities in “P. butanovora.”
Although methane monooxygenase (MMO) has been shown to oxidize the different isomeric forms of DCE (6, 22, 38, 39), our observations indicate that BMO responded differently to the oxidation of DCEs than did MMO. For example, 1,2-trans DCE was oxidized at a lower rate by BMO than either 1,1-DCE or 1,2-cis DCE, resulting in a much smaller amount of 1,2-trans DCE turnover being required to inactivate BMO than 1,2-cis DCE. These data contrast with an earlier report from studies carried out on Methylosinus trichosporium OB3b, in which 1,2-cis DCE oxidation inactivated MMO and reduced cell viability more rapidly than did oxidation of 1,2-trans DCE (39). The different sensitivities of M. trichosporium OB3b to DCEs was attributed to differential activity of MMO toward the epoxides of the cis and trans DCEs. Further studies are required to compare in more detail the effects of 1,2-trans DCE, 1,2-cis DCE, and their epoxides on BMO and MMO.
We also obtained evidence for the potential of 1,2-DCE isomers as tools for dissecting BMO gene regulation. Although it was shown previously that butane, 1-butanol, and butyraldehyde are positive effectors of BMO transcription in “P. butanovora” (34), it was not possible to unequivocally separate substrate from product induction. The same problem was encountered with Xanthobacter sp. strain Py2, in which both 1,2-cis and 1,2-trans DCEs partially induced ethene monooxygenase activity (10). The lack of butane-dependent induction of lacZ in the reporter strain of “P. butanovora,” coupled with the ability of 1-butanol to induce lacZ, implies that BMO gene expression is up regulated in response to the products of alkane oxidation. This idea is consistent with the fact that ethene oxide is an inducer of lacZ while ethene is not and that 1,2-cis DCE is an inducer of the wild type but not of the lacZ reporter strain. Our data illustrated, however, that 1,2-trans DCE behaved uniquely by inducing as much lacZ expression as the natural product, 1-butanol. At this time, however, we cannot discount the possibility that induction by 1,2-trans DCE represents another undefined form of gene regulation, as was suggested for toluene monooxygenase induction by PCE and TCE in P. stutzeri OX1 (33). Further work on the regulation of the BMO operon by substrates and products of BMO activity is under way in our laboratory.
Acknowledgments
This research was supported by a grant from the U.S. Environmental Protection Agency-sponsored Western Region Hazardous Substance Research Center under agreement R-828772, National Institutes of Health grant no. 5RO1 GM56128-06, and the Oregon Agricultural Experiment Station. D.M.D. acknowledges partial financial support from a teaching assistantship and a Janet Ford Scholarship from the Department of Microbiology.
REFERENCES
- 1.Agency for Toxic Substances and Disease and Registry. 1995. Toxicological profile for trichloroethylene. U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga.
- 2.Anzai, Y., H. Kim, J. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 50:1563-1589. [DOI] [PubMed] [Google Scholar]
- 3.Arp, D. J.1999. Butane metabolism by butane-grown ′Pseudomonas butanovora'. Microbiology 145:1173-1180. [DOI] [PubMed] [Google Scholar]
- 4.Arp, D. J., C. M. Yeager, and M. R. Hyman. 2001. Molecular and cellular fundamentals of aerobic cometabolism of trichloroethene. Biodegradation 12:81-103. [DOI] [PubMed] [Google Scholar]
- 5.Beeman, R. E., and C. A. Bleckman. 2002. Sequential anaerobic-aerobic treatment of an aquifer contaminated by halogenated organics: field results. J. Contam. Hydrol. 57:147-159. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H.-L., and L. Alvarez-Cohen. 1996. Biodegradation of individual and multiple chlorinated apliphatic hydrocarbons by methane-oxidizing cultures. Appl. Environ. Microbiol. 62:3371-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, K. H., and L. Alvarez-Cohen. 1999. Evaluation of toxic effects of aeration and trichloroethylene oxidation on methanotrophic bacteria grown with different nitrogen sources. Appl. Environ. Microbiol. 65:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman, N. V., T. E. Mattes, J. M. Gossett, and J. C. Spain. 2002. Biodegradation of cis-dichloroethene as the sole carbon source by a β-proteobacterium. Appl. Environ. Microbiol. 68:2726-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan, M. E., and P. L. McCarty. 1995. Methanotrophic chloroethene transformation capacities and 1,1-dichloroethene transformation product toxicity. Environ. Sci. Technol. 29:2741-2747. [DOI] [PubMed] [Google Scholar]
- 10.Ensign, S. A. 1996. Aliphatic and chlorinated alkenes and epoxides as inducers of alkene monooxygenase and epoxidase activities in Xanthobacter strain Py2. Appl. Environ. Microbiol. 62:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folsom, B. R., P. J. Chapman, and P. H. Pritchard. 1990. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl. Environ. Microbiol. 56:1279-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman, D. L., A. S. Danko, and M. F. Varce. 2001. Substrate interactions during aerobic biodegradation of methane, ethane, vinyl chloride, and 1,2-dichloroethenes. Water Sci. Technol. 43:333-340. [PubMed] [Google Scholar]
- 13.Gornall, A. G., C. J. Bardawill, and M. M. David. 1949. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177:751-766. [PubMed] [Google Scholar]
- 14.Gossett, J. M. 1987. Measurement of Henry's law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 21:2002-2008. [Google Scholar]
- 15.Halsey, K. H., L. A. Sayavedra-Soto, P. J. Bottomley, and D. J. Arp. 2005. Trichloroethylene degradation by butane-oxidizing bacteria causes a spectrum of toxic effects. Appl. Microbiol. Biotechnol. [Online.] doi: 10.1007/s00253-005-1944-z. [DOI] [PubMed]
- 16.Hamamura, N., R. T. Storfa, L. Semprini, and D. J. Arp. 1999. Diversity in butane monooxygenases among butane-grown bacteria. Appl. Environ. Microbiol. 65:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamamura, N., C. Page, T. Long, L. Semprini, and D. J. Arp. 1997. Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora, and Mycobacterium vaccae JOB5 and methane-grown Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 63:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry, S. M., and D. Grbic-Galic. 1991. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl. Environ. Microbiol. 57:236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins, G. D., and P. L. McCarty. 1995. Field evaluation of in situ aerobic cometabolism of trichloroethylene and three dichloroethylene isomers using phenol and toluene as primary substrates. Environ. Sci. Technol. 29:1628-1637. [DOI] [PubMed] [Google Scholar]
- 20.Huang, L., C. Colas, and P. R. Ortiz de Monetellano. 2004. Oxidation of carboxylic acids by horseradish peroxidase results in prosthetic heme modification and inactivation. J. Am. Chem. Soc. 126:12865-12876. [DOI] [PubMed] [Google Scholar]
- 21.Hyman, M. R., S. A. Russell, R. L. Ely, K. K. Williamson, and D. J. Arp. 1995. Inhibition, inactivation, and recovery of ammonia-oxidizing activity in cometabolism of trichloroethene by Nitrosomonas europaea. Appl. Environ. Microbiol. 61:1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen, D., B. G. Grobben, R. Hoekstra, R. Oldenhuis, and B. Witholt. 1988. Degradation of 1,2-trans dichloroethene by mixed cultures of methanotrophic bacteria. Appl. Environ. Biotechnol. 29:392-399. [Google Scholar]
- 23.Kastner, M. 1991. Reductive dechlorination of tri- and tetrachloroethylenes depends on transition from aerobic to anaerobic conditions. Appl. Environ. Microbiol. 57:2039-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, Y., D. J. Arp, and L. Semprini. 2002. Kinetic and inhibition studies for the aerobic cometabolism of 1,1,1-trichloroethane, 1,1-dichloroethylene and 1,1-dichloroethane by a butane-grown mixed culture. Biotechnol. Bioeng. 80:498-508. [DOI] [PubMed] [Google Scholar]
- 25.Kim, Y., L. Semprini, and D. J. Arp. 1997. Aerobic cometabolism of chloroform and 1,1,1-trichloroethane by butane-grown microorganisms. Bioremediation J. 1:135-148. [Google Scholar]
- 26.Leahy, J. G., A. M. Byrne, and R. H. Olsen. 1996. Comparison of factors influencing trichloroethylene degradation by toluene-oxidizing bacteria. Appl. Environ. Microbiol. 62:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major, D. W., M. L. McMaster, E. E. Cox, E. A. Edwards, S. M. Dworatzek, E. R. Hendrickson, M. G. Starr, J. A. Payne, and L. W. Buonamici. 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ. Sci. Technol. 36:5106-5116. [DOI] [PubMed] [Google Scholar]
- 28.Maymo-Gatell, X., I. Nijenhuis, and S. H. Zinder. 2001. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by “Dehalococcoides ethenogenes”. Environ. Sci. Technol. 35:516-521. [DOI] [PubMed] [Google Scholar]
- 29.McCarty, P. L. 1993. In situ bioremediation of chlorinated solvents. Curr. Opin. Biotechnol. 4:323-330. [Google Scholar]
- 30.McClay, K., S. H. Streger, and R. J. Steffan. 1995. Induction of toluene oxidation activity in Pseudomonas mendocina KR1 and Pseudomonas sp. strain ENVPC5 by chlorinated solvents and alkanes. Appl. Environ. Microbiol. 61:3479-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Raner, G. M., A. J. Hatchell, P. E. Morton, D. P. Ballou, and M. J. Coon. 2000. Stopped-flow spectrophotometric analysis of intermediates in the peroxo-dependent inactivation of cytochrome P450 by aldehydes. J. Inorg. Biochem. 81:153-160. [DOI] [PubMed] [Google Scholar]
- 33.Ryoo, D., H. Shim, F. L. Arenghi, P. Barbieri, and T. K. Wood. 2001. Tetrachloroethylene, trichloroethylene, and chlorinated phenols induce toluene-o-xylene monooxygenase activity in Pseudomonas stutzeri OX1. Appl. Microbiol. Biotechnol. 56:545-549. [DOI] [PubMed] [Google Scholar]
- 34.Sayavedra-Soto, L. A., C. M. Byrd, and D. J. Arp. 2001. Induction of butane consumption in ′Pseudomonas butanovora'. Arch. Microbiol. 176:114-120. [DOI] [PubMed] [Google Scholar]
- 35.Semprini, L. 1997. Strategies for the aerobic co-metabolism of chlorinated solvents. Curr. Opin. Biotechnol. 8:296-308. [DOI] [PubMed] [Google Scholar]
- 36.Shingleton, J. T., B. M. Applegate, A. C. Nagel, P. R. Bienkowski, and G. S. Sayler. 1998. Induction of the tod operon by trichloroethylene in Pseudomonas putida TVA8. Appl. Environ. Microbiol. 64:5049-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strand, S. E., M. D. Bjelland, and H. D. Stensel. 1990. Kinetics of chlorinated hydrocarbon degradation by suspended cultures of methane-oxidizing bacteria. Res. J. Water Pollut. Control Fed. 62:124-129. [Google Scholar]
- 38.van Hylckama Vleig, J. E. T., W. de Koning, and D. B. Janssen. 1996. Transformation kinetics of chlorinated ethenes by Methylosinus trichosporium OB3b and detection of unstable epoxides by on-line gas chromatography. Appl. Environ. Microbiol. 62:3304-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hylckama Vlieg, J. E. T., W. de Koning, and D. B. Janssen. 1997. Effect of chlorinated ethene conversion on viability and activity of Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 63:4961-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Yeager, C. M., K. M. Arthur, P. J. Bottomley, and D. J. Arp. 2004. Trichloroethylene degradation by toluene-oxidizing bacteria grown on non-aromatic substrates. Biodegradation 15:19-28. [DOI] [PubMed] [Google Scholar]
- 42.Yeager, C. M., P. J. Bottomley, and D. J. Arp. 2001. Cytotoxicity associated with trichloroethylene oxidation in Burkholderia cepacia G4. Appl. Environ. Microbiol. 67:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]