Abstract
Numerous Salmonella enterica food-borne illness outbreaks have been associated with contaminated vegetables, in particular sprouted seeds, and the incidence of reported contamination has steadily risen. In order to understand the physiology of S. enterica serovar Newport on plants, a screen was developed to identify transposon mutants that were defective in attachment to alfalfa sprouts. Twenty independent mutants from a pool of 6,000 were selected for reduced adherence to alfalfa sprouts. Sixty-five percentage of these mutants had insertions in uncharacterized genes. Among the characterized genes were strains with insertions in the intergenic region between agfB, the surface-exposed aggregative fimbria (curli) nucleator, and agfD, a transcriptional regulator of the LuxR superfamily, and rpoS, the stationary-phase sigma factor. Both AgfD and RpoS have been reported to regulate curli and cellulose production and RpoS regulates other adhesins such as pili. The intergenic and rpoS mutants were reduced in initial attachment to alfalfa sprouts by 1 log unit compared to the wild type. Mutations of agfA, curli subunit, and agfB in S. enterica serovar Enteritidis differentially affected attachment to plant tissue. The agfA mutation was not reduced in ability to attach to or colonize alfalfa sprouts, whereas the agfB mutation was reduced. Thus, agfB alone can play a role in attachment of S. enterica to plant tissue. These results reveal that S. enterica genes important for virulence in animal systems are also required for colonization of plants, a secondary host that can serve as a vector of S. enterica from animal to animal.
Numerous Salmonella enterica and Escherichia coli O157:H7 (EHEC) outbreaks have been associated with contaminated sprouts and the incidence of reported contamination has remained steady (9). Previously, we showed procedures used in production of sprouted seeds encourage proliferation of human pathogens (11). Furthermore, S. enterica preferentially attached to the alfalfa sprout roots where the S. enterica cells formed large aggregates. These studies revealed that replacement of irrigation water reduced the number of EHEC cells on sprouts compared to S. enterica. Subsequent experiments showed that S. enterica was not removed from alfalfa sprouts with rinsing, whereas EHEC was easily removed, therefore suggesting differential attachment capacities between the bacteria (8).
Bacterial attachment to plants has predominantly been studied for its role in virulence of plant pathogens. Recently, the surface interactions between bacteria and plant are being studied prior to the development of disease symptoms. Agrobacterium tumefaciens, which causes tumorigenic diseases in many plant species, requires a Ca2+-dependent adhesin (35), a repertoire of proteins encoded by att genes (22, 23), exo- and capsular polysaccharides (30), and cellulose fibrils (21, 22) to attach to roots. Other rhizosphere inhabitants also utilize cellulose (5) and fimbriae (18, 39, 40). However, only one study has begun to address the fundamental questions of what allows animal bacterial pathogens to associate with plants initially and how they remain attached (14). These experiments showed that attachment of Listeria monocytogenes to radish tissue was dependent on flagellar motility.
The intent of the present study was to identify genes required for attachment of S. enterica serovar Newport to plant tissue. We chose to use alfalfa sprouts as a model system for attachment because S. enterica has been isolated from alfalfa sprouts and seeds and caused numerous salmonellosis outbreaks in association with ingestion of alfalfa sprouts. In addition, alfalfa sprouts grow relatively quickly, lending themselves well to in situ experiments, and sprouts usually are not cooked before consumption. Therefore, this system is relevant to public health.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The strains used in the present study are listed in Table 1. Bacteria were grown in, or plated on, LB medium (Difco/Becton Dickinson, Franklin Lakes, NJ). Kanamycin was obtained from Sigma (St. Louis, MO) and, when required, was incorporated into the medium at 40 mg/liter. Salmonella-Shigella (SS) Agar (Difco), a Salmonella semiselective indicator medium, was used to determine S. enterica populations during the non-attachment mutant enrichment, mutant sprout water growth assays, and S. enterica serovar Enteritidis assays. Congo red indicator plates were made of LB base medium containing 20 μg of Congo red (Sigma)/ml and 10 μg of Coomassie brilliant blue (Sigma)/ml. Curli-producing bacteria form red colonies, whereas nonproducing cells form beige colonies. Cellulose indicator plates were made of LB base medium containing 50 μg of calcofluor white (fluorescent brightener 28; Sigma)/ml. Cellulose-producing bacteria form colonies that fluoresce under UV light.
TABLE 1.
Strains used in this study
| Strain or plasmid | Identification | Description | Source or reference |
|---|---|---|---|
| E. coli | pLBT | Donor strain; mini-Tn10:lac:kan | 2 |
| S. enterica Newport | 96Eo1152C-TX | Isolated from alfalfa sprouts | 17 |
| JDB 279 | rpoS::Tn10:lac:kan | This study | |
| JDB 287 | Tn10:lac:kan insertion in the agfD/agfB intergenic region | This study | |
| JDB 423 | rpoS::Tn10:lac:kan complimented with pJDB1 | This study | |
| S. enterica Enteritidis | 27655-3b | Clinical | 12 |
| ΔagfA | Produces no agfA | 41 | |
| ΔagfB | Produces no agfB | 41 | |
| Plasmid pJDB1 | rpoS pCR2.1 from 1655 | This study |
Transposon mutagenesis.
The donor strain, E. coli(pLBT), and the recipient, S. enterica Newport 96Eo1152C-TX, were grown overnight in LB at 37°C, diluted to an optical density at 600 nm of 0.2 in sterile water, and mixed together at a ratio of 1:1, and then 25 μl was spotted on LB agar and grown overnight at 37°C. The cells were suspended in sterile water, a dilution series was plated onto SS agar containing kanamycin, and the cells were grown overnight at 37°C. Six thousand black colonies, presumably S. enterica Newport, resistant to kanamycin were randomly selected to comprise the mutant pool.
Alfalfa sprout attachment and colonization assays.
All experiments were performed at least twice at room temperature. Alfalfa seeds were surface sanitized with 3% calcium hypochlorite as previously described (8) and sprouted in petri plates (50 seeds/plate) with 25 ml of irrigation water that was exchanged daily for sterile water. Seeds and sprouts were constantly shaken at 40 rpm in petri plates or tubes. For individual strain attachment assays, overnight bacterial streak cultures were suspended in sterile water by sterile swab to an optical density at 600 nm of 0.2 and diluted to a concentration of ∼104 CFU/ml. Sanitized seeds were incubated in the bacterial suspension for 1 h, and then the suspension was replaced with sterile water. Individual seeds/sprouts were homogenized in 500 μl of phosphate buffer solution (34), plated on LB agar, incubated overnight at 37°C, and bacterial cells were enumerated to determine the number of attached cells.
Selection of attachment mutants.
To enrich for attachment mutants, sprouts were grown for 3 days prior to inoculation. Fifty-three-day-old sprouts were placed in a conical 50-ml tube and a pool of random mini-Tn10:lac:kan insertion mutants of S. enterica Newport were added. The mutant pool that had been grown overnight at 37°C in LB containing kanamycin was diluted in sterile water to an optical density at 600 nm of 0.2 and diluted to a concentration of ∼106 CFU/ml. The sprouts and bacteria were incubated for 4 h at 25°C and shaken in an orbital shaker at 40 rpm. The cell suspension from this tube was poured into a second sprout-containing tube and incubated for another 4 h. After the second 4-h incubation, the cell suspension was transferred to a sterile flask and 20 ml of LB medium plus kanamycin was added to the sprout irrigation water. This enriched culture was incubated overnight and then used to repeat the attachment and regrowth of unattached cells. In this fashion, the selection procedure was continued for 10 consecutive days. On each day, the numbers of S. enterica Newport cells in the cell suspension was monitored by dilution plating on SS agar. After 10 days, 600 colonies were chosen and screened individually for attachment to sprouts.
Statistics.
Where indicated, Student t tests were performed to determine the significance between the average populations of strains per alfalfa sprout.
Growth assay.
To determine whether a nonattaching phenotype was due either to a reduced ability to attach or colonize plant tissue or to a growth or survival defect, the growth of planktonic mutant and wild-type cells in the sprout irrigation water was measured. Fifty-three-day-old sprouts were placed in a conical tube, bacterial cells were added (∼104 CFU/ml), and the sprouts and bacteria were incubated for 4 h at 25°C. An aliquot of water was sampled every hour for 4 h, and bacterial populations were enumerated on SS plates.
Cloning and sequencing of the regions surrounding mini-Tn10:lac:kan insertions.
To establish that each strain contained only one insertion, chromosomal DNA was digested with HindIII and screened by Southern blot hybridization with a probe containing the kanamycin resistance gene of mini-Tn10:lac:kan. The region upstream of the insertions was cloned by digestion of the chromosomal DNA with EcoRI, ligation into pBluescript II KS(+), and selection in E. coli TOP10 (Invitrogen, Carlsbad, CA). The region upstream of the insertions was sequenced by using the primer LBT, which is complementary to sequence contained in the transposon (2). Sequences were compared to the published sequences of S. enterica Typhimurium (GenBank accession no. NC_003197) and S. enterica serovar Typhi (GenBank accession no. NC_004631) by a BLAST search (3).
Quantitative reverse transcription-PCR (qRT-PCR).
Alfalfa seeds were surface sanitized and inoculated as described above. To harvest planktonic cells for RNA extraction, sprout irrigation water was removed, the bacterial cells were pelleted by centrifugation for 45 min at 45,440 × g and 4°C due to the ephemeral nature of the bacterial pellet, and the supernatant was discarded. To harvest bacterial cells attached to plant tissue for RNA extraction, alfalfa sprouts were rinsed three times with 20 ml of sterile water and placed in a 50-ml conical tube with 20 ml of sterile water. The tubes were sonicated for 1 min at 250 W in a water bath sonicator. The detached cells were pelleted as described above, the supernatant was discarded, and the cells were resuspended in 1.5 ml of RNAprotect Bacterial Reagent (QIAGEN, Valencia, CA). Total RNA was extracted with RNeasy kit (QIAGEN) according to the manufacturer's instructions. After elution, the tubes containing the extracted nucleic acids were heated 4 min at 95°C and immediately transferred to ice water. Contaminating DNA was removed by using Ambion DNA-free (Ambion, Austin, TX). The RNA was stored at −80°C.
qRT-PCR primers (Operon; QIAGEN) are listed in Table 2, along with their target gene and annealing temperatures. qRT-PCRs were prepared as follows (final volumes per sample): 25 μl of 2× SYBR Green qRT-PCR master mix (Stratagene, La Jolla, CA), 2 μl of each primer (0.4 mM), 0.5 μl of fluorescein (10 pM), 0.125 μl of StrataScript RT/RNase block enzyme mixture (Stratagene), 1 μl of RNA template (∼200 ng of total RNA), and 19.375 μl of RNase-free water. A four-step RT-PCR protocol was used for the iCycler iQ (Bio-Rad, Hercules, CA): (i) the addition of reverse transcriptase (30 min at 45°C); (ii) denaturation (10 min at 95°C); (iii) an amplification and extension program repeated 40 times (30 s at 95°C, 45 s at appropriate annealing temperature, and 30 s at 72°C, with a single fluorescence measurement); and (iv) a melting curve program of 1 min at 95°C and 1 min at 55°C, followed by an increase from 55 to 80°C, with a heating rate of 5°C per 10 s, and a continuous fluorescence measurement. PCR products were confirmed by sequence analysis.
TABLE 2.
PCR primers and annealing temperatures used in this study
| Primer | Gene | Sequence (5′ to 3′) | Tmaa (°C) |
|---|---|---|---|
| LBT | TTTTTACACTGATGAATGTTCCGTT | 55 | |
| agfBF RT | agfB | TCCTGGTCTTCAGTAGCGTAA | 54.7 |
| agfBR RT | TATGATGGAAGCGGATAAGAA | ||
| agfDF RT | agfD | TCCTGGTCTTCAGTAGCGTAA | 59.1 |
| agfDR RT | TATGATGGAAGCGGATAAGAA | ||
| rplUF RT | rplU | CTGTTGAGTTCGCTGAAGTGC | 57.2 |
| rplUR RT | CAGCATTTGTGATAGCATTCGTCG |
Tma, annealing temperature used in PCR.
rplU was used as internal control since it is constitutively expressed under a wide range of conditions (20). To calculate the change in fold gene expression in comparison to rplU, we used the 2−X method (26), where X is calculated as the gene-of-interest CT − the rplU CT, and the cycle threshold (CT) value was determined for each gene at 1,000 relative fluorescence units. For this calculation to be valid, the amplification efficiencies of the target and reference must be approximately equal. To validate the use of this calculation, we determined by template dilution that our amplification efficiencies were similar for rplU, agfD, and agfB (results not shown).
RESULTS AND DISCUSSION
We used 3-day-old alfalfa sprouts as an affinity matrix to remove the attachment-competent cells from a pool of random mini-Tn10:lac:kan (2) S. enterica Newport mutants. Although not all cells that can attach do so (8), additional cycles of sequential sprout attachment and regrowth of attachment-defective cells were done to enrich for nonattaching cells. Even in a suspension of wild-type S. enterica, at least 50% of the total cells applied are removed by washing. In order to produce a population enriched for attachment-defective mutants, we used a library of 6,000 random, pooled insertions of S. enterica Newport. On each day, the numbers of S. enterica Newport cells in the wash fluids were monitored. On day 1, the pooled wash fluids contained 44% of the total input cells. On day 10, 99% of the input cells were removed by washing; therefore, 600 colonies were chosen randomly from the day-10 unattached populations, for further screening. This enrichment was similar to that used for L. monocytogenes when screened for mutants deficient in attachment to radish tissue (14).
Each of the 600 strains was tested individually for the ability to attach to sprouts by the assay used originally to select them. Thirty-two strains were reduced at least 1 log unit compared to the wild-type strain and were analyzed by Southern blot hybridization to verify that each strain had a single unique insertion (not shown). The regions upstream of the insertions were cloned and sequenced, and BLAST (3) was used to compare the sequences to the complete genome sequences of S. enterica serovars Typhimurium and Typhi, as well as the NCBI sequence database. Of the 20 unique insertions identified, 65% were located in uncharacterized genes, and two were in sequences not found in the NCBI database and thus might represent sequences unique to S. enterica Newport (Table 3). The disrupted genes were of diverse functions, including those involved in growth, regulation, and chemotaxis, as well as those encoding surface proteins and those associated with bacteriophage. Future work will include complementation and characterization of the mutants not addressed here.
TABLE 3.
Identification of S. enterica Newport regions disrupted by mini-Tn10:lac:kan insertions
| Function | Gene | Functiona |
|---|---|---|
| Regulation | rpoS | Global stress regulator |
| Intergenic agfB-agfD | Regulates agfD | |
| Growth | sfcA | NAD-linked malate dehydrogenase |
| adhP | Alcohol dehydrogenase | |
| trpB | Tryptophan beta chain | |
| Chemotaxis | yeaJ | Methyl-accepting chemotaxis* |
| Membrane-associated proteins | gtrB | Glycosyl transferase |
| STM0278 | Periplasmic* | |
| STM3650 | Periplasmic* | |
| STM0565 | Periplasmic* | |
| stcC | Outer membrane* | |
| STM3832 | Membrane transport* | |
| Unknown | STM0650 | Hydrolase* |
| STM0564 | Oxidoreductase* | |
| YbeQ | TPR (tetratricopeptide) repeat* | |
| STY2035 | Bacteriophage† | |
| SEN uniqueb | SEN_01 | |
| SEN_02 |
*, a BLAST search of S. enterica serovar Typhimurium strain LT2 identified a putative protein. †, there was no match in a BLAST search of S. enterica Typhimurium (a similar sequence was found only in serovar Typhi).
No sequence matched any Salmonella strain.
Aggregative fimbriae are important for attachment to plants.
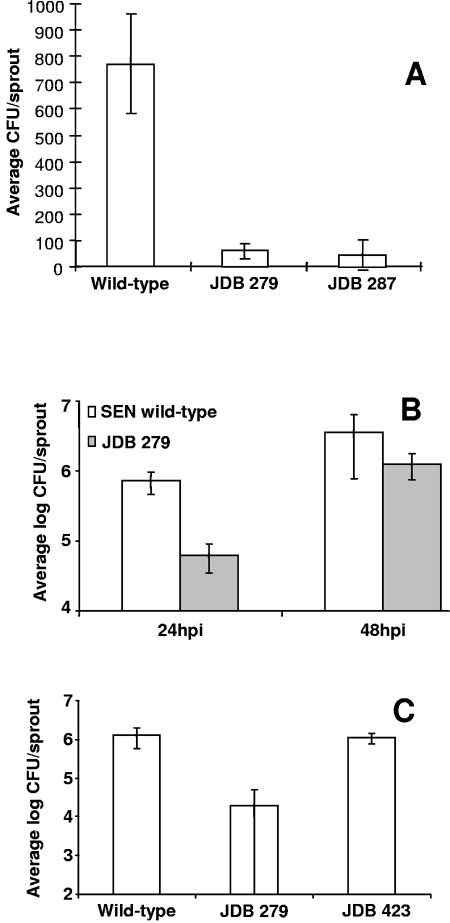
A random insertion (JDB 287) was reduced in attachment by 1 log unit compared to the wild type (Fig. 1A). The insertion was located 217 nucleotides (nt) and 305 nt upstream of the transcriptional start sites of agfD, a transcriptional regulator of the LuxR superfamily, and agfB, the surface-exposed curli nucleator, respectively. AgfD has been reported to regulate curli and cellulose production, both of which are required for multicellular behavior in S. enterica (33). The area of insertion is required for agfD regulation by OmpR, H-NS, and IHF (13). Notably, the mini-Tn10:lac:kan adds approximately 5 kb into the agfD-agfB intergenic region required for direct association with the regulators H-NS and IHF.
FIG. 1.
Adhesion of S. enterica Newport to alfalfa sprouts. (A) Cells of the S. enterica Newport rpoS mutant (JDB 279) and Tn10:lac:kan insertion in the agfD/agfB intergenic region (JDB 287) were recovered from 3-day-old alfalfa sprouts and compared to wild-type S. enterica Newport after 4-h adhesion assays. The data are averages of three experiments. (B) Cells of JDB 279 recovered from alfalfa sprouts 24 and 48 h postinoculation compared to wild-type S. enterica Newport. (C) Cells of JDB 279 and JDB 423 recovered from alfalfa sprouts 24 h postinoculation compared to wild-type S. enterica Newport. The data for panels B and C are representative experiments with two internal replicates and five sprout samples taken from each. The standard deviations are represented as error bars in all panels.
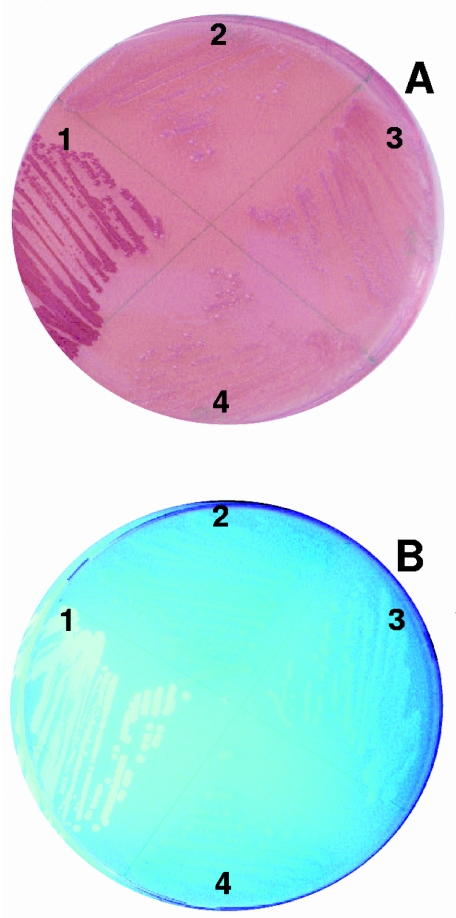
We hypothesized that any three-dimensional structure of the DNA that affects expression of agfD would be disrupted in JDB 287. We tested the ability of JDB 287 to produce curli on Congo red indicator plates and cellulose on agar with calcofluor under permissible conditions. When compared to the wild type, JDB 287 produced neither curli nor cellulose because the colonies on Congo red indicator plates were beige and did not fluoresce on calcofluor plates (Fig. 2A and B).
FIG. 2.
Phenotypic characterization of S. enterica wild-type (section 1), JDB 287 (section 2), JDB 279 (section 3), and JDB 423 (section 4) Newport strains grown on Congo red indicator media (A) and LB no salt containing calcofluor (B) for 48 h at 28°C.
Furthermore, we used qRT-PCR to monitor RNA expression of agfD and agfB under curli-permissible conditions on LB agar and on alfalfa sprouts and in irrigation water. When agfD transcription was measured in JDB 287, we found it was downregulated 10-fold compared to the wild type for all of the conditions we tested (Table 4). Furthermore, agfB expression was significantly reduced in this intergenic mutant on media and in association with plants, with 100-fold expression reduction (Table 4). This was expected since AgfD regulates agfB transcription (33). Thus, the insertion in JDB 287 affected agfD, which is responsible for both curli and cellulose, and both may play a role in bacterial attachment to plants.
TABLE 4.
Average expression of S. enterica Newport genes relative to rplU in cells on LB agar, in sprout irrigation water, or attached to alfalfa sprouts at 24 h postinoculation
| Medium or condition | Gene | Avg expression (SD) of S. enterica gene
|
|
|---|---|---|---|
| 96Eo1152C-TX (wild type) | JDB 287 (agfD-agfBA intergenic region) | ||
| LB agar | agfD | 0.5 (0.5) | 0.02 (0.008) |
| agfB | 1,236 (300) | 9 (5.1) | |
| Irrigation water | agfD | 7.7 (0.4) | 0.6 (0.5) |
| agfB | 102,609 (40,253) | 128 (0.00) | |
| Alfalfa sprout | agfD | 0.6 (0.6) | 0.07 (0.03) |
| agfB | 724 (0.00) | 15.5 (7.3) | |
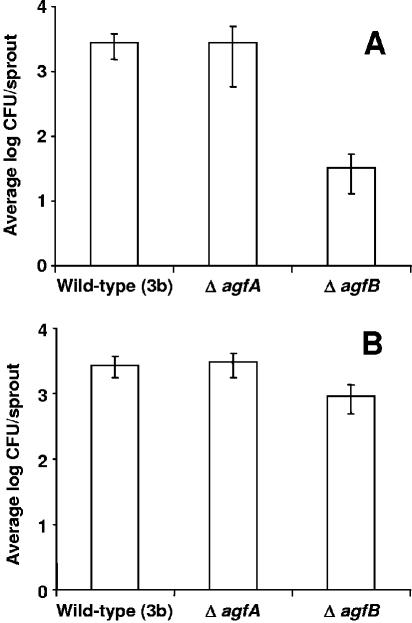
Despite repeated attempts, we were unable to delete the S. enterica Newport agf genes. Therefore, to help elucidate the role of curli in attachment and colonization of plant tissue, we obtained isogenic mutation strains of S. enterica Enteritidis agfA and agfB (Table 1). AgfA is the secreted curli subunit (12) and AgfB is the surface-exposed nucleator (41) which are assembled by the extracellular nucleation/precipitation pathway (15). To test whether curli are important for initial attachment of S. enterica to plant tissue, 3-day-old sprouts were irrigated with wild-type 27655-3b, ΔagfA, or ΔagfB strains. After 4 h, the populations of wild-type and ΔagfA were similar (P = 0.9), whereas ΔagfB was reduced by 2 logs (Fig. 3A; P < 0.05). To determine the role of curli in attachment and colonization, seeds were inoculated with each strain for 1 h and replaced with sterile water; at 24 h and at 48 h postinfection, the populations of the wild-type and ΔagfA strains were similar (P = 0.9), whereas the ΔagfB strain was reduced by 1 log (Fig. 3B; P < 0.05). These results are similar to those with both JDB 287 and JDB 279 in that the adhesion of S. enterica was not eliminated with disruption of either agfA or agfB. Deletion of agfB affected initial attachment, as well as attachment and colonization over time, whereas deletion of agfA did not. This was an unexpected result since neither mutant of agfA or agfB displays an aggregative phenotype on agar plates under curli-permissible conditions (confirmed in the present study) or aggregative fimbriae when examined under electron microscopy (41).
FIG. 3.
Adhesion of S. enterica serovar Enteritidis to alfalfa sprouts. (A) Attachment of cells to 3-day-old alfalfa sprouts in a 4-h attachment assay; (B) attachment and colonization 24 h postinoculation. The data are representative experiments with 10 sprout samples taken for each strain. The standard deviations are represented as error bars in all panels.
In E. coli, csgB (agfB equivalent) has been described as the cell-bound curli nucleator which anchors curli to the cell surface (15). We hypothesize that this surface-bound protein may facilitate initial attachment of S. enterica to plant tissue in the absence of agfA in the agfA mutant and allow for initial attachment to plant tissue without production of curli. The agfB mutant, which lacks curli and the surface-bound protein, was reduced in initial attachment, as well as colonization, over time; this may be due to the necessity of plant-colonizing bacteria to form aggregates for successful survival (25). Thus, S. enterica cells deficient in curli (JDB 287 and JDB 279) and agfB mutants would be disadvantaged in their ability to attach to one another and form aggregates (6, 29).
The importance of curli may also be supported by previous studies of S. enterica and EHEC attachment to alfalfa sprouts (8). We observed differences between the abilities of S. enterica and EHEC to attach to sprouts; the number of S. enterica cells that can attach was similar to plant-associated bacteria, whereas EHEC could easily be rinsed away. We hypothesized that the differences observed in the ability of EHEC and S. enterica to adhere could be attributed to the lack of curli and cellulose production in EHEC. The EHEC strains used for these studies did not encode a functional csgD (agfD in S. enterica); the gene that encodes the positive regulator for curli and cellulose, due to single-base-pair mutations in the promoter (38). Moreover, nonpathogenic E. coli that exhibited similar ability to attach to alfalfa sprouts as S. enterica also tested positive for curli production (J. D. Barak et al., unpublished observations), suggesting bacteria that can produce curli are more likely to attach at high populations to plant tissue.
The role of cellulose in attachment and colonization of S. enterica to plants was not studied directly by mutant analysis; however, neither JDB 287 nor JDB 279 produced cellulose, whereas the S. enterica Enteritidis curli mutants did. However, comparison of the ability to attach or colonize plant tissue revealed very little difference between JDB 287, JDB 279 (Fig. 1A), ΔagfA, and ΔagfB (Fig. 3) strains, suggesting a limited direct role for cellulose in these studies.
rpoS is required for S. enterica attachment to plants.
Another previously characterized gene identified in our random screen was rpoS, which was also reduced in attachment by one log (Fig. 1A). In S. enterica, rpoS, the general stress response regulator sigma factor, is a global regulator required for Salmonella virulence in animals. RpoS plays an important role in biofilm formation (27), as well as in the regulation of agfD (31), other adhesins (28), and other genes (16, 19).
The role of rpoS in S. enterica attachment to plant tissue appeared to be during initial attachment, similar to its role in biofilm formation (1). When sprout colonization was monitored over time, the populations of the rpoS mutant JDB 279 on sprouts were reduced by at least 1 log compared to the wild type at 24 h postinoculation but reached levels similar to those of the wild type by 48 h postinoculation (Fig. 1B), suggesting that RpoS regulates mechanisms required for initial attachment to plant tissue. When the rpoS mutation in JDB 279 was complemented on a plasmid (pJDB1), the ability to attach 24 h postinoculation was restored to wild-type levels (Fig. 1C). A strain carrying plasmid vector control did not restore attachment. To demonstrate that this nonattaching phenotype was due to a reduced ability to attach or colonize plant tissue and not a growth or survival defect, growth curves of planktonic mutant and wild-type cells in the sprout irrigation water were monitored. The numbers of mutant and wild-type cells were indistinguishable, indicating that the defect was in attachment to plant tissue (not shown).
The JDB 279 strain was also defective in curli and cellulose production, as demonstrated by the lack of production on agar plates at permissive conditions (Fig. 2A and B, section 3). When the rpoS mutation in JDB 279 was complemented on a plasmid (pJDB1), however, the ability to produce curli and cellulose was not restored (Fig. 2). This was unexpected, since rpoS is known to regulate curli production through the transcription of agfD (10). However, since rpoS regulates multiple systems and rescuing JDB279 by complementing it with the plasmid-encoded rpoS allowed for wild-type levels of sprout attachment, we hypothesize that S. enterica utilizes other adhesins or mechanisms in addition to curli to attach to plants. This hypothesis is further supported by our sprout attachment data for JDB 287, JDB 279, and the S. enterica Enteritidis agfA and agfB mutants that showed that no strain was completely deficient in attachment but only reduced. Multicellular behavior in S. enterica involves uncharacterized factors in addition to curli and cellulose. These molecules form a close bacterial network embedded in a matrix of acidic polysaccharides (36). Deletion of rpoS in S. enterica Typhimurium has minimal effect on biofilm formation though curli are absent (32), suggesting other adhesins or mechanisms are utilized for initial attachment and biofilm development.
Our sprout attachment data support the conclusion that RpoS contributes to S. enterica attachment to plants, perhaps due to the disruption of adhesin regulation in the rpoS mutant. A similar requirement for RpoS in plant-microbe interactions is also found in plant pathogens. For example, in the plant pathogen Erwinia carotovora subsp. carotovora, rpoS is needed for survival in the competitive environment of the leaf surface during stress conditions (4). An E. carotovora subsp. carotovora rpoS mutant was unable to colonize tobacco plants to similar populations as had the wild type, suggesting a role for rpoS in epiphytic fitness on plants. Furthermore, a role for rpoS in colonization of roots has been suggested for Pseudomonas putida (24) where rpoS mutant strains are reduced in their ability to colonize bean and cucumber seedlings.
Common themes between animal and plant pathogens.
The rise in incidence of outbreaks caused by S. enterica-contaminated produce illustrates the importance of understanding the fundamental mechanisms of S. enterica survival, growth, and virulence as they relate to the food supply. This is the first report to begin to study the details of the molecular interactions of S. enterica with plant tissue. The results of the present study reveal that some S. enterica genes required for virulence in animals are also required for attachment to plant tissue. These results add to the common themes and strategies for bacterial fitness in association with eukaryotic hosts that are emerging in animal and plant pathogens (7, 37) by demonstrating that curli and gene products regulated by rpoS are required for S. enterica attachment to plant tissue.
Sixty-five percent of the S. enterica genes identified as potentially contributing to bacterial adhesion to plant tissue have no previously reported function. This demonstrates the importance of investigating bacterial interactions with secondary hosts, such as plants, since many S. enterica genes may play a larger role in survival in the environment or on secondary hosts than they do in virulence in animal model systems. Understanding the functions of these genes is important since the role on secondary hosts or in the environment throughout the disease cycle may be crucial for intervention strategies or disease reduction. For our initial work, we focused on two strains with mutations in genes of known functions, both to confirm that our selection worked as expected and to tie the functions of these genes in bacterial interactions with a secondary host to what is already known about the regulatory roles of these genes in growth media and in animal hosts. Future studies will examine the many strains isolated with mutations in genes of unknown function.
Acknowledgments
This research was funded by U.S. Department of Agriculture Agricultural Research Service CRIS project 5325-42000-040.
We thank William Kay for the gift of the S. enterica serovar Enteritidis strains. We thank Jeff Palumbo for critical discussion of the manuscript and William Miller for help with figure production.
REFERENCES
- 1.Adams, J. L., and R. J. McLean. 1999. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 65:4285-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertson, N. H., S. Stretton, S. Pongpattanakitshote, J. Ostling, K. C. Marshall, A. E. Goodman, and S. Kjelleberg. 1996. Construction and use of a new vector/transposon, pLBT::mini-Tn10:lac:kan, to identify environmentally responsive genes in a marine bacterium. FEMS Microbiol. Lett. 140:287-294. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, R. A., V. Koiv, C. Norman-Setterblad, and M. Pirhonen. 1999. Role of RpoS in virulence and stress tolerance of the plant pathogen Erwinia carotovora subsp. carotovora. Microbiology 145:3547-3556. [DOI] [PubMed] [Google Scholar]
- 5.Ausmees, N., H. Jonsson, S. Hoglund, H. Ljunggren, and M. Lindberg. 1999. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology 145:1253-1262. [DOI] [PubMed] [Google Scholar]
- 6.Austin, J. W., G. Sanders, W. W. Kay, and S. K. Collinson. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295-301. [DOI] [PubMed] [Google Scholar]
- 7.Baker, B., P. Zambryski, B. Staskawicz, and S. P. Dinesh-Kumar. 1997. Signaling in plant-microbe interactions. Science 276:726-733. [DOI] [PubMed] [Google Scholar]
- 8.Barak, J. D., L. C. Whitehand, and A. O. Charkowski. 2002. Differences in attachment of Salmonella enterica serovars and Escherichia coli O157:H7 to alfalfa sprouts. Appl. Environ. Microbiol. 68:4758-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 10.Brown, P. K., C. M. Dozois, C. A. Nickerson, A. Zuppardo, J. Terlonge, and R. Curtiss. 2001. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 41:349-363. [DOI] [PubMed] [Google Scholar]
- 11.Charkowski, A. O., J. D. Barak, C. Z. Sarreal, and R. E. Mandrell. 2002. Differences in growth of Salmonella enterica and Escherichia coli O157:H7 on alfalfa sprouts. Appl. Environ. Microbiol. 68:3114-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinson, S. K., L. Emody, K. H. Muller, T. J. Trust, and W. W. Kay. 1991. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 173:4773-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstel, U., C. Park, and U. Romling. 2003. Complex regulation of csgD promoter activity by global regulatory proteins. Mol. Microbiol. 49:639-654. [DOI] [PubMed] [Google Scholar]
- 14.Gorski, L., J. D. Palumbo, and R. E. Mandrell. 2003. Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Appl. Environ. Microbiol. 69:258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammar, M., Z. Bian, and S. Normark. 1996. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:6562-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez-Ruiz, M., V. Robbe-Saule, D. Hermant, S. Labrude, and F. Norel. 2000. Identification of RpoS σ(S)-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:5749-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inami, G. B., and S. E. Moler. 1999. Detection and isolation of Salmonella from naturally contaminated alfalfa seeds following an outbreak investigation. J. Food Prot. 62:662-664. [DOI] [PubMed] [Google Scholar]
- 18.Korhonen, T. K., K. Haahtela, M. Romantschuk, and D. H. Bamford. 1986. Role of fimbriae and pili in the attachment of Klebsiella, Enterobacter, and Pseudomonas to plant surfaces, p. 229-241. In B. Lugtenberg (ed.), Recognition in microbe-plant symbiotic and pathogenic interactions, vol. H4. Springer-Verlag, Heidelberg, Germany. [Google Scholar]
- 19.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 20.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 21.Matthysse, A. G. 1983. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J. Bacteriol. 154:906-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthysse, A. G., and S. McMahan. 1998. Root colonization by Agrobacterium tumefaciens is reduced in cel, attB, attD, and attR mutants. Appl. Environ. Microbiol. 64:2341-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthysse, A. G., H. A. Yarnall, and N. Young. 1996. Requirement for genes with homology to ABC transport systems for attachment and virulence of Agrobacterium tumefaciens. J. Bacteriol. 178:5302-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, C. D., Y. C. Kim, and A. J. Anderson. 2001. Competitiveness in root colonization by Pseudomonas putida requires the rpoS gene. Can. J. Microbiol. 47:41-48. [DOI] [PubMed] [Google Scholar]
- 25.Monier, J. M., and S. E. Lindow. 2003. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA 100:15977-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45-e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raina, S., D. Missiakas, L. Baird, S. Kumar, and C. Georgopoulos. 1993. Identification and transcriptional analysis of the Escherichia coli htrE operon which is homologous to pap and related pilin operons. J. Bacteriol. 175:5009-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisner, A., J. A. Haagensen, M. A. Schembri, E. L. Zechner, and S. Molin. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933-946. [DOI] [PubMed] [Google Scholar]
- 30.Reuhs, B. L., J. S. Kim, and A. G. Matthysse. 1997. Attachment of Agrobacterium tumefaciens to carrot cells and Arabidopsis wound sites is correlated with the presence of a cell-associated, acidic polysaccharide. J. Bacteriol. 179:5372-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romling, U., W. Bokranz, W. Rabsch, X. Zogaj, M. Nimtz, and H. Tschape. 2003. Occurrence and regulation of the multicellular morphotype in Salmonella serovars important in human disease. Int. J. Med. Microbiol. 293:273-285. [DOI] [PubMed] [Google Scholar]
- 33.Romling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behavior of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 35.Smit, G., S. Swart, B. J. Lugtenberg, and J. W. Kijne. 1992. Molecular mechanisms of attachment of Rhizobium bacteria to plant roots. Mol. Microbiol. 6:2897-2903. [DOI] [PubMed] [Google Scholar]
- 36.Solano, C., B. Garcia, J. Valle, C. Berasain, J. M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43:793-808. [DOI] [PubMed] [Google Scholar]
- 37.Staskawicz, B. J., M. B. Mudgett, J. L. Dangl, and J. E. Galan. 2001. Common and contrasting themes of plant and animal diseases. Science 292:2285-2289. [DOI] [PubMed] [Google Scholar]
- 38.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2001. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:2367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vesper, S. J. 1987. Production of pili (fimbriae) by Pseudomonas fluorescens and correlation with attachement to corn roots. Appl. Environ. Microbiol. 53:1397-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vesper, S. J., and W. D. Bauer. 1986. Role of pili (fimbriae) in attachment of Bradyrhizobium japonicum to soybean roots. Appl. Environ. Microbiol. 52:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White, A. P., S. K. Collinson, P. A. Banser, D. L. Gibson, M. Paetzel, N. C. Strynadka, and W. W. Kay. 2001. Structure and characterization of AgfB from Salmonella enteritidis thin aggregative fimbriae. J. Mol. Biol. 311:735-749. [DOI] [PubMed] [Google Scholar]