Abstract
There are well documented costs of mating in insects but little evidence for underlying mechanisms. Here, we provide experimental evidence for a hormone-based mechanism that reduces immunity as a result of mating. We examined the mealworm beetle Tenebrio molitor and show that (i) mating reduces a major humoral immune effector-system (phenoloxidase) in both sexes, and (ii) that this down-regulation is mediated by juvenile hormone. Because both juvenile hormone and phenoloxidase have highly conserved functions across all insects, the identified mechanism is similarly likely to be highly conserved. The positive physiological function of mating-induced juvenile hormone secretion is gamete and accessory gland production: we propose that its negative effects on immune function are the consequence of physiological antagonism. Therefore, we have identified a physiological tradeoff between mating and immunity. Our results suggest that increasing mating success can result in increasing periods of immune suppression, which in turn implies that reproductively successful individuals may be more vulnerable to infection by, and the negative fitness effects of, pathogens.
Mating bears life-history costs (e.g., refs. 1–3), but potential mechanism(s) that underpin, or contribute to the expression of, these costs are poorly understood, except where the costs arise directly from sexual conflicts (1, 4, 5). Ecological costs of multiple mating (6) include increased predation risk and increased exposure to parasites. Such consequences of mating are potentially manifest as decreased longevity, a well documented correlate of increased copulation success (3, 5, 6). In this paper, we examine mating physiology in the mealworm beetle Tenebrio molitor L. and provide evidence for a general physiological mechanism that reduces immune function as a result of mating. Recent work has documented significant mating-induced reductions in immune function in a damselfly (7) and in Drosophila melanogaster (8). These reductions did not seem to be the consequence of the energetic requirements of reproductive behavior (7), and a previous study (9) suggested they may not be the consequence of the energetic demands of gamete production either. It is generally assumed that physiological (and evolutionary) trade-offs are mediated by hormones (10), and there is good, but scarce, evidence in insects that the conserved insect hormone “juvenile hormone” (11) is involved in such trade-offs (12, 13). We examined whether juvenile hormone was responsible for mating-induced down-regulation in immune function in T. molitor. Our study took three consecutive steps to identify the underlying mechanism.
First, we examined whether mating reduced immune function. We measured hemocyte load (the total number of immunocytes in the hemolymph), an assay of insect cellular immunity that has been shown to be related to parasite resistance (14–16) as well as phenoloxidase (PO; a key, functionally conserved component of humoral immunity in insects; ref. 17). These components of innate immunity (18) provide measures of cellular and humoral immune function, respectively, but are also essential components of the cellular encapsulation response, the major insect immune response (18).
Second, we examined how mating-induced changes in the endocrine system influence immune function. Behaviorally influenced control of reproductive physiology in insects is mediated largely by the corpora allata (19), a paired endocrine organ attached to the brain. Activity in this organ is stimulated by mating (11, 19), and the secretory products are known to control physiological function in several tissues (11, 19). To uncouple the direct physical and physiological consequences of copulation (e.g., harassment, the effects of physiologically active ejaculatory products, etc.) from the internal state changes stimulated by copulation and mediated via the corpora allata, we conducted a “traditional” allograft experiment (20, 21) in which we transplanted corpora allata from either mated or virgin beetles into virgin recipients. Immune function was measured in all recipients.
Third, we examined whether juvenile hormone was a critical component of the observed mating-induced effects. Juvenile hormone is the most important physiologically active compound secreted by the corpora allata (11), and several studies have shown that titers of this hormone are elevated after mating (22, 23). Moreover, high titers of juvenile hormone have been implicated in humoral immune suppression in insects (24). We used a specific juvenile hormone inhibitor to block juvenile hormone synthesis in transplanted corpora allata (25) and thereby assessed whether juvenile hormone down-regulates immune function.
Materials and Methods
Insects.
All beetles were derived from a stock culture maintained at the University of Sheffield. Pupae were removed from the culture on the day of pupation, weighed, sexed, and then kept individually until used in experiments. Upon eclosion, the solitary adult was placed in a Petri dish with ad libitum access to water and rat chow and maintained at 23 ± 1°C and 12-h light/12-h dark cycle. This procedure guaranteed the beetles had not mated before they were allocated to treatments. Beetles of each sex were randomly allocated to the treatments when aged between 7 and 14 days. Mated beetles copulated once and then were isolated.
Experiment 1.
We first examined whether mating affected immune function by allocating age-controlled virgin, sexually mature T. molitor to a treatment or control group at random. Animals in the treatment group mated once [duration (± SE) 76.4 ± 2.1 sec, n = 93], were immediately isolated, and, 24 h later, had blood removed to measure hemocyte load and PO activity. Animals in the control group were treated identically to the treatment group but were not mated. Hemolymph samples were obtained by perfusing the abdomen of the beetle with 1 ml of ice-cold sodium cacodylate buffer. The sample was split, and one half was used for assaying PO (see below). The remaining 500 μl were transferred to a lycene-coated multiwell slide, which was incubated at 24 ± 2°C for 30 min (the time taken for the hemocytes to settle and adhere to the slide) in a chamber with ≈100% room humidity. The hemocytes then were fixed in 3% (wt/vol) paraformaldehyde for 2 min, washed three times in buffer, stained with 4′,6-diamidino-2-phenylindole (DAPI), and examined with a compound fluorescence microscope (Diaplan, Leitz) fitted with a digital camera. The fluorescing cell nuclei were counted by using OPTIMAS V.6.1 image analysis software. Because animals were perfused, it was not possible to estimate the repeatability of spatial sampling. Instead, we conducted seven successive perfusions on the same animal and calculated the variation in percent of hemocytes collected in the first perfusion. We were able to collect 88% ± 1.5% (n = 10) of hemocytes with the first perfusion. Repeatabilities (26) for the measurement technique were calculated by taking three subsamples from the first perfusion from each of 100 beetles. There was a significant (F = 14.411, P < 0.0001) within-individual repeatability of 0.82.
PO activity was assayed by using the protocol of Barnes and Siva-Jothy (27). In brief, hemolymph was collected as outlined above and immediately frozen to −90°C. Frozen samples were thawed and centrifuged (15 min, 2,800 × g, 4°C) and the supernatant was mixed with 1 ml of ice-cold sodium cacodylate buffer containing 3 mM l-dopa. The cold reaction mixture was placed in a heated (30°C) spectrophotometer (Amersham Pharmacia), and the reaction was allowed to proceeded for 30 min. Readings were taken every 2 min at 490 nm. The activity of the enzyme was measured as the slope [absorbance (OD) against time] of product formation during the first 5 min of the linear phase of the reaction commencing 5 min after the start of the reaction. Repeatabilities (26) for this measurement technique were calculated by taking two sub samples from each of 20 beetles. There was a significant (F = 29.45, P < 0.0001) within-individual repeatability of 0.93.
Experiment 2.
Corpora allata were removed from unmated or single-mated donors (donors were generated as in the experiment above) and transplanted into virgin same-sex recipients. Corpora allata transplantation has been used as an anatomical and physiological manipulation among and within insect species for many years (20, 21). We dissected donors in Grace's medium by decapitating the live beetle, removing the dorsum of the head capsule, and removing the corpora allata with fine bow-spring scissors. Removed corpora allata were either incubated in sterile culture dishes (Nunc) at room temperature or transplanted immediately. When transplanted, they were sucked whole into a 29G hypodermic needle (U-100 microfine; Becton Dickinson) with 3 μl of Grace's medium and injected whole into the abdominal hemocoel of the recipient. After injection, the needle was flushed through to check that the transplantation was successful; recipients that had been injected but that had not received the allograft were removed from the protocol along with the failed allograft. In the absence of intervening in vitro incubation, the procedure (from dissection to injection) took between 2 and 5 min. Insects have poor graft recognition abilities, even at the xenograft level (21), so this manipulation is unlikely to produce confounding immunological effects. PO activity was measured in blood collected 3 h after transplantation, but 24 h after the donor mated.
Experiment 3.
Inhibition of juvenile hormone in the corpora allata of mated donors was achieved as follows. After mating (18 h), the corpora allata were removed and randomly allocated to treatments: they were incubated at 21 ± 2°C for 3 h either in Grace's insect medium with 11 μg/ml−1 Fluvastatin or Grace's medium only. Fluvastatin (Novartis Pharma, Basel, Switzerland) is a commercially produced synthetic hydroxymethylglutaryl-CoA reductase inhibitor that has specific inhibitory activity toward juvenile hormone (25). It's inhibitory effects on juvenile hormone persist for 24 h after treatment (23). After incubation the corpora allata were washed three times in Grace's medium and then injected whole into the abdominal hemocoel of recipients. PO activity was measured 3 h after the transplant (i.e., 24 h after the donor had mated).
Results and Discussion
Experiment 1.
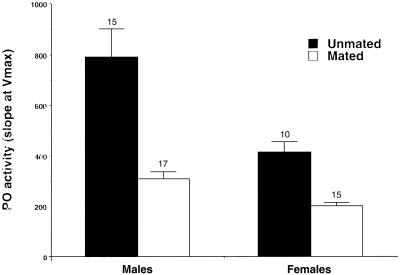
PO activity was significantly lower 24 h after mating in treatment males and females, compared with unmated males and females (treatment F1,53 = 4.14, P = 0.0468; sex F1,53 = 1.423, P = 0.238; sex.treatment F1,53 = 0.362, P = 0.55, Fig. 1). By contrast, hemocyte load was not affected by mating (treatment F1,55 = 0.035, P = 0.853; sex F1,55 = 12.334, P = 0.001; sex.treatment F1,55 = 0.010, P = 0.922), but there was a strong effect of sex (see ref. 28 for a discussion of sexual dimorphism in insect immune traits). Lowered PO activity is likely to have important immunological consequences in insects (29, 30) and is known to reduce refractoriness to parasites (31, 32).
Figure 1.
Activity (measured as the change in absorbance over time during the fastest linear phase of the reaction = OD × 103 min−1) of PO in mated and unmated male and female T. molitor 24 h after mating. Mating-induced down-regulation in PO activity recovered 36 h after mating [mean activity before mating in virgin animals 350.7 ± 42.5 (mean ± SE); mean activity 36 h after first mating 341.3 ± 64.5; F1,39 = 0.016, P = 0.900]. Numbers above bars indicate sample sizes.
Experiment 2.
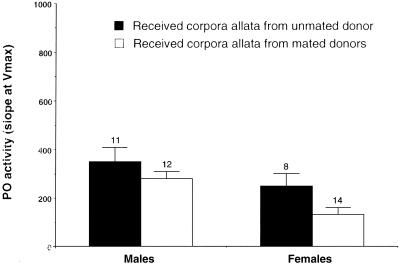
Unmated individuals that received corpora allata from a mated donor showed significantly reduced PO activity compared with recipients of corpora allata from unmated donors (treatment F1,41 = 5.90, P = 0.0196; sex F1,41 = 9.489, P = 0.004; sex.treatment F1,41 = 0.461, P = 0.501; Fig. 2). We conclude that mating induces the corpora allata to release one, or more, immunosuppressive signals/compounds.
Figure 2.
Activity of PO in virgin male and female T. molitor that received corpora allata from same-sex mated donors (black bars) or same-sex unmated donors (white bars). Numbers above bars indicate sample sizes.
Experiment 3.
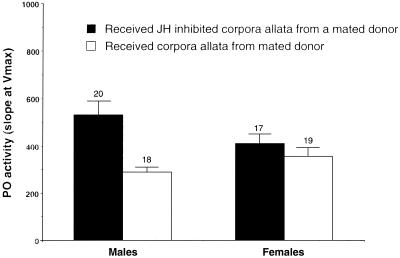
If juvenile hormone secretion is involved in mediating mating-induced immune suppression, we predicted no mating-induced PO down-regulation in recipients of juvenile hormone-inhibited corpora allata. Virgin insects were allocated at random to one of two groups. Animals in the treatment group received corpora allata that had been removed from a mated donor and had then been incubated in juvenile hormone inhibitor. Virgins in the control group received corpora allata from mated donors that had been treated identically but had not been exposed to inhibitor. Virgins in the control group had significantly less PO activity 3 h after transplantation (but 24 h after donor mated), compared with the treatment (treatment F1,70 = 9.04, P = 0.0037, sex F1,70 = 0.25, P = 0.6189, sex.treatment: F1,70 = 4.11, P = 0.0464, Fig. 3). We conclude that mating induces the corpora allata to release juvenile hormone, which plays a role in down-regulating PO activity.
Figure 3.
Activity of PO in virgin male and female T. molitor that received corpora allata from mated donors. Black bars represent the means for individuals that received corpora allata which were incubated in Fluvastatin, a specific juvenile hormone (JH) synthesis inhibitor. White bars represent the means for individuals that received mated corpora allata that had undergone similar incubation, but had not been exposed to the inhibitor. Numbers above bars indicate sample sizes.
Our results indicate that measurable postmating reduction in humoral PO activity is mediated, at least in part, by juvenile hormone secretion from the corpora allata. Female T. molitor remate every other day to maintain maximum egg output (33). Therefore, females may have reduced function in a major immune effector system for at least half their adult life, an effect that may occur over longer relative time-scales in reproductively successful males (see Fig. 1).
There are at least two important, nonexclusive implications arising from these results. First, the mechanism we have identified may contribute to the expression of the longevity cost associated with mating in insects. Examination of monarch butterflies (13) has shown that high juvenile hormone titers reduce longevity: all insects produce juvenile hormone (11) and utilize PO in humoral immunity (17, 18). Mating induced juvenile hormone secretion functions to switch on physiological processes associated with gametogenesis and spermatophore production (19), processes vital for female and male fitness. Antagonism between these reproductive functions and the hormone's effects on PO is the basis of a physiological tradeoff that may contribute to decreased longevity by means of weakened immunity. Moreover, there is recent evidence that mating suppresses antibacterial activity (8), another important compartment of the insect immune system (17).
Second, any decrease in immune function linked to mating will potentially enhance the transmission success of sexually transmitted diseases. Mating induced down-regulation of immune function may provide a window of opportunity which sexually transmitted and/or dormant pathogens exploit.
Acknowledgments
We thank Mark Fellowes, Hanno Sandvik, Sophie Armitage, John Thompson, Stuart Wigby, and Yannick Moret for helpful comments and/or assistance in the laboratory. J.R. was supported by the Deutsche Forschungsgemeinschaft and a Marie-Curie Fellowship. M.T.S.-J. was supported by Natural Environment Research Council (London) Grant GR3/12121. We thank Novartis Pharma AG, Basel, Switzerland for their generosity in supplying Fluvastatin.
Abbreviation
- PO
phenoloxidase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chapman T, Liddle F, Kalb M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 2.Cordero C. Behav Ecol Sociobiol. 2000;48:458–462. [Google Scholar]
- 3.Cordts R, Partridge L. Anim Behav. 1996;52:269–278. [Google Scholar]
- 4.Arnqvist G, Rowe L. Proc R Soc London Ser B. 1995;261:123–127. [Google Scholar]
- 5.Crudgington C, Siva-Jothy M T. Nature (London) 2000;407:855–856. doi: 10.1038/35038154. [DOI] [PubMed] [Google Scholar]
- 6.Arnquist G, Nilsson T. Anim Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. [DOI] [PubMed] [Google Scholar]
- 7.Siva-Jothy M T, Tsubaki Y, Hooper R. Physiol Entomol. 1998;23:274–277. [Google Scholar]
- 8.McKean K, Nunney L. Proc Natl Acad Sci USA. 2001;98:7904–7909. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Vorhies W A. Nature (London) 1992;360:456–458. doi: 10.1038/360456a0. [DOI] [PubMed] [Google Scholar]
- 10.Williams G C. Adaptation and Natural Selection. Princeton: Princeton Univ. Press; 1966. [Google Scholar]
- 11.Nijhout H F. Insect Hormones. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 12.Zera A, Harshman L G. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
- 13.Herman W S, Tatar M. Proc R Soc London Ser B. 2001;268:2509–2514. doi: 10.1098/rspb.2001.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz J, Wiesner A, Götz P, Sauer K-P. Dev Comp Immunol. 2000;24:1–12. doi: 10.1016/s0145-305x(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 15.Kraaijeveld A R, Limentani E, Godfray H C J. Proc R Soc London Ser B. 2001;268:259–261. doi: 10.1098/rspb.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellowes M D E, Godfray H C J. Heredity. 2000;84:1–8. doi: 10.1046/j.1365-2540.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 17.Brey P T, Hultmark D, editors. Molecular Mechanisms of Immune Responses in Insects. London: Chapman & Hall; 1998. [Google Scholar]
- 18.Pathak J, editor. Insect Immunity. Dordrecht, The Netherlands: Kluwer; 1993. [Google Scholar]
- 19.Wigglesworth V B. The Principles of Insect Physiology. London: Methuen; 1965. [Google Scholar]
- 20.Wigglesworth V B. The Physiology of Insect Metamorphosis. Cambridge, U.K.: Cambridge Univ. Press; 1954. [Google Scholar]
- 21.Lackie A M. In: Hemocytic and Humoral Immunity in Arthropods. Gupta A P, editor. New York: Wiley; 1986. pp. 191–226. [Google Scholar]
- 22.Couche G, Gillott C, Tobe S, Feyereisen R. Can J Zool. 1985;63:2789–2792. [Google Scholar]
- 23.Duportets L, Dufour M C, Couillaud F, Gadenne C. J Exp Biol. 1998;201:2425–2432. doi: 10.1242/jeb.201.16.2425. [DOI] [PubMed] [Google Scholar]
- 24.Hiruma K, Riddiford L. J Insect Morphol Embryol. 1993;22:103–117. [Google Scholar]
- 25.Debernard S, Rossignol F, Couillaud F. Gen Comp Endocrinol. 1994;95:92–98. doi: 10.1006/gcen.1994.1105. [DOI] [PubMed] [Google Scholar]
- 26.Lessells C M, Boag B T. Auk. 1987;104:116–121. [Google Scholar]
- 27.Barnes A I, Siva-Jothy M T. Proc R Soc London Ser B. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolff J. Proc R Soc London Ser B. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson K, Cotter S, Reeson A, Pell J. Ecol Lett. 2001;4:637–649. [Google Scholar]
- 30.Rizki R M, Rizki T M. J Insect Physiol. 1990;36:523–529. [Google Scholar]
- 31.Nigam Y, Maudlin L, Welburn S, Ratcliffe N. J Invert Pathol. 1997;69:279–281. doi: 10.1006/jipa.1996.4652. [DOI] [PubMed] [Google Scholar]
- 32.Shiao S, Higgs S, Adelman Z, Christensen B M, Liu S M, Chen C C. Insect Mol Biol. 2001;10:315–321. doi: 10.1046/j.0962-1075.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 33.Drnevich J, Papke R, Rauser C, Rutowski R. J Insect Behav. 2001;14:215–230. [Google Scholar]