Abstract
The objective of this work was to assess the effect of dilute bovine manure (1.0% and 0.1%) versus that of no manure on attachment and subsequent detachment of Cryptosporidium parvum oocysts to soil. Manure enhanced the attachment of oocysts to soil particles; the maximum attachment was observed with 0.1% manure. Oocyst attachment was partially reversible; maximum detachment was observed with dilute manure. These results indicate that oocyst attachment to soil is substantially affected by bovine manure in a complex manner and should have implications for how oocysts may be transported through or over soils.
There is concern regarding the contamination of surface waters and groundwaters with Cryptosporidium parvum. Several waterborne outbreaks of cryptosporidiosis have occurred in the past decade, the most severe in Milwaukee, WI, where over 400,000 people were infected (15). Dairy/beef animals are widely considered to be a major source of C. parvum oocysts. Based on a survey of 7,369 calves from 1,103 dairy farms (located in 28 states), Garber et al. (10) concluded that virtually all herds with >100 cows are infected with C. parvum. Neonatal calves are particularly susceptible to infection and can excrete several billion oocysts if they develop cryptosporidiosis. Recent studies indicate that postweaned and adult, asymptomatic cattle also excrete oocysts (9). Although excretion rates (oocysts/gram feces) are lower, the total number of oocysts excreted may still be substantial due to the quantity of feces produced.
Watershed monitoring studies indicate that contamination can occur via surface transport of oocysts from manures applied to land or fecal excretion or via vertical transport via preferential flow to groundwater (e.g., karst groundwater). Hansen and Ongerth (11) and Ong et al. (19) reported that oocyst concentrations downstream of dairy/beef operations were greater than those upstream, while Kuczynska et al. (14) documented the presence of oocyts in karst groundwater in a region devoted primarily to cattle grazing. Brush et al. (5) and Harter et al. (12) have described the leaching of oocysts through sands and sediments as convective dispersion transport in conjunction with sorption-desorption processes. Laboratory studies have also demonstrated the potential for oocyst runoff (2) or leaching (16, 17), although oocyst numbers in runoff or leachate were dramatically attenuated by soils.
Most studies have been conducted with purified oocysts in distilled water or oocysts in calf diarrhea. However, purified oocysts or oocysts from calf diarrhea are a highly unlikely source for water contamination. A much more likely scenario is oocyst contamination from the application to land of calf manures mixed with adult cow manure or fecal deposition on pastures by calves. Oocysts must first be “released” from the manure matrix before they can be transported to surface water or groundwater. Bradford and Schijven (3) have investigated the release of oocysts from dairy cow manure during simulated rainfall. They observed that oocyst numbers were highly correlated with turbidity (i.e., from fiber or microbial biomass), indicating that oocyst “release” was concomitant with manure dissolution.
Bovine manure is a complex matrix consisting of microbial biomass, dietary fiber, bedding materials, urine, and fecal mucus. The single largest component of manure is fiber, both dietary and from bedding (composed of cellulose, hemicellulose, and lignin). Van Kessel and Reeves (21) reported that the fiber content of dry manure was ca. 55% (mean value for 107 manures), although values were variable depending on management practices. Based on visual inspection, bovine manure contains a wide range of fiber sizes, from microscopic to macroscopic. The second largest component of manure is microbial biomass plus sloughed intestinal cells. According to Salo (20), this accounts for ca. 30% of dry fecal matter. The remaining dry matter is predominantly inorganic material (21). Manures also contain glycoproteins (i.e., mucus), which account for up to 40% of total N (1). Based on a mean total N content of ca. 4% (21), manure can contain up to ca. 1.5% mucus.
Manure can potentially affect oocyst attachment to soils in a variety of ways. Attachment or adhesion of oocysts to dietary or bedding fiber in manure prior to application to land may inhibit attachment to soil particles. The difficulties reported by many researchers in extracting oocysts from manure/feces (for examples, see references 6, 13, and 22), particularly at low concentrations, suggest that there is some association between oocysts and manure fiber. Alternatively, the high microbial populations in manure may compete for attachment sites on soils, thereby preventing oocyst attachment. Finally, the presence of mucus may serve to “bind” oocysts to soil particles, fiber particulates, or both. The objective of this study was to assess the effect of bovine manure on the oocysts' initial attachment to and subsequent detachment from soil particles.
Manure samples were collected from the Beltsville Area Research Center dairy farm on two separate occasions. The manure had dry matter contents of ca. 10%. Manure was diluted 10-fold with deionized-distilled (DD) water and blended at high speed for 2 min (10% manure). The manure suspension was subsequently diluted 10-fold with DD water and vortexed for ca. 30 seconds (1% manure). Both 1% and 10% manure suspensions were inoculated with purified C. parvum oocysts; purified C. parvum oocysts were obtained from infected calves as previously described (8). Oocysts were suspended in distilled water to give a concentration of ca. 105 oocysts ml−1 manure suspension. Oocyst suspensions were prepared approximately 30 min prior to use.
One milliliter of a 1% or 10% manure-oocyst suspension was added to 9 ml of a 1% (wt/vol) suspension of sandy loam or clay loam soil in DD water, resulting in suspensions consisting of ca. 0.9% soil and either 0.1% manure (0.01% manure solids) or 1.0% manure (0.1% manure solids). Twelve tubes were prepared for each soil and consisted of (i) four tubes with oocysts but without manure, (ii) four tubes with oocysts and 0.1% manure, and (iii) four tubes with oocysts and 1.0% manure. After soil and manure suspensions were combined, the tubes were vortexed thoroughly and incubated for 2 h on their sides with a gentle shaking motion at ca. 8°C. After being shaken the tubes were centrifuged for 10 min at 100 × g to pellet the soil-manure, and the top 9 ml of supernatant was removed using a glass pipette. Nine milliliters of distilled water was added to the tubes, and the tubes were vortexed for ca. 30 seconds and then incubated a second time as previously described. Oocysts in supernatants from the first and second incubations were enumerated using an immunofluorescence assay (14).
An additional set of four tubes that consisted of oocysts in DD water (ca. 105 oocysts ml−1) without manure or soil was prepared. After the tubes were shaken and centrifuged, oocyst concentrations in the top 9 ml and bottom 1 ml of water were analyzed. Oocyst concentrations were identical in the two water fractions, indicating that centrifugation for 10 min at 100 × g was not responsible for the observed differences in oocyst distributions within the tubes.
Two independent experiments were conducted with manure collected at different times. The same experimental procedure was used for both experiments. Since results were generally comparable, data from the first and second incubations have been combined.
Statistical analyses were done with software SPLUS (MathSoft, 1999). Significance of differences between average values was tested with the Welch modified t test. Analysis of variance was applied to see whether soil, manure content, or resuspension was a significant factor affecting the attachment of partition coefficient values.
In the absence of manure, the percentages of oocyst attachment to sandy loam and clay loam soil were 72.0% and 93.1%, respectively; differences were statistically significant (P ≤ 0.001; Table 1). This is consistent with previous work by Kuczynska and Shelton (13) in which percentages of oocyst recovery from soils were inversely correlated with clay content. Presumably, the increased number of soil particles and/or surface area (per gram of soil) presents a greater number of potential attachment sites. This is also consistent with the results of Atwill et al. (2), who observed an ca. 2- to 3-log (99 to 99.9%) reduction in oocysts transported per meter of loam or sandy loam soil. This contradicts the findings of Dai and Boll (7), who observed no attachment of oocysts to clay particles. However, the maximum soil concentration tested in their experiments was 2 mg ml−1, which occurs only in pristine waters; hence, this has no relevance to agricultural runoff, where typical sediment loads are 1% (10 g ml−1).
TABLE 1.
Effect of dilute bovine manure on oocyst attachment to soil particles
| Treatment | % Attachmenta to:
|
|
|---|---|---|
| Sandy loam | Clay loam | |
| Water | 72.0 ± 3.6A | 93.1 ± 2.9D |
| Manure | ||
| 1.0% | 86.7 ± 2.2B | 93.1 ± 3.8D |
| 0.1% | 97.4 ± 1.8C | 97.7 ± 2.0C |
Values represent the mean ± standard deviation from two experiments (n = 8). Different letters following values indicate statistical significance of differences between average values evaluated at the 0.05 significance level.
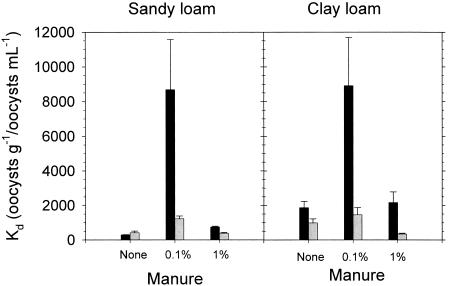
In general, manure enhanced the attachment of oocysts to soil particles, indicating that some component of manure facilitated oocyst attachment; collectively, differences were statistically significant (P ≤ 0.001) (Table 1). The highest attachment percentage (and Kd value) (Fig. 1) was observed with the 0.1% manure suspension, suggesting that there is some optimal concentration of the “facilitating” component in manure between 0 and 1.0%. It is unclear why oocyst attachment decreased with increasing manure concentration. Perhaps the “facilitating” component was offset by higher biomass concentrations in the 1.0% manure suspension which competed with oocysts for soil attachment sites.
FIG. 1.
Effect of dilute manure versus no manure on oocyst attachment to sandy loam or clay loam soil. ▪, Kd values for first incubation (initial attachment);  , Kd values for second incubation (detachment). Bars represent standard errors (n = 8).
, Kd values for second incubation (detachment). Bars represent standard errors (n = 8).
Soil-manure-oocyst pellets were resuspended in freshwater and incubated a second time to assess the tendency for detachment. Kd values, defined as the ratio of oocysts attached to soil (oocysts g−1) to oocysts in solution (oocysts ml−1) are presented for both incubations in Fig. 1. Note that total oocyst numbers decreased between incubations due to removal of suspended oocysts after the first incubation. Analysis of variance showed that manure content and resuspension both significantly affected Kd values (P < 0.001). Average Kd values before and after resuspension were not significantly different in sandy loam and clay loam in the absence of manure. However, resuspension significantly decreased average Kd values in the presence of manure (P < 0.05), indicating that oocyst attachment was readily reversible. The magnitude of detachment was consistently greater with the 0.1% manure suspension than with the 1.0% manure suspension. A higher percentage of oocysts initially attached to soil particles, and remained attached after resuspension, in the presence of 0.1% manure.
Although the precise mechanism(s) of oocyst attachment is unclear, previous research is suggestive. Brush et al. (4) examined the impact of different purification methods on oocyst surface charge. They observed that purification using milder methods yielded oocysts with a neutral surface charge, while purification using harsher methods yielded oocysts with a net negative surface charge. They concluded that oocysts in the environment likely have minimal surface charge. Brush et al. (4) also investigated the “stickiness” of oocysts by examining their adhesion to inert polystyrene beads. They observed 50 to 90% adhesion in solutions with ionic strength typical of agricultural runoff (<5 mM). This is consistent with previous research documenting that the oocyst outer wall is composed largely of glycoproteins (18), which promote adhesion. In the current study, oocysts were purified via sucrose flotation and discontinuous CsCl centrifugation. Although the surface charge of oocysts was not measured, the use of a mild purification method likely produced oocysts with minimal surface charge. In addition, manure and soil solutions were prepared using DD water, giving a low-ionic-strength solution. Consequently, our data are consistent with those of Brush et al. and suggest that adhesion is the predominant mechanism of oocyst attachment to soil particles.
In conclusion, these results indicate that oocyst attachment to soil is substantially affected by bovine manure in a complex manner. The extent of oocyst attachment to soil particles and the tendency to remain attached appear to be correlated with manure dilution. Consequently, rates of manure dissolution control not only oocyst “release” but also transient attachment to soil particles. Further research is needed to more thoroughly elucidate the impact of manure dissolution on oocyst transport to surface waters or groundwaters from manure applied to land or fecal deposition.
Acknowledgments
We thank Valerie McPhatter for technical assistance.
REFERENCES
- 1.Anonymous. 1985. Nitrogen metabolism in the large intestine, p. 53-56. In Leonard S. Bull, William Chalupa, Fredric N. Owens, Larry D. Satter, Charles J. Sniffen, Allen H. Trenkle, and Dale R. Waldo (ed.), Ruminant nitrogen usage. The National Academy of Sciences, Washington, D.C.
- 2.Atwill, E. R., L. Hou, B. M. Karle, T. Harter, K. W. Tate, and R. A. Dahlgren. 2002. Transport of Cryptosporidium parvum oocysts through vegetated buffer strips and estimated filtration efficiency. Appl. Environ. Microbiol. 68:5517-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, S. A., and J. Schijven. 2002. Release of Cryptosporidium and Giardia from dairy calf manure: impact of solution salinity. Environ. Sci. Technol. 36:3916-3923. [DOI] [PubMed] [Google Scholar]
- 4.Brush, C. F., M. F. Walter, L. J. Anguish, and W. C. Ghiorse. 1998. Influence of pretreatment and experimental conditions on electrophoretic mobility and hydrophobicity of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 64:4439-4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brush, C. F., W. C. Ghiorse, L. J. Anguish, J.-Y. Parlange, and H. G. Grimes. 1999. Transport of Cryptosporidium parvum oocysts through saturated columns. J. Environ. Qual. 28:809-815. [Google Scholar]
- 6.Bukhari, Z., and H. V. Smith. 1995. Effect of three concentration techniques on viability of Cryptosporidium parvum oocysts recovered from bovine feces. J. Clin. Microbiol. 33:2592-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai, X., and J. Boll. 2003. Evaluation of attachment of Cryptosporidium parvum and Giardia lamblia to soil particles. J. Environ. Qual. 32:296-304. [DOI] [PubMed] [Google Scholar]
- 8.Fayer, R., and W. Ellis. 1993. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J. Parasitol. 79:771-774. [PubMed] [Google Scholar]
- 9.Fayer, R., J. M. Trout, T. K. Graczyk, and E. J. Lewis. 2000. Prevalence of Cryptosporidium, Giardia and Eimeria infections in post-weaned and adult cattle on three Maryland farms. Vet. Parasitol. 93:103-112. [DOI] [PubMed] [Google Scholar]
- 10.Garber, L. P., M. D. Salman, H. S. Hurd, T. Keefe, and J. L. Schlater. 1994. Potential risk factors for Cryptosporidium infection in dairy calves. J. Am. Vet. Med. Assoc. 205:86-91. [PubMed] [Google Scholar]
- 11.Hansen, J. S., and J. E. Ongerth. 1991. Effects of time and watershed characteristics on the concentration of Cryptosporidium oocysts in river water. Appl. Environ. Microbiol. 57:2790-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harter, H., S. Wagner, and E. R. Atwill. 2000. Colloid transport and filtration of Cryptosporidium parvum in sandy soils and aquifer sediments. Environ. Sci. Technol. 34:62-70. [Google Scholar]
- 13.Kuczynska, E., and D. R. Shelton. 1999. Method for detection and enumeration of Cryptosporidium parvum oocysts from feces, manure, and soils. Appl. Environ. Microbiol. 65:2820-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczynska, E., D. G. Boyer, and D. R. Shelton. 2003. Comparison of immunofluorescent antibody assay (IFA) and immunomagnetic-electrochemiluminescence (IM-ECL) in detection of Cryptosporidium parvum in karst water samples. J. Microbiol. Methods 53:17-26. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. A. Addiss, K. R. Fox, J. R. Rose, and J. P. Davis. 1994. A massive outbreak of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 16.Mawdsley, J. L., A. E. Brooks, and R. J. Merry. 1996. Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biol. Fertil. Soils. 21:30-36. [Google Scholar]
- 17.Mawdsley, J. L., A. E. Brooks, R. J. Merry, and B. F. Pain. 1996. Use of a novel soil tilting table apparatus to demonstrate the horizontal and vertical movement of the protozoan pathogen Cryptosporidium parvum in soil. Biol. Fertil. Soils. 23:215-220. [Google Scholar]
- 18.Nanduri, J., S. Williams, T. Aji, and T. P. Flanigan. 1999. Characterization of an immunogenic glycocalyx on the surfaces of Cryptosporidium parvum oocysts and sporozoites. Infect. Immun. 67:2022-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong, C., W. Moorehead, A. Ross, and J. Isaac-Renton. 1996. Studies of Giardia spp. and Cryptosporidium spp. in two adjacent watersheds. Appl. Environ. Microbiol. 62:2798-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salo, M. L. 1965. Determination of carbohydrate fractions in animal foods and faeces. Acta Agral. Fenn. 105:1-102. [Google Scholar]
- 21.Van Kessel, J. S., and J. B. Reeves III. 2002. Nitrogen mineralization potential of dairy manures and its relationship to composition. Biol. Fertil. Soils. 36:118-123. [Google Scholar]
- 22.Xiao, L., and R. P. Herd. 1993. Quantitation of Giardia and Cryptosporidium oocysts in fecal samples by direct immunofluorescence assay. J. Clin. Microbiol. 31:2944-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]