Abstract
Sperm competition theory predicts that males should strategically allocate their sperm reserves according to the level of sperm competition, defined as the probability that the sperm of two males compete for fertilizing a given set of ova. Substantial evidence from numerous animal taxa suggests that, at the individual level, sperm expenditure increases when the risk of sperm competition is greater. In contrast, according to the “intensity model” of sperm competition [Parker, G. A., Ball, M. A., Stockley, P. & Gage, M. J. G. (1996) Proc. R. Soc. London Ser. B 263, 1291–1297], when more than two ejaculates compete during a given mating event, sperm expenditure should decrease as the number of competing males increases. Empirical evidence supporting this prediction, however, is still lacking. Here we measured sperm expenditure in two gobiid fishes, the grass (Zosterisessor ophiocephalus) and black goby (Gobius niger), in which up to six sneakers can congregate around the nest of territorial males and release their sperm when females spawn. We show that, in accordance with theory, sneaker males of both species release fewer sperm as the number of competitors increases.
In many animal taxa females mate multiply and sperm competition is therefore an important evolutionary force in sexually reproducing animals (1). The most common adaptation to high levels of sperm competition in males is represented by an increase in sperm expenditure at mating to increase their probability of fertilizing the eggs (2, 3). However, ejaculates can be energetically costly to produce (4–7), and males are expected to allocate sperm strategically in response to varying levels of sperm competition (2, 8). Specifically, theory predicts that ejaculate expenditure should depend on the number of males competing for fertilization (9, 10). When the probability of competition between a maximum of two ejaculates is low, male gametic expenditure is predicted to increase with sperm competition risk (the so-called “risk model”; ref. 10). Support for this prediction comes from several within-species studies (11–18). However, in other instances, such as the group-spawning fishes (19), several ejaculates compete simultaneously for the same set of eggs. Under such circumstances, theoretical models predict that an individual male that faces variable levels of sperm competition among successive spawns or matings should release fewer sperm as the estimated number of competitors at a given spawning increases above two, because returns are diminishing for providing more sperm (the so-called “intensity model”; refs. 9 and 20). This counterintuitive prediction is due to the fact that in spawns with several competitors the chances of encountering an unfertilized egg are too low to favor the release of additional sperm. In other words, if a male can strategically allocate sperm among spawns, an increase in output will profit more when the intensity of sperm competition is low.
Although fish are anatomically capable of adjusting their sperm release between spawns (6, 17), experimental evidence supporting the intensity model is still lacking, probably because it is difficult to determine the actual number of males in a spawning group and to attribute the sperm released in the spawn to a specific individual (21). Here we overcome these problems by using two shallow water gobiid fishes, the grass goby and the black goby, in which large, territorial males defend a nest in which females lay their eggs (22). During spawning sneaker males, which are usually younger and smaller than territorial ones, attempt to release their sperm either in the nest or in close proximity to it (22). Sperm competition is intense in these two species, because about 70% of the spawns have sneakers and up to six sneakers have been observed during spawning (23, 24). Here we present the results of an experiment in which we measured the sperm expenditure of a focal sneaker male during simulated spawning events in which we experimentally manipulated the number of attending sneakers.
Methods
The fish used in this experiment were either collected from the Venice Lagoon, Italy, under a scientific fishing permit from the Regione Veneto or provided by local professional fishers. They were acclimated for 2 days in large outdoor tanks (120 liters) at the Chioggia Hydrobiological Station of the University of Padova, and released back into the wild after being used in the tests. Territorial and sneaker males were separated on the basis of body size, presence of sexual secondary characters, and ejaculate characteristics (22–24). Sexual maturity of males used in the experiments was confirmed by the release of a droplet of seminal fluid after the application of a gentle pressure on the abdomen.
A territorial male was allowed to settle 1 day in the compartment containing an artificial nest (Fig. 1). The experiment commenced with the introduction of the focal sneaker in the small central compartment and 0, 1, 2, or 4 competitor sneakers in the two side compartments, according to the experimental treatment. After 5 min of acclimation, 100 μl (grass goby) or 10 μl (black goby) of urine of territorial males were released into the compartment of the focal sneaker. For this purpose, five territorial males of each species were humanely killed and their urine samples were collected and pooled. Aliquots of these pools were stored at −20°C until used. Fresh aliquots were prepared every week. The urine of territorial males contains pheromones that are released by the male during courtship and induce the female to spawn (25). Previous observations demonstrated that such quantities of urine were sufficient to induce females to spawn and sneakers to release sperm (ref. 25; A.P., M.S., and M.B.R., unpublished observations).
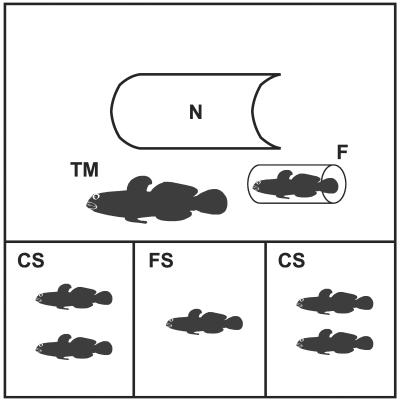
Figure 1.
Plan view of the experimental tank (60 × 36 × 40 height cm). The tank was divided into four compartments: the large compartment (40 × 36 cm) contained a plastic nest (20 cm long and with a diameter of 15 cm, N), a territorial male (TM) and a ripe female (F), confined in a transparent Plexiglas tube. The focal sneaker (FS) was placed in the central compartment (20 × 12 × 40 height cm), whereas 0, 1, 2, or 4 competitor sneakers (CS) were placed in the two side compartments.
After 5 min, a ripe female was placed in the compartment of the territorial male. The female was confined in a transparent Plexiglas tube that was placed in front of the nest. Territorial males started to court the female immediately and release sperm in the nest (mean concentration of sperm after 30 min, grass goby = 275.0 sperm per ml ± 82.8 SEM, n = 28; black goby = 234.4 sperm per ml ± 41.5 SEM, n = 16). After 10 min, the female was moved into the nest, and an aliquot of 500 μl of female ovarian fluid was released in the compartment of the focal sneaker. Ovarian fluid is released by females during egg laying, and acts as a spawning cue for sneakers (A.P., M.S., and M.B.R., unpublished observations). Ovarian fluid was collected from the genital papilla of ripe females that were previously anesthetized, by exerting a gentle pressure on their abdomen. Aliquots of ovarian fluid were prepared from the pool of the ovarian fluids of five females, stored at −20°C, and used within 1 week. After that, a new ovarian fluid stock was prepared from five other females. Stripped females were then released back to the point of capture. Simulated spawnings were used because natural spawnings usually last 4–12 h, and females can enter and exit the nest several times during spawning (26), which would have introduced large variance in the characteristics of the spawnings that may have influenced the behavior of the focal sneakers. Thirty minutes after the introduction of the female, 80 ml of water was sampled from the compartment of the focal sneaker, and one of the authors (M.S.) determined sperm concentration blind of the experimental group (24). Previous observations demonstrated that in both species the spermatozoa remain viable in water up to 2 h, without changing their concentration over time (23).
Eight and five replicates were run in each condition for the grass goby (total n = 32) and the black goby (total n = 20), respectively. Focal sneakers were tested only once and all replicates were therefore independent observations. Experiments were performed May 18–31, 2001 (grass goby) and July 29 through August 17, 2001 (black goby). Mean total length ± SD (range) of the fish used was as follows: territorial male: grass goby, 18.4 ± 0.37 cm (18.1–19.3); black goby, 11.1 ± 0.72 cm (10.5–12.3); focal sneaker: grass goby, 9.7 ± 1.03 cm (7.5–11.3); black goby, 7.9 cm ± 0.65 (5.6–8.2); female: grass goby, 11.2 ± 0.79 cm (9.2–13.3); black goby, 11.1 ± 0.72 cm (10.5–12.3). Total length of territorial males, females, and focal sneakers did not differ among treatment groups (ANOVA, all P > 0.19). Competitor sneakers were randomly chosen from a stock of sneakers (n = 30 and n = 25 for the grass and the black goby, respectively). After each test, stimulus sneakers were put back into the stock tank, and, therefore, some of them were used more than once.
Data were checked for normality and homogeneity of the variance. Where not otherwise stated, means ± SEM are reported. Statistical analyses were done by using SPSS 10.1.
Results
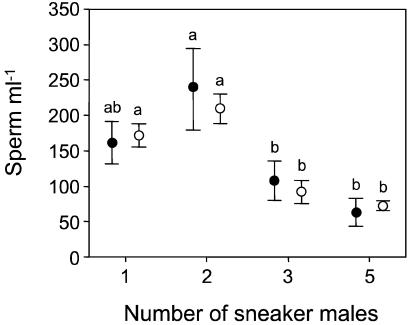
Overall, sperm expenditure of the focal sneaker was 142.0 ± 20.8 sperm per ml (n = 32) and 136.1 ± 14.8 sperm per ml (n = 20) for grass and black goby, respectively. All focal sneakers released sperm during the trial with the exception of one grass goby in the experimental group with three sneakers. In both species, the sperm expenditure of the focal sneaker differed significantly according to the number of attending sneakers (ANOVA, grass goby: F3,28 = 4.22, P = 0.014; black goby: F3,16 = 17.10, P < 0.001; Fig. 2). Removing the data of the grass goby sneaker that did not release sperm did not qualitatively change the results (F3,27 = 4.02, P = 0.017). The body size (total length) of the focal sneaker had no effect on the sperm output (analysis of covariance, grass goby: number of sneakers, F3,27 = 5.00, P = 0.007; total length, F1,27 = 2.78, P = 0.11; model, F4,27 = 4.06, P = 0.01; black goby: number of sneakers, F3,15 = 19.29, P < 0.0001; total length, F1,15 = 2.31, P = 0.15; model, F4,15 = 14.46, P < 0.0001). The relationship between sperm output and the number of sneakers is best described by a cubic regression (grass goby, R2 = 0.31, Y = −326.6 + 762.4N − 309.5N2 + 34.5N3, P = 0.014; black goby, R2 = 0.76, Y = −191.6 + 582.9N − 248.0N2 +28.4N3, P < 0.001, where N is the number of sneakers; CURVEFIT procedure, SPSS; ref. 27), indicating that sperm output tended to increase in the presence of two sneakers and progressively decrease when three and five sneaker males were present.
Figure 2.
Mean (±SEM) concentration of sperm shed by the focal sneaker during a simulated spawning in relation to the number of sneakers attending the spawning (black circles = grass goby; white circles = black goby). Different letters indicate significantly different groups within species (ANOVA, Student–Newman–Keuls post hoc test, P < 0.05).
Discussion
In accordance with theory (9, 20), these results represent direct evidence that males respond to the high intensity of sperm competition by reducing their sperm expenditure. Previous studies attempting to measure sperm expenditure in relation to varying intensity of sperm competition did not support the intensity model (17), or produced only indirect evidence (15, 28). Recently, it was shown that one of three cricket species studied decreased its sperm allocation under the high intensity of sperm competition, but that study only considered one condition of intensity (six rivals; ref. 29). More specifically, studies on fishes that have focused on within-species patterns of sperm expenditure have tended to compare males exhibiting alternative mating tactics (30–35) rather than individual males facing varying levels of sperm competition among a series of spawns (but see refs. 17 and 21).
In the present study it was possible to control for all possible confounding variables and vary only the number of males attending the spawning. The pattern of sperm release found in these two gobies was entirely consistent with the theoretical one predicted by the model of Parker et al. (9, 20). In particular, the sperm expenditure peaked when two sneakers competed, and then decreased with three and five sneaker competitors, as predicted by the model when the mean number of males competing at spawnings is greater than two. Sneakers, however, were always competing with the territorial male; therefore, the situation tested here is partly different from that (a fair lottery) envisaged in the theoretical models, unless the fraction of eggs cuckolded by sneakers in a spawning does not increase with the number of sneakers, as observed in the Atlantic salmon (Salmo salar; ref. 36). If this pattern of paternity sharing also applies to the fishes studied here, the sperm allocation strategy adopted by goby sneakers at a given spawning should be influenced only by the presence of other sneaker competitors and therefore be qualitatively equivalent to that predicted for “true” group-spawning fish. Consistent with this interpretation, territorial males do not seem to vary their sperm expenditure with the number of sneakers (A.P., M.S., and M.B.R., unpublished observations). Another factor that may affect sperm allocation decisions by sneakers may be the distance from the spawning female at which they are able to release their sperm. In the present experiment sneakers could not enter the nest, but previous unpublished observations suggest that the number of sperm released by a sneaker is higher when he is able to enter the nest.
Sperm allocation strategies such as those described here are expected to evolve if males are able to estimate the number of competitors and have the opportunity to allocate their sperm strategically. These assumptions seem to apply in the two gobiid species used here. Spawnings last up to 10 h and sneakers congregate around nests (23). As a consequence, it is likely that sneakers can assess the intensity of sperm during spawnings. Moreover, the number of sneaker competitors can change within a single event, because the territorial male often chases away sneakers or other sneakers can join the spawning and nearly one third of spawnings occur without the presence of sneakers (23, 24), which suggests that sneakers have the option not to compete with other sneakers and that sperm competition level can vary not only between but also within spawnings. Although grass and black goby sneakers have large testes (22), a careful allocation of their sperm stores may therefore be advantageous and may have selected for their ability to tailor their sperm expenditure according to the number of competitor males. Although this was beyond the scope of this study, our results suggest that sneakers can assess the level of sperm competition visually, because olfactory cues (territorial male's urine and female ovarian fluid) were kept constant and chemical communication between sneakers was prevented. Gobiids emit sounds during spawning (37, 38), and acoustic communication between sneakers cannot be excluded given the experimental design adopted here.
Acknowledgments
We thank Jon Evans, Francis Neat, Geoff Parker, Guglielmo Marin, and two anonymous reviewers for their helpful comments on previous versions of the manuscript. We are grateful to Carlotta Mazzoldi for helping with the experiments and to the staff of the Chioggia Hydrobiological Station for assistance. This study was funded by grants from the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (Cofin2000) and the University of Padova (Institutional Research Funds ex60% 1999–2001) (to A.P. and M.B.R.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Birkhead T R, Møller A P, editors. Sperm Competition and Sexual Selection. London: Academic; 1998. [Google Scholar]
- 2.Parker G A. Proc R Soc London Ser B. 1990;242:120–126. [Google Scholar]
- 3.Parker G A. In: Sperm Competition and Sexual Selection. Birkhead T R, Møller A P, editors. London: Academic; 1998. pp. 1–54. [Google Scholar]
- 4.Dewsbury D A. Am Nat. 1982;119:601–610. [Google Scholar]
- 5.Nakatsuru K, Kramer D L. Science. 1982;216:753–755. doi: 10.1126/science.216.4547.753. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro D Y, Marconato A, Yoshikawa T. Ecology. 1994;75:1334–1344. [Google Scholar]
- 7.Olsson M, Madson T, Shine R. Proc R Soc London Ser B. 1997;264:455–459. [Google Scholar]
- 8.Parker G A. Proc R Soc London Ser B. 1990;242:127–133. [Google Scholar]
- 9.Parker G A, Ball M A, Stockley P, Gage M J G. Proc R Soc London Ser B. 1996;263:1291–1297. [Google Scholar]
- 10.Parker G A, Ball M A, Stockley P, Gage M J G. Proc R Soc London Ser B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gage M J G. Anim Behav. 1991;42:1036–1037. [Google Scholar]
- 12.Simmons L W, Craig M, Llorens T, Schinzig M, Hosken D J. Proc R Soc London Ser B. 1993;251:183–186. [Google Scholar]
- 13.Cook P A, Wedell N. Proc R Soc London Ser B. 1996;263:1047–1051. [Google Scholar]
- 14.Gage M J G, Barnard C J. Behav Ecol Sociobiol. 1996;38:349–353. [Google Scholar]
- 15.Simmons L W, Kvarnemo C. Proc R Soc London Ser B. 1997;264:1203–1208. [Google Scholar]
- 16.Hunter F M, Harcourt R, Wright M, Davis L S. Proc R Soc London Ser B. 2000;267:1541–1545. doi: 10.1098/rspb.2000.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller R C. Proc R Soc London Ser B. 1998;265:2365–2371. [Google Scholar]
- 18.Nicholls E H, Burke T, Birkhead T R. Proc R Soc London Ser B. 2001;268:1265–1270. doi: 10.1098/rspb.2001.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner R R. Environ Biol Fishes. 1995;44:337–345. [Google Scholar]
- 20.Ball M A, Parker G A. J Theor Biol. 1997;186:459–466. doi: 10.1006/jtbi.1997.0406. [DOI] [PubMed] [Google Scholar]
- 21.Petersen C W, Warner R R. In: Sperm Competition and Sexual Selection. Birkhead T R, Møller A P, editors. London: Academic; 1998. pp. 435–463. [Google Scholar]
- 22.Marconato A, Rasotto M B, Mazzoldi C. Environ Biol Fishes. 1996;46:321–327. [Google Scholar]
- 23.Mazzoldi C. Ph.D. thesis. Padova, Italy: Univ. of Padova; 1999. [Google Scholar]
- 24.Mazzoldi C, Scaggiante M, Ambrosin E, Rasotto M B. Mar Biol (Berlin) 2000;137:1041–1048. [Google Scholar]
- 25.Colombo L, Marconato A, Colombo Belvedere P, Friso C. Boll Zool. 1980;47:355–364. [Google Scholar]
- 26.Scaggiante M, Mazzoldi C, Petersen C W, Rasotto M B. J Exp Zool. 1999;283:81–90. [Google Scholar]
- 27.Norušis M J. SPSS for Windows Base System User's Guide. Chicago: SPSS; 1993. , Version 6.0. [Google Scholar]
- 28.Wedell N, Cook P A. Proc R Soc London Ser B. 1999;266:1033–1039. [Google Scholar]
- 29.Schaus J M, Sakaluk S K. Behav Ecol. 2001;12:740–745. [Google Scholar]
- 30.Robertson D R, Warner R R. Smithson Contrib Zool. 1978;255:1–26. [Google Scholar]
- 31.Warner R R, Robertson D R. Smithson Contrib Zool. 1978;254:1–24. [Google Scholar]
- 32.Gross M R. Z Tierpsychol. 1982;60:1–26. [Google Scholar]
- 33.Petersen C W. Oecologia. 1990;83:62–67. doi: 10.1007/BF00324635. [DOI] [PubMed] [Google Scholar]
- 34.Gage M J G, Stockley P, Parker G A. Philos Trans R Soc London B. 1995;350:391–399. [Google Scholar]
- 35.Pilastro A, Bisazza A. Proc R Soc London Ser B. 1999;266:1887–1891. [Google Scholar]
- 36.Thomaz D, Beall E, Burke T. Proc R Soc London Ser B. 1997;264:219–226. [Google Scholar]
- 37.Lindstrom K, Lugli M. Environ Biol Fishes. 2000;58:411–424. [Google Scholar]
- 38.Lugli M, Torricelli P, Pavan G, Mainardi D. Mar Freshw Behav Physiol. 1997;29:109–126. [Google Scholar]