Abstract
The occurrence of Cryptosporidium oocysts in feces from a population of wild eastern grey kangaroos inhabiting a protected watershed in Sydney, Australia, was investigated. Over a 2-year period, Cryptosporidium oocysts were detected in 239 of the 3,557 (6.7%) eastern grey kangaroo fecal samples tested by using a combined immunomagnetic separation and flow cytometric technique. The prevalence of Cryptosporidium in this host population was estimated to range from 0.32% to 28.5%, with peaks occurring during the autumn months. Oocyst shedding intensity ranged from below 20 oocysts/g feces to 2.0 × 106 oocysts/g feces, and shedding did not appear to be associated with diarrhea. Although morphologically similar to the human-infective Cryptosporidium hominis and the Cryptosporidium parvum “bovine” genotype oocysts, the oocysts isolated from kangaroo feces were identified as the Cryptosporidium “marsupial” genotype I or “marsupial” genotype II. Kangaroos are the predominant large mammal inhabiting Australian watersheds and are potentially a significant source of Cryptosporidium contamination of drinking water reservoirs. However, this host population was predominantly shedding the marsupial-derived genotypes, which to date have been identified only in marsupial host species.
Cryptosporidium, an apicomplexan protozoan parasite, is a causative agent of enteric disease in humans and other animals. Transmission occurs via ingestion of the infectious oocysts, which are shed in the feces of an infected host. The presence of Cryptosporidium oocysts in feces is used to diagnose Cryptosporidium infection. Cryptosporidium oocysts are environmentally robust, and waterborne transmission has emerged as a public health problem worldwide (8).
Wildlife species, including deer (19) raccoons (24), squirrels (2), chipmunks (19), bank voles and other wild rodents (6, 7, 26), and birds (11), have been identified as significant sources of Cryptosporidium contamination in watersheds. Further, many of these wildlife host species have been implicated in transmission cycles involving zoonotic Cryptosporidium species (2, 5, 19). In Australia the numerically dominant mammals inhabiting watersheds are marsupials, and it is unknown if Australian marsupials are involved in transmission of zoonotic Cryptosporidium species. Observation of Cryptosporidium in Australian marsupials is limited to information gained from animals in captivity or undergoing rehabilitation. Characterization of Cryptosporidium oocysts collected from marsupial species have identified two marsupial-derived genotypes that have a broad marsupial host range (15, 21, 34). The Cryptosporidium “marsupial” genotype I has been identified in captive koalas (Phascolarctos cinereus) (isolates K1 and K3) (16), a juvenile red kangaroo (Macropus rufus) (isolate K2) (34), a captive yellow-footed rock wallaby (Petrogale xanthopus), and eastern grey kangaroos (Macropus giganteus) (isolate EGK2) (21). Two further Cryptosporidium genotypes have been identified in wild eastern grey kangaroos, a variant of the Cryptosporidium “marsupial” genotype I (isolate EGK1) and the Cryptosporidium marsupial genotype II (21). Understanding patterns and degrees of Cryptosporidium oocyst shedding by native animals, their potential contribution to water contamination, and the significance to human health is important for formulating catchment management strategies, including risk assessment and interpretation of results from raw water analyses.
The eastern grey kangaroo Macropus giganteus is the most abundant large (height, 2 to 2.5 m) marsupial species inhabiting watersheds in eastern Australia. The population of eastern grey kangaroos in New South Wales was estimated by a New South Wales National Parks and Wildlife Service aerial survey to be greater than 3 million in the year 2000. This paper describes the results of a 2-year survey of a wild population of eastern grey kangaroos inhabiting a protected watershed in Sydney, Australia, with the aim to understand the potential for this marsupial species as a source of Cryptosporidium contamination of the environment.
MATERIALS AND METHODS
Fecal sample collection.
Eastern grey kangaroo fecal samples were collected from the Warragamba Special Area within the Sydney Hydrological Catchment. The Warragamba Special Area is a protected drinking water catchment of approximately 258,400 ha, which represents approximately 28% of the total watershed. Management strategies for the Warragamba Special Area include the exclusion of humans (except management staff) and the reduction of the number of feral animals inhabiting the watershed, but native Australian wildlife are not subject to population control.
Kangaroo fecal samples were collected on the basis of morphology and the sighting of kangaroos immediately prior to collection. Feces are typically deposited as discrete piles containing 7 to 10 pellets, but depending on the food source, pellets can be compressed into cylinders (27). Fecal samples that exhibited a loose watery consistency were also collected.
A sampling frame was designed to aim for a prevalence estimate with a 95% confidence interval and an absolute precision of 5%, based on an initial prevalence estimate of 25%. To achieve the sampling aim, 323 samples were required for each sampling period (25). Due to kangaroo feeding behavior (typically, large numbers of kangaroos congregate at feeding sites and slowly graze across the paddocks), a collection strategy was adopted to minimize the chance of repeat sampling: (i) samples were collected from sites which were initially selected according to eastern grey kangaroo abundance and continual kangaroo presence and at distances greater than the kangaroos’ home range; (ii) the number of samples collected at each site was half of the number of grazing kangaroos observed at the time of collection; (iii) samples were collected along a transect line at a minimum of 1-m intervals; (iv) transect lines at sampling locations were approximately 2 m apart; and (v) only discrete fecal piles, likely to be from an individual animal, were collected. Samples were stored at 4°C until analyzed.
Cryptosporidium purification, identification, and enumeration.
Purification, identification, and enumeration of Cryptosporidium oocysts from fecal samples were performed using immunomagnetic separation, flow cytometry, and immunofluorescence assay (IMS/FC/IFA) as previously described (20) (20). Briefly, a fecal slurry was prepared for each sample by suspending fecal material (1 g) in 9 ml of dispersion solution (Tween 80 at 0.05% in distilled H2O). An aliquot (1 ml) of the slurry was then exposed to paramagnetic beads coated with monoclonal antibody CRY104 (Macquarie Research Ltd., Sydney, Australia), which is specific to the Cryptosporidium oocyst wall (30). Cryptosporidium oocysts bind to the paramagnetic beads, allowing removal of fecal debris. Oocysts were further purified using fluorescence-activated cell sorting, which allowed for collection onto an 8-mm Isopore membrane (Millipore, Sydney, Australia). Fluorescence microscopy was used to identify and enumerate oocysts.
To monitor the recovery of oocysts by IMS/FC/IFA, a minimum of two Cryptosporidium-spiked kangaroo fecal samples were included in analyses of fecal samples from each collection period. The Cryptosporidium-negative kangaroo fecal samples were obtained from a captive eastern grey kangaroo colony which was regularly monitored for Cryptosporidium oocyst shedding. Control fecal samples from the captive kangaroo colony were spiked with 200 Cryptosporidium oocysts as previously described (20).
Oocyst confirmation and viability.
A dual staining method incorporating DAPI (4′,6′-diamidino-2-phenylindole) and fluorescence in situ hybridization (FISH) was used to confirm internal morphology and viability of the oocysts. FISH reactions were performed using two oligonucleotide probes targeting sequences of the 18S rRNA (CRY1 [5′-CGGTTATCCATGTAAGTAAAG-3′] [28] and CRY2 [5′-GATATGTCACATTAATTGTGATCC-3′] [M. Dorsch, personal communication]), which were conjugated at the 5′ ends to Texas Red (Proligo, France). Staining reactions were performed on 8-mm Isopore membranes (Millipore, Australia), and incubations were performed in plastic contact lens cases (Bausch and Lomb, Sydney, Australia). Oocysts were permeabilized by incubation in 0.1 M HCl at room temperature for 20 min followed by incubation in a solution of 50% phosphate-buffered saline (0.01 M phosphate buffer, 2.7 mM potassium chloride, and 137 mM sodium chloride, pH 7.4) (Sigma, Australia) and 50% ethanol at 80°C for 20 min. After permeabilization, the membrane was transferred to a new contact lens holder containing hybridization buffer (300 μl) (0.9 M NaCl, 20 mM Tris-HCl, pH 7.5, 0.1% sodium dodecyl sulfate), 1 pmol/μl of both oligonucleotides CRY1 and CRY2, and DAPI (5 μg/ml). Oocysts were incubated at 80°C for 2 min and then at 48°C for 1 h. Membranes were placed over a vacuum manifold and rinsed with hybridization buffer (1 ml). Oocysts were stained with fluorescein isothiocyanate-labeled CRY104 monoclonal antibody (20) and then mounted onto a glass slide.
Membranes were examined by epifluorescence microscopy at a magnification of ×400. Presumptive oocysts identified by IFA were confirmed as Cryptosporidium by the presence of four DAPI-stained sporozoite nuclei (excitation at 365 nm). FISH-positive oocysts were identified by a bright red fluorescence (excitation at 580 nm). The percentages of DAPI- and FISH-positive oocysts were determined by the number of oocysts with corresponding fluorescence divided by the total number of oocysts detected by IFA.
Fecal pellet consistency and weight.
The consistency of feces containing Cryptosporidium oocysts was noted as either formed pellets or loose (for watery samples). The average fresh weight of fecal pellets, to the nearest 0.01 g, was determined using the pellets remaining in each sample after removal of 1 g for fecal screening. All Cryptosporidium-positive samples and 215 negative samples were weighed; the weights of samples with fewer than three pellets or a loose consistency were not determined.
Genotype distribution.
Previous multilocus analyses of six Cryptosporidium isolates arising from this study identified three isolates representative of two Cryptosporidium “marsupial” genotypes: Cryptosporidium marsupial genotype I isolates EGK1 and EGK2 and the Cryptosporidium marsupial genotype II (21). The frequencies of these isolates in the kangaroo population were determined by partial 18S rRNA gene sequencing of 51 isolates representing 10 of the 11 fecal sampling periods. The samples selected for sequencing had >5,000 oocysts/g feces. A 1,022-bp PCR product of the 18S rRNA gene was generated using the primers 18SF1 (AAC CTG GTT GAT CCT GCC AGT AGT C) (21) and C18SR3 (AGG AGT AAG GAA CAA CCT CC) (34), PCR conditions and sequencing were as previously described (21).
Epidemiological indices and statistical analysis.
The prevalence of Cryptosporidium oocysts in kangaroo feces was estimated by the division of the number of Cryptosporidium-positive fecal samples by the total number of fecal samples tested. The binomial distribution was used to estimate confidence intervals for each sampling period (23). Chi-square and Fisher's exact tests were used to determine if a significant difference in the number of positive samples occurred between sample periods.
The mean intensity of oocyst shedding was determined by the average of the number of oocysts detected in samples positive for Cryptosporidium, and the mean abundance of oocysts was determined by the average number of oocysts detected in all samples screened. To examine the distribution of the numbers of oocysts shed in feces, the parameter k of the negative binomial distribution was estimated from k = mean squared/variance (4). Statistical tests (correlation coefficient, chi-square analysis, Fisher's exact tests, and t tests) were performed using MINITAB statistical software (MINITAB Ltd., United Kingdom) or Quantitative Parasitology software (22).
RESULTS
Prevalence and shedding intensity of Cryptosporidium oocysts.
Over the 2-year sampling period, 239 Cryptosporidium-positive samples were detected from screening 3,557 eastern grey kangaroo fecal samples. The prevalence of Cryptosporidium oocysts ranged from 0.32% to 28.5% at different sampling times, the overall prevalence was 6.72% (Table 1). Prevalence was at its peak during the late summer (February) and autumn (March to May) sampling months and at the lowest throughout winter (June to August) and spring (September to November) (Table 1). There were significant differences between prevalence estimates for the 11 sampling periods (P < 0.001).
TABLE 1.
Prevalence estimates and confidence intervals for Cryptosporidium in eastern grey kangaroos
| Collection date (mo/yr) | No. of samples positive/no. tested | Prevalence (%) | 95% confidence interval of mean prevalence (%) |
|---|---|---|---|
| 05/2000 | 26/91 | 28.6 | 19.3-37.9 |
| 07/2000 | 14/272 | 5.15 | 2.52-7.77 |
| 09/2000 | 5/319 | 1.57 | 0.20-2.93 |
| 10/2000 | 1/310 | 0.32 | −0.31-0.95 |
| 12/2000 | 4/350 | 1.14 | 0.03-2.26 |
| 02/2001 | 38/356 | 10.7 | 7.47-13.9 |
| 04/2001 | 28/335 | 8.36 | 5.39-11.3 |
| 06/2001 | 36/412 | 8.74 | 6.01-11.5 |
| 08/2001 | 18/344 | 5.23 | 2.87-7.59 |
| 02/2002 | 19/355 | 5.35 | 3.01-7.69 |
| 03/2002 | 50/413 | 12.1 | 8.96-15.3 |
| All samples | 239/3557 | 6.72 | 8.17-10.4 |
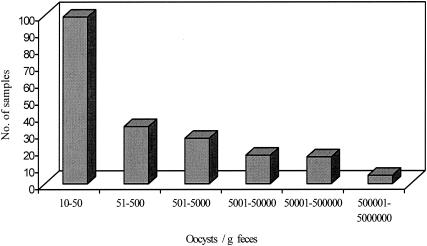
The number of oocysts detected in kangaroo feces ranged from below 20 to 2.0 × 106 oocysts/g feces (wet weight). The majority of samples contained low numbers of oocysts, and the distribution of oocyst shedding was highly aggregated (k < 1), with the majority of samples containing low oocyst numbers (Fig. 1).
FIG. 1.
Distribution of oocyst numbers shed in 239 positive fecal samples from eastern grey kangaroos. The distribution of Cryptosporidium in this host is highly aggregated (k < 1).
The mean shedding intensity and mean abundance for all sample periods are shown in Table 2. The average percent recovery rate for the IMS/FC/IFA method, determined from 26 spiked negative samples, was 77.3% (standard deviation, 9.21%).
TABLE 2.
Intensity of shedding of Cryptosporidium oocysts in positive eastern grey kangaroo fecal samples
| Sample time (mo/yr) | Mean shedding intensity, oocysts/g (SD) | Mean abundance, oocysts/g (SD) |
|---|---|---|
| 05/2000 | 80,856 (391,603) | 23,101 (209,635) |
| 07/2000 | 1,189 (2,187) | 61 (546) |
| 09/2000 | 600,134 (894,315) | 9,406 (125,038) |
| 10/2000 | <20 (NAa) | |
| 12/2000 | 2,293 (3,830) | 26 (431) |
| 02/2001 | 100,191 (242,135) | 10,694 (84,086) |
| 04/2001 | 2,826 (3,594) | 236 (1,287) |
| 06/2001 | 12,392 (31,838) | 1,082 (9,929) |
| 08/2001 | 37,290 (96,975) | 1,951 (23,135) |
| 02/2002 | 13,400 (42,092) | 717 (9,960) |
| 03/2002 | 78,083 (176,941) | 6,931 (67,440) |
| All samples | 59,948 (231,517) | 4,061 (62,005) |
NA, not applicable.
Genotype frequency and distribution.
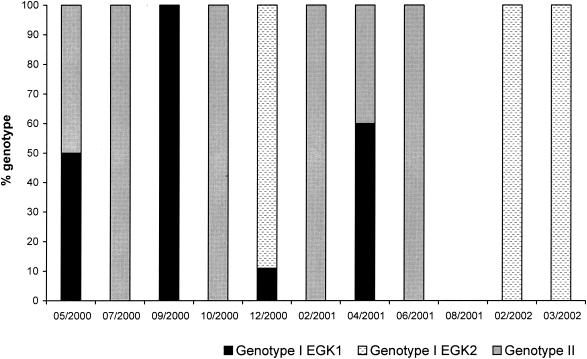
Genotyping at the 18S rRNA gene locus for 51 samples allowed for identification of the Cryptosporidium “marsupial” genotype I isolates EGK1 and EGK2 in 18% and 43% of samples, respectively. The Cryptosporidium marsupial genotype II was present in 39% of samples. For 7 of the 10 sampling periods a single Cryptosporidium genotype was identified, and mixed genotypes were found at other times (Fig. 2).
FIG. 2.
Distribution of Cryptosporidium marsupial genotypes for each fecal sample collection period. (No samples were collected in August 2001 due to bushfires preventing access.)
Oocyst species confirmation.
Of the 239 samples identified as containing presumptive oocysts, 210 were confirmed as Cryptosporidium by DAPI staining. FISH-positive oocysts were identified in 84% of fecal samples. The numbers of FISH-positive oocysts per sample ranged from 0 to 100%. The majority of presumptive oocysts negative with DAPI (97%) were identified in samples with oocyst numbers below 30/g feces. Similarly, 95% of samples containing oocysts negative by FISH were from fecal samples with oocyst numbers below 30/g feces. A correlation (r = 0.82) was found between DAPI- and FISH-positive oocysts.
Fecal sample weight and consistency.
To determine if samples with high oocyst counts were associated with diarrhea or small fecal pellets, the sample consistency and average pellet weight were recorded. Of the 239 Cryptosporidium-positive samples, the weights of 17 could not be determined; 8 samples contained too little material, and 9 samples had a loose consistency. Of the samples with a loose consistency, six had an oocyst count of fewer than 200/g feces. The remaining three samples had counts of 3,300, 250,000, and 300,000 oocysts/g feces. There was no correlation between the number of oocysts per gram of feces and fecal pellet weight (r = −0.177). Pellets that were positive for Cryptosporidium were significantly (P < 0.001) smaller (mean weight, 1.59 ± 0.852 g; n = 222) than pellets not containing Cryptosporidium (mean weight, 2.07 ± 1.24 g; n = 215).
DISCUSSION
Previously, Cryptosporidium has been reported only in eastern grey kangaroos in captivity in Berlin, Germany (13). The present study demonstrates that a population of wild eastern grey kangaroos inhabiting a watershed in Australia are susceptible to Cryptosporidium infections. Although pathology data were not acquired from eastern grey kangaroos it is assumed that oocysts in kangaroo feces arose from infection. Further, the sampling approach strengthened the likelihood that each fecal sample tested was from a different individual; therefore, it was assumed that the data reflect infection in the kangaroo population.
Although Cryptosporidium oocysts were detected in eastern grey kangaroo feces at all sampling times throughout the 2-year study period, peaks in prevalence were observed during three consecutive autumn sampling periods, suggesting that Cryptosporidium is seasonal in this host. Similar patterns of increased Cryptosporidium prevalence during autumn have been described for captive wildlife (10) and wild rodent species (7).
Domestic animal studies have further demonstrated that Cryptosporidium is more prevalent in juvenile animals (3, 9, 16a, 29, 33). Similarly, Cryptosporidium in marsupials is common in captive and hand-reared juveniles; for example, it was found in a juvenile koala from Western Australia (U. Ryan, personal communication) and in a juvenile red kangaroo and four juvenile western grey kangaroos from South Australia (C. Irving, personal communication).
Cryptosporidium prevalence peaks in eastern grey kangaroos at a time when the number of susceptible kangaroos in the study population would be high due to weaning of young. Although eastern grey kangaroos breed all year, there is a peak of births during spring and early summer; these offspring are weaned 18 months after birth, coinciding with the autumn months. Fecal samples positive for Cryptosporidium were significantly smaller, suggesting that these samples were from juvenile kangaroos. Age susceptibility creates the potential to increase parasite prevalence in the total host population through greater exposure to infective oocysts shed by juveniles. Kangaroos will defecate several times during grazing periods (14), and the likely transmission route would be via ingestion of oocysts while grazing. This is further supported by the detection of Cryptosporidium oocysts in fecal pellets of different sizes and weights, indicating that both juvenile and adult kangaroos were shedding oocysts.
The intensity of shedding of Cryptosporidium oocysts by eastern grey kangaroos ranged from as few as 10 to 2.0 × 106 oocysts/g feces, and oocyst shedding was aggregated, with the majority of samples containing low numbers of parasites and few samples containing a high parasite load. An aggregated parasite distribution is characteristic within host populations (1). Hence, for accurate estimates of prevalence, diagnostic methods need to be able to detect as few parasites as possible and sampling of large numbers is required.
Cryptosporidium infections in eastern grey kangaroos appeared to be asymptomatic. Feces with a loose consistency were found to constitute only 0.35% of samples, and diarrhea was not associated with the numbers of oocysts shed. Fecal samples with low numbers of oocysts were larger, indicating that they were most likely to be from adults. The persistence of low-level, asymptomatic infections in adults may provide a reservoir of this parasite in the kangaroo population. In a similar way, adult cattle maintain low-level, asymptomatic infections (9).
Multilocus analysis of six Cryptosporidium isolates from this study population identified three types of Cryptosporidium in eastern grey kangaroo feces (21). Further genotyping at the 18S rRNA gene locus of 51 Cryptosporidium samples from different collection periods indicated seasonal variation in the occurrence of Cryptosporidium genotypes in the kangaroo population. For each sampling period, one of the three Cryptosporidium isolates (assigned to two genotypes) dominated the positive fecal samples. The Cryptosporidium marsupial genotype I isolate EGK1 and marsupial genotype II were identified during all four seasons, whereas Cryptosporidium marsupial genotype I isolate EGK2 was detected only in the late summer and early autumn months. With such little information and in a complex natural system, it is difficult to determine the factors that may contribute to seasonal variation of Cryptosporidium genotypes observed in this host species. One possibility is the low host specificity of the parasite. All Cryptosporidium “marsupial” genotype isolates described for this kangaroo population have been identified in other marsupial hosts, including representatives of different marsupial orders (15, 20, 34). The presence of multiple marsupial hosts within a habitat and the potential for interchange between these host species would result in complex transmission patterns. Further, it is possible that the marsupial-derived genotypes vary in their susceptibility to environmental conditions such as temperature, which may result in characteristic temporal infection patterns. Both of these factors remain to be investigated.
We have established that eastern grey kangaroos inhabiting the Sydney Hydrological Catchment have the potential to excrete relatively high numbers of Cryptosporidium oocysts and are a potential source of Cryptosporidium in source drinking waters. The mean oocyst shedding intensity in infected eastern grey kangaroos was approximately 60,000 oocysts/g feces. Based on a mean fecal output of 1.5 kg feces/day (14), the estimated average daily environmental loading of Cryptosporidium from the eastern grey kangaroo population inhabiting the Warragamba Special Area is 9 × 107 oocysts/infected kangaroo/day. However, to date, marsupial-derived Cryptosporidium genotype oocysts have not been detected in raw water samples from the Sydney Hydrological Catchment (12), although it should be noted that few samples have been genotyped due to the relatively large numbers of oocysts required for current genotyping methods, and mixed genotypes in water filtrates may be incorrectly identified.
To date, there have been no reported outbreaks of cryptosporidiosis in Sydney due to the drinking water supply (8), nor have marsupial-derived genotypes been identified in humans. Conversely, the emergence of zoonotic species and genotypes such as C. meleagridis, C. felis, C. canis, and the Cryptosporidium cervine and pig genotypes (17, 18, 31, 32) indicates that apparently host-specific Cryptosporidium species or genotypes should not be excluded as potential human pathogens. The epidemiological information from this study has broadened our understanding of potential sources of Cryptosporidium in watersheds in Australia and will be valuable to water testing utilities and the catchment management organizations that monitor and maintain Australian drinking water sources.
Acknowledgments
This work was supported by a linkage grant from Sydney Water Corporation (SWC) and the Australian Research Council.
We thank Raj Shanker and Peter Cox (SWC) for assistance during the project and for review of the manuscript, respectively. We gratefully acknowledge Liz Barnes for her assistance with statistical analysis and for manuscript review. Access to the Warragamba Special Area was provided by the Sydney Catchment Authority (SCA). We also thank Brian Waldron and Glen Capararo from the SCA for their invaluable assistance throughout the project.
REFERENCES
- 1.Anderson, R. M. 1993. Epidemiology, p. 75-114. In F. E. G. Cox (ed.), Modern parasitology. Blackwell Science, London, United Kingdom.
- 2.Atwill, E. R., S. M. Camargo, R. Phillips, L. H. Alonso, K. W. Tate, W. A. Jensen, J. Bennet, S. Little, and T. P. Salmon. 2001. Quantitative shedding of two genotypes of Cryptosporidium parvum in California ground squirrels Spermophilus beecheyi. Appl. Environ. Microbiol. 67:2840-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwill, E. R., E. Johnson, D. J. Klingborg, G. M. Veserat, G. Markegard, W. A. Jensen, D. W. Pratt, R. E. Delmas, H. A. George, L. C. Forero, R. L. Philips, S. J. Barry, N. K. McDougald, R. R. Gildersleeve, and W. E. Frost. 1999. Age, geographic and temporal distribution of fecal shedding of Cryptosporidium in cow-calf herds. Am. J. Vet. Res. 60:420-425. [PubMed] [Google Scholar]
- 4.Barger, I. A. 1985. The statistical distribution of trichostrongylid nematodes in grazing lambs. Int. J. Parasitol. 15:645-649. [DOI] [PubMed] [Google Scholar]
- 5.Bodley-Tickell, A. T., S. E. Kitchen, and A. P. Sturdee. 2002. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res. 36:1880-1886. [DOI] [PubMed] [Google Scholar]
- 6.Bull, S. A., R. M. Chalmers, A. P. Sturdee, and T. D. Healing. 1998. A survey of Cryptosporidium species in Skomer bank voles Clethrionomys glareolus skomerensis. J. Zool. (London) 244:119-122. [Google Scholar]
- 7.Chalmers, R. M., A. P. Sturdee, S. A. Bull, A. Miller, and S. E. Wright. 1997. The prevalence of Cryptosporidium parvum and C. muris in Mus domesticus, Apodemus sylvaticus and Clethrionomys glareolus in an agricultural system. Parasitol. Res. 83:478-482. [DOI] [PubMed] [Google Scholar]
- 8.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30: 1305-1322. [DOI] [PubMed] [Google Scholar]
- 9.Fayer, R., J. M. Trout, T. K. Graczyk, and E. J. Lewis. 2000. Prevalence of Cryptosporidium, Giardia and Eimeria infections in post-weaned and adult cattle on three Maryland farms. Vet. Parasitol. 93:103-112. [DOI] [PubMed] [Google Scholar]
- 10.Gracenea, M., M. S. Gomez, J. Torres, E. Carne, and J. Fernandez-Moran. 2002. Transmission dynamics of Cryptosporidium in primates and herbivores at the Barcelona Zoo: a long-term study. Vet. Parasitol. 104:19-26. [DOI] [PubMed] [Google Scholar]
- 11.Graczyk, T. K., R. Fayer, J. M. Trout, E. J. Lewis, C. A. Farley, I. Sulaiman, and A. A. Lal. 1998. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta canadensis). Appl. Environ. Microbiol. 64:2736-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins, P., P. Swanson, and U. Morgan. 2001. Genotypes of Cryptosporidium in Sydney's Catchment. Water 28:38-41. [Google Scholar]
- 13.Jacob, W. 1992. Cryptosporidein- und andere Kokzidienoozysten bei Zoo- und wildtiere im nach Ziehl-Neelsen gefarbten Kotausstrich. Erhandlungen Berichte Erkrg. Zootiere 5:291-299. [Google Scholar]
- 14.Johnson, C. N., P. J. Jarman, and C. J. Southwell. 1987. Macropod studies at Wallaby Creek New South Wales Australia V. Patterns of defecation by eastern grey kangaroos and red-necked wallabies. Aust. Wildl. Res. 14:133-138. [Google Scholar]
- 15.Morgan, U. M., C. C. Constantine, D. A. Forbes, and R. C. A. Thompson. 1997. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 83:825-830. [PubMed] [Google Scholar]
- 16.Morgan, U. M., L. Xiao, R. Fayer, A. A. Lal, and R. C. A. Thompson. 1999. Variation in Cryptosporidium: towards a taxonomic revision of the genus. Int. J. Parasitol. 29:1733-1751. [DOI] [PubMed] [Google Scholar]
- 16a.Noordeen, F., A. C. M. Faizal, R. P. V. J. Jajapakse, N. U. Horadagoda, and A. Arulkanthan. 2001. Excretion of Cryptosporidium oocysts by goats in relation to age and season in the dry zone in Sri Lanke. Vet. Parasitol. 99:79-85. [DOI] [PubMed] [Google Scholar]
- 17.Ong, C. S. L., D. L. Eisler, V. W. K. Alikhani, J. T. Fung, J. Tomblin, W. R. Bowie, and J. L. Isaac-Renton. 2002. Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases: first report of human infections with a cervine genotype. Emerg. Infect. Dis. 8:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedraza-Diaz, S., C. Amar, A. M. Iversen, P. J. Stanley, and J. McLauchlin. 2001. Unusual Cryptosporidium species recovered from human faeces: first description of Cryptosporidium felis and Cryptosporidium ‘dog type’ from patients in England. J. Med. Microbiol. 50:293-296. [DOI] [PubMed] [Google Scholar]
- 19.Perz, J. F., and S. M. Le Blancq. 2001. Cryptosporidium parvum infection involving novel genotypes in wildlife from lower New York State. Appl. Environ. Microbiol. 67:1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power, M. L., S. R. Shanker, N. C. Sangster, and D. A. Veal. 2003. Evaluation of a combined immunomagnetic separation/flow cytometry technique for epidemiological investigations of Cryptosporidium in domestic and Australian native animals. Vet. Parasitol. 112:21-31. [DOI] [PubMed] [Google Scholar]
- 21.Power, M. L., M. B. Slade, N. C. Sangster, and D. A. Veal. 2004. Genetic characterisation of Cryptosporidium from a wild population of eastern grey kangaroos Macropus giganteus inhabiting a water catchment. Infect. Gen. Evol. 4:59-67. [DOI] [PubMed] [Google Scholar]
- 22.Rozsa, L., J. Reiczigel, and G. Majoros. 2000. Quantifying parasites in samples of hosts. J. Parasitol. 86:228-232. [DOI] [PubMed] [Google Scholar]
- 23.Smith, R. D. 1995. Veterinary clinical epidemiology, 2nd ed. CRC Press, Boca Raton, Fla.
- 24.Snyder, D. E. 1988. Indirect immunofluorescent detection of oocysts of Cryptosporidium parvum in the feces of naturally infected raccoons (Procyon lotor). J. Parasitol. 74:1050-1052. [PubMed] [Google Scholar]
- 25.Thrusfield, M. 1995. Veterinary epidemiology, 2nd ed. Blackwell Science, Oxford, United Kingdom.
- 26.Torres, J., M. Gracenea, M. S. Gomez, A. Arrizabalaga, and O. Gonzalez-Moreno. 2000. The occurrence of Cryptosporidium parvum and C. muris in wild rodents and insectivores in Spain. Vet. Parasitol. 92:253-260. [DOI] [PubMed] [Google Scholar]
- 27.Triggs, B. 1996. Tracks, scats and other traces. A field guide to Australian mammals. Oxford Univeristy Press, Oxford, United Kingdom.
- 28.Vesey, G., N. Ashbolt, E. J. Fricker, D. Deere, K. L. Williams, D. A. Veal, and M. Dorsch. 1998. The use of a ribosomal RNA targeted oligonucleotide probe for fluorescent labeling of viable Cryptosporidium parvum oocysts. J. Appl. Microbiol. 85:429-440. [DOI] [PubMed] [Google Scholar]
- 29.Wade, S. E., H. O. Mohammed, and S. L. Schaaf. 2000. Prevalence of Giardia sp., Cryptosporidium parvum and Cryptosporidium muris (C. andersoni) in 109 dairy herds in five counties of southeastern New York. Vet. Parasitol. 93:1-11. [DOI] [PubMed] [Google Scholar]
- 30.Weir, C., G. Vesey, M. Slade, B. Ferrari, D. A. Veal, and K. Williams. 2000. An immunoglobulin G1 monoclonal antibody highly specific to the wall of Cryptosporidium oocysts. Clin. Diagn. Lab. Immunol. 7:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao, L., C. Bern, M. Arrowood, I. Sulaiman, L. Zhou, V. Kawai, A. Vivar, A. A. Lal, and R. H. Gilman. 2002. Identification of the Cryptosporidium pig genotype in a human patient. J. Infect. Dis. 185:1846-1848. [DOI] [PubMed] [Google Scholar]
- 32.Xiao, L., C. Bern, J. Limor, I. M. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183: 492-497. [DOI] [PubMed] [Google Scholar]
- 33.Xiao, L., and R. P. Herd. 1994. Epidemiology of equine Cryptosporidium and Giardia infections. Equine Vet. J. 26:14-17. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. A. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]