Abstract
Enterococcus faecalis is a gram-positive commensal bacterium of the gastrointestinal tract and an important opportunistic pathogen. Despite the increasing clinical significance of the enterococci, genetic analysis of these organisms has thus far been limited in scope due to the lack of advanced genetic tools. To broaden the repertoire of genetic tools available for manipulation of E.faecalis, we investigated the use of phosphoribosyl transferases as elements of a counterselection strategy. We report here the development of a counterselectable markerless genetic exchange system based on the upp-encoded uracil phosphoribosyl transferase of E. faecalis. Whereas wild-type E. faecalis is sensitive to growth inhibition by the toxic base analog 5-fluorouracil (5-FU), a mutant bearing an in-frame deletion of upp is resistant to 5-FU. When a cloned version of upp was ectopically introduced into the deletion mutant, sensitivity to 5-FU growth inhibition was restored, thereby providing the basis for a two-step integration and excision strategy for the transfer of mutant alleles to the enterococcal chromosome by recombination. This method was validated by the construction of a ΔsrtA mutant of E. faecalis and by the exchange of the surface protein Asc10, encoded on the pheromone-responsive conjugative plasmid pCF10, with a previously isolated mutant allele. Analysis of the ΔsrtA mutant indicated that SrtA anchors Asc10 to the enterococcal cell wall, facilitating the pheromone-induced aggregation of E. faecalis cells required for high-frequency conjugative plasmid transfer in liquid matings. The system of markerless exchange reported here will facilitate detailed genetic analysis of these important pathogens.
Enterococcus faecalis is a gram-positive commensal bacterium normally associated with humans as a member of the intestinal microflora (40). However, E. faecalis is also an opportunistic pathogen of increasing clinical significance, particularly as an etiological agent of nosocomial infections (34). The species exhibits relatively high-level intrinsic resistance to many antibiotics and is well known for its promiscuity, resulting in horizontal transfer of mobile genetic elements carrying antibiotic resistance markers (18) and consequently making treatment of enterococcal infections an increasingly difficult problem for clinicians armed with currently available therapeutic agents. New therapeutic targets and strategies are needed to combat enterococcal infections caused by multiresistant strains often found in hospital environments. The ability to develop such therapeutics demands an intimate understanding of enterococcal physiology and genetics. Experimental techniques that permit researchers to perform sophisticated genetic analyses of enterococci are crucial for the development of a comprehensive understanding of enterococcal biology.
One essential genetic technique is the replacement of a wild-type chromosomal allele of a gene of interest with a cloned allele that has been manipulated in vitro (termed allelic exchange [24]; in some cases, it is also known as markerless genetic exchange because no antibiotic resistance marker is retained by the mutants [32]). This technique can be used to transfer various types of mutant alleles of a gene of interest, including, for example, in-frame deletions and point mutations, to the wild-type chromosomal locus. One advantage of allelic exchange is the placement of a mutant allele in its natural chromosomal context, an optimal situation for genetic analysis of the effects of the mutation. Furthermore, if markerless exchange is used, multiple mutations can be serially introduced into the same strain without the addition of distinct antibiotic resistance markers that might not be available or might otherwise interfere with subsequent genetic analysis. Allelic exchange is typically performed by using a two-step integration-segregation strategy, in which a nonreplicating plasmid harboring a cloned copy of the gene with the desired mutation is first integrated into the chromosome via Campbell-type homologous recombination with selection for a plasmid-encoded marker. After removal of the selective condition, the resulting merodiploid recombinants can resolve the duplication via a second recombination event between the duplicated chromosomal segments, yielding a recombinant carrying either the wild-type allele or the mutated allele, depending on the site of recombination. Because the desired recombinants have lost the plasmid backbone, no direct phenotypic selection is usually possible. Thus, identification of these recombinants can be a cumbersome process. To facilitate the identification of recombinants that have lost the integrated plasmid, a counterselectable marker is often incorporated into the plasmid backbone. This strategy permits selection, rather than laborious screening, to be used for isolation of recombinants that have resolved the merodiploid state (24).
A variety of counterselection strategies have been developed for genetic manipulation of prokaryotic organisms. These strategies include inhibition of growth in the presence of sucrose, mediated by the sacB gene product (35), and inhibition of growth in the presence of lipophilic chelators such as fusaric acid, mediated by gene products encoding tetracycline resistance (23). In both of these examples, the growth of clones carrying the respective gene products is inhibited on medium containing the appropriate counterselective agent. An analogous strategy has been developed using various genes involved in purine and pyrimidine base salvage pathways, such as the genes encoding phosphoribosyl transferases (PRTases) (3, 9, 10, 30, 32, 39). PRTases recycle free purine or pyrimidine bases by converting them to the corresponding nucleotide monophosphates, thereby sparing the cell the burden of synthesizing these molecules de novo. However, PRTases may also act on base analogs, creating unnatural nucleotides that can be toxic to the cell. Accordingly, PRTase-defective mutants are resistant to the toxic effects of such analogs. Ectopic integration of a plasmid-encoded functional copy of the PRTase into a PRTase mutant host cell, by recombination at a duplicated sequence, provides the basis for a counterselection strategy. Once such a strain has been constructed, selection for resistance to the appropriate base analog yields recombinants in which the integrated plasmid has been excised, with concomitant loss of the cloned PRTase. Two variations on this theme have recently been reported: the hypoxanthine PRTase (hpt) has been successfully exploited for markerless exchange in the archaeon Methanosarcina acetivorans (32), and the uracil PRTase (upp) has been similarly used in the gram-positive bacterium Bacillus subtilis (9).
Genetic analysis of enterococci has thus far been limited in scope due to the lack of advanced genetic tools. Although some basic tools have been developed for manipulation of E.faecalis, the effectiveness of currently available tools is limited. For example, Tn917 has been used for transposon mutagenesis of E. faecalis (45), but a recent study revealed that Tn917 insertions are highly biased near the region of the chromosomal replication terminus. Furthermore, such insertions were obtained in only ∼25% of all predicted genes (12). For directed mutagenesis of chromosomal genes in E. faecalis, a nonreplicating delivery plasmid, pTEX4577, has been used (33). However, this approach suffers from two drawbacks: (i) due to low frequencies of electroporation and recombination in E. faecalis, recombinants are difficult to obtain (zero to five recombinants per microgram of plasmid DNA), and (ii) recombinants generated by this approach result from single-crossover recombination of intact, circular plasmids, which creates polar mutations. Furthermore, the integrated plasmid can be prone to excision (and concomitant regeneration of the wild-type allele) under some experimental conditions. Another approach to performing allelic exchange in E. faecalis relies on the use of the temperature-sensitive replicon, pG+host, to deliver substrate DNA for recombination (1), but such pG+host-based approaches have met with limited success in our hands. A variation on this theme, originally developed for use in lactococci (21), can be used in E. faecalis, but this system relies on blue/white screening to identify the desired recombinants and thus lacks the benefits provided by a functional counterselectable marker, which has not been developed for E.faecalis.
To address this deficiency, we investigated the use of PRTases of E. faecalis as elements of a counterselection strategy. We describe here the development of a counterselectable markerless exchange system based on the upp-encoded uracil PRTase of E. faecalis. We used this system to introduce defined mutations into both the chromosome of E. faecalis and the enterococcal pheromone-responsive conjugative plasmid, pCF10. Analysis of one mutant constructed with this system, harboring an in-frame deletion of the chromosomally encoded srtA gene, suggests that the pCF10-encoded surface protein Asc10 is anchored to the enterococcal cell wall by the SrtA sortase. The ΔsrtA mutant secretes Asc10 into the culture supernatant and is impaired in Asc10-mediated aggregation. We also introduced a previously described mutant allele of Asc10 into the Asc10-encoding prgB locus of pCF10, enabling us to analyze the effect of the mutation when Asc10 is expressed in its natural plasmid-encoded context. This system of markerless exchange should be of general use for enterococcal researchers and may be adapted for use in other gram-positive organisms.
MATERIALS AND METHODS
Bacterial strains, growth media, and chemicals.
Bacterial strains used in the present study are listed in Table 1. Bacteria were stored at −80°C in Todd-Hewitt broth (THB; prepared according to the manufacturer's instructions) supplemented with 30% glycerol. Unless otherwise indicated, all culture media were purchased from Difco and all chemicals were purchased from Sigma (St. Louis, MO). Base analogs surveyed for the inhibition of E. faecalis included: 8-mercaptoguanosine, 2-aminopurine, 8-aza-2,6-diaminopurine, 2-mercaptopurine, and 5-fluorouracil. For routine use, 5-fluorouracil (5-FU) was prepared fresh prior to each experiment by dissolution in dimethyl sulfoxide (DMSO) at 75mg/ml and added to preautoclaved culture media at indicated concentrations. Additional culture media were as follows: Trypticase soy broth without dextrose (TSB), prepared according to the manufacturer's instructions without additional exogenous carbohydrate; M17 broth (M17), prepared according to the manufacturer's instructions without additional exogenous carbohydrate; brain heart infusion (BHI), prepared according to the manufacturer's instructions; M9YE medium (8), a semidefined M9-based medium supplemented with 0.3% yeast extract and 1% Casamino Acids (but prepared without MgSO4 or CaCl2). When required for selective growth of E. faecalis, erythromycin (Em) was used at 10μg/ml, tetracycline (Tc) at 10 μg/ml, and chloramphenicol (Cm) at 20 μg/ml. Other supplements, when required for E. faecalis, were X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) at 250 μg/ml, synthetic cCF10 pheromone peptide at 12.5 ng/ml (for induction of Asc10 expression from pCF10) unless otherwise noted, and nisin at 25 ng/ml (for induction of gene expression from pMSP3535 and its derivatives). When required for selective growth of Escherichia coli, Em was used in BHI at 100 μg/ml. Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). DNA sequencing was performed at the Advanced Genetic Analysis Center, University of Minnesota.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics or descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| OG1RF | Reference strain | 7, 14 |
| CK102 | OG1RF upp1::erm | This study |
| CK104 | OG1RF Δupp2 | This study |
| CK107 | CK104 ΔsrtA2 | This study |
| E. coli | ||
| DH5α | E. coli cloning host | |
| EC1000 | E. coli cloning host, provides RepA in trans | 21 |
| Plasmids | ||
| pFW11 | Cloning vector, source of promoter for P-upp; Spr | 31 |
| pFW14 | Cloning vector; Cmr | 31 |
| pFW15 | Cloning vector; Emr | 31 |
| pMSP3535 | Nisin-inducible expression vector; Emr | 4 |
| pVE6007 | Supplies RepA(ts); Cmr | 22 |
| pORI280 | Requires RepA in trans for replication; Emr | 21 |
| pAM401ts | Temperature-sensitive shuttle plasmid; Cmr | 46 |
| pCJK2 | P-upp expression cassette cloned into pORI280 | This study |
| pCJK8 | Δupp2 allele in pORI280 | This study |
| pCJK11 | upp::erm cassette in pAM401ts | This study |
| pCJK14 | ΔsrtA2 allele in pCJK2 | This study |
| pCJK15 | E. faecalis srtA cloned into pMSP3535 | This study |
| pCJK2-1 | prgBΩ1638 cloned into pCJK2 | This study |
| pCF10 | Pheromone-inducible conjugative plasmid | 6 |
| pCF10-1 | pCF10 carrying prgBΩ1638 | This study |
| pCWΩ1638 | pMSP3535 carrying prgBΩ1638 | 43 |
| pCWΩ3599 | pMSP3535 carrying prgBΩ3599 (lacks cell wall anchor) | 43 |
| pCJK21 | Spectinomycin resistance gene cloned into XbaI/XhoI sites of pMSP3535; Emr Spr | This study |
| pCJK22 | E. faecalis upp cloned into pCJK21 | This study |
Spr, spectinomycin resistance; Emr, erythromycin resistance; Cmr, chloramphenicol resistance.
Construction of plasmids.
Primers used in the present study are listed in Table 2. All plasmids derived from pORI280 were propagated in E. coli strain EC1000, which supplies RepA in trans to allow replication of these plasmids. All other plasmids were propagated in E. coli DH5α.
TABLE 2.
Primers used in this study
| Primer | Sequencea |
|---|---|
| upp_upF | GCTTTTGACGTCGCATTGGATCCAAGTTTTGCTG |
| upp_upR | AATCATCTCGAGTTTGTGTTGAATTAGCGGATGATC |
| upp_downF | TAGATGGCATGCTCGAGGAAGACGGCTATATCGTTCCTG |
| upp_downR | TTACTTACTAGTAGATCTGACAGCCACAAACAGGCGTTG |
| upp_upF_SphI | GCTTTTGCATGCGCATTGGATCCAAGTTTTGCTG |
| PfwUPP_F | ATGGGAAAATTTCAAGTAATTGATCATC |
| PfwUPP_NcoR | TTTTTTCCATGGATTCTTTTTCATAGCTCCTCCTTTG |
| Pfw_XbaF | TCGACGTCTAGATCCTCGAGCTCTAGATCTTAAGC |
| Pfw_R | GATGATCAATTACTTGAAATTTTCCCATATATATATCCTCCTCACTATTTTGATTAG |
| SrtA_upF | TTGGAATCTAGAAATAACACCTTCTTGCAAGATACCTTTC |
| SrtA_upR | TTTTTTCTGCAGTGGGCGCATATTTTCCCTCCTTTTAATG |
| SrtA_dnF | CAAAAACTGCAGGCCGATTGGGTGGCTTAATAAAAAAATC |
| SrtA_dnR | TCAGCACCCGGGAGCCACTGGGAAGTTGGCAGTCAG |
| 3535_SrtAF | AAATAGGCATGCCAAATAAATTGTGGTAAACTTGATACATT |
| 3535_SrtAR | TTTTGCTCTAGAGAAACAGCAAGAACGCCAAGTGATG |
| 3535_uppF | TTAGGAACTAGTGAATTCAACTTGTGTCCTAGGCTC |
| 3535_uppR | ACCGTTTCTAGACATAATCGGTTACCTAACGGTT |
| upp_oup | GTGGCCCATTGGAACATGTGATTGC |
| upp_odn | AGGATTTCGCCGTTGACACCCATG |
| srtA_oup | CTTGCTAAGTGAATCTTCCACCAAG |
| srtA_odn | CGTCAGAAAGTCCAACAAGCCGGT |
| Brbs | AACTGCAGATCGAGGAGAATGATACATGAATC |
| PrgB10466 rev | TTATTTTGTTTCTTTTCTACGTTTA |
Restriction sites used for cloning of PCR amplicons are indicated in boldface.
A plasmid used to replace E. faecalis upp with an Em resistance gene (pCJK11) was constructed by first amplifying fragments of OG1RF chromosomal DNA flanking the upp gene using PCR with primer pairs upp_upF/upp_upR (upstream fragment) and upp_downF/upp_downR (downstream fragment). The resulting amplicons contained 598 and 656 bp of E. faecalis DNA, respectively. These amplicons included flanking sequences and sequences encoding the first 15 residues and the last 20 residues of the upp open reading frame (ORF), respectively. The PCR amplicons were sequentially cloned into pFW15 (31) using the primer-encoded AatII/XhoI restriction sites for the upstream fragment and the primer-encoded SphI/SpeI restriction sites for the downstream fragment (Fig. 1). In the resulting construct, the upstream and downstream fragments flank the plasmid-encoded Em resistance gene, creating the upp::erm cassette. This cassette was removed on a SalI/SpeI fragment and cloned into similarly digested pAM401ts (46), creating pCJK11.
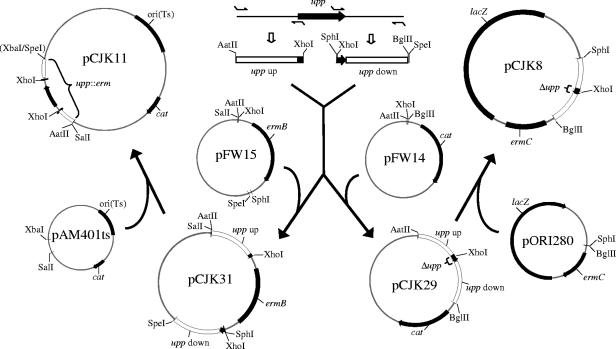
FIG. 1.
Construction of pCJK11 and pCJK8. From each side of E. faecalis upp, a chromosomal fragment encompassing a short segment of the end of upp and sequences flanking upp were amplified by PCR from OG1RF chromosomal DNA, yielding the “upp up” (upstream) and “upp down” (downstream) amplicons. These amplicons were sequentially cloned into pFW15 on opposite sides of the erm determinant (using primer-encoded restriction sites) to create the upp::erm cassette (left half of figure), which was subsequently subcloned into the temperature-sensitive replicon, pAM401ts, to create pCJK11. Thus, this plasmid encodes a upp allele in which 83% of the gene has been replaced with the erm determinant. The two PCR amplicons were also sequentially cloned into pFW14 such that they are adjacent to each other, thereby creating the Δupp allele (right half of figure; 83% of upp is deleted in this construct), which was subsequently amplified and subcloned into pORI280 to create pCJK8. Replication of pORI280-derived plasmids is dependent on the provision of the RepA replication initiation protein in trans. Thus, pCJK8 cannot replicate in E. faecalis. Note that the plasmids are not shown to scale.
A plasmid used to create an in-frame deletion of E. faecalis upp (pCJK8; Fig. 1) was constructed using the same upp-flanking PCR amplicons from above. As noted above, these amplicons contained sequences encoding several amino acids at the beginning and end of the upp ORF, which were retained in the deletion construct in an effort to avoid any unanticipated perturbations of the expression of adjacent genes. The two PCR amplicons were sequentially cloned into pFW14 (31) using the primer-encoded AatII/XhoI restriction sites for the upstream fragment and the primer-encoded XhoI/BglII restriction sites for the downstream fragment. In the resulting construct, the XhoI site forms the fusion point between the two flanking fragments and is in-frame with the remaining codons of the upp ORF, such that 2 XhoI-encoded amino acids are inserted into the 35-amino-acid product that is produced from the Δupp2 allele. More than 83% of the upp gene was eliminated in this construct. The Δupp2 allele was amplified by PCR from this plasmid using the primer pair upp_upF_SphI/ upp_downR, digested with SphI/BglII, and cloned into similarly digested pORI280, creating pCJK8.
The markerless exchange integration plasmid pCJK2 (Fig. 2) was constructed by first creating the P-upp expression cassette using overlap-extension PCR (15) in two steps. In the first step, primers PfwUPP_F and PfwUPP_NcoR were used to amplify upp from E. faecalis chromosomal DNA, and primers Pfw_XbaF and Pfw_R were used to amplify the constitutive promoter upstream of the spectinomycin resistance gene of pFW11 (31). In the second step, primers Pfw_XbaF and PfwUPP_NcoR were used to amplify the P-upp expression cassette using the combined amplicons from step 1 as a template. The resulting amplicon was digested with XbaI and NcoI and ligated into similarly digested pORI280. The integrity of the P-upp cassette in this plasmid was confirmed by nucleotide sequencing. An undesirable consequence of this cloning strategy was that most of the unique restriction sites in the multiple cloning site of pORI280 were eliminated. To address this problem, the P-upp expression cassette was excised by digestion with BglII and religated into similarly digested pORI280, creating pCJK2.
FIG. 2.
Construction of a counterselectable integration plasmid, pCJK2, and derivatives. A constitutive promoter (P) was amplified by PCR from pFW11 and fused to the E. faecalis upp ORF to create the P-upp cassette, using overlap-extension PCR. This cassette was cloned into pORI280, generating pCJK2. Unique restriction sites that can be used to clone target DNA into the integration plasmid are indicated. To construct a plasmid for deletion of E. faecalis srtA, a chromosomal fragment encompassing a short segment of the end of srtA and sequences flanking srtA were amplified by PCR from either side of srtA from OG1RF chromosomal DNA, yielding the “srtA up” (upstream) and “srtA down” (downstream) amplicons. These amplicons were sequentially cloned into pCJK2 such that they are adjacent to each other, thereby creating the ΔsrtA allele. To construct a plasmid for exchange of wild-type prgB on pCF10 with the prgB1638 allele, prgB1638 was excised from pCWΩ1638 and subcloned into pCJK2, creating pCJK2-1. All pORI280-derived plasmids cannot replicate in E. faecalis.
A plasmid used to create an in-frame deletion of E. faecalis srtA by markerless exchange (pCJK14; Fig. 2) was constructed by first amplifying fragments of OG1RF chromosomal DNA flanking the srtA gene using PCR with the primer pairs SrtA_upF/SrtA_upR (upstream fragment) and SrtA_dnF/SrtA_dnR (downstream fragment). The resulting amplicons contained 820 and 728 bp of E.faecalis DNA, respectively. The amplicons included flanking sequences and sequences encoding the first three residues and the last five residues of the srtA ORF, respectively, which were retained in the deletion construct in an effort to avoid any unanticipated effects on expression of adjacent genes. The two PCR amplicons were sequentially cloned into pCJK2 to create pCJK14, using the primer-encoded XbaI/PstI restriction sites for the upstream fragment and the primer-encoded PstI/XmaI restriction sites for the downstream fragment. In the final construct, the PstI site forms the fusion point between the two flanking fragments and is in-frame with the few remaining codons of the srtA ORF, such that two PstI-encoded amino acids are inserted into the eight-amino-acid product that is produced from the ΔsrtA2 allele. More than 96% of the srtA gene was eliminated in this construct.
A plasmid used to transfer prgBΩ1638 to pCF10 by markerless exchange (pCJK2-1; Fig. 2) was constructed by first excising the prgBΩ1638 allele from plasmid pCWΩ1638 (43) on a BsrGI/BlpI fragment. The ends were made blunt by treatment with T4 polymerase, and the resulting fragment was ligated to SmaI-digested pCJK2, yielding pCJK2-1. This plasmid carries an internal fragment of prgB containing the Ω1638 insertion flanked by ∼1.5 kb of prgB upstream of the insertion and ∼2.2 kb of prgB downstream of the insertion.
Plasmid pMSP3535 is an expression vector containing a nisin-inducible promoter (4). A spectinomycin resistance cassette was excised from plasmid pMSP3545S (4a) by digestion with XbaI and XhoI. This fragment was ligated to similarly digested pMSP3535, yielding pCJK21. To create an expression plasmid for complementation of the upp deletion in CK104, E. faecalis upp was amplified from OG1RF chromosomal DNA using primers 3535_uppF and 3535_uppR. The resulting amplicon was digested at the primer-encoded SpeI and XbaI sites, followed by ligation to similarly digested pCJK21, yielding pCJK22. To create an expression plasmid for complementation of the srtA deletion in CK107, E. faecalis srtA was amplified from OG1RF chromosomal DNA using primers 3535_SrtAF and 3535_SrtAR. The resulting amplicon was digested with SphI/XbaI and cloned into similarly digested pMSP3535, yielding pCJK15. Transformation of E. faecalis by pCJK11, pMSP3535, and pMSP3535 derivatives was carried out via electroporation as previously described (2).
Construction of a upp gene replacement mutant.
A mutant in which E. faecalis upp was replaced with an Em resistance gene (strain CK102) was constructed by using the temperature-sensitive plasmid pCJK11 (Fig. 1). OG1RF carrying pCJK11 was cultured overnight at 30°C in THB supplemented with Cm (for maintenance of the plasmid). Aliquots of the culture were plated on THB agar supplemented with Em at 42°C. Colonies that arose were initially replica plated to the same medium and regrown at 42°C before replica plating to THB supplemented with Cm at 42°C to identify isolates that retained the Em marker but had lost the Cm marker encoded in the plasmid backbone. Two such isolates were identified after screening approximately 6,000 colonies. Genomic DNA from these isolates was analyzed by using PCR (as described below) to verify the replacement of upp with the Em resistance gene.
Markerless genetic exchange.
Fig. 3 provides an overview of the recombination process. The transformation procedure of Law et al. (20) was used to introduce plasmids pCJK8, pCJK14, and pCJK2-1 into their respective E. faecalis host strains for integration by recombination. This procedure uses plasmid pVE6007 to transiently supply the temperature-sensitive RepA protein (which is required for replication of pORI280 derivatives) for a short period after electroporation, such that the integration plasmid may undergo a few rounds of replication. The temperature is then raised, inactivating RepA to prevent further replication of either pVE6007 or the pORI280-derived integration plasmid. A brief version of the transformation procedure follows: the appropriate host strain for integration of a particular plasmid (OG1RF for pCJK8, CK104 for pCJK14, or CK104/pCF10 for pCJK2-1) carrying pVE6007 was cultured overnight in THB supplemented with Cm at 30°C. The culture was diluted tenfold in the same medium and incubated at 30°C until the A600 reached 0.5, at which time the cells were harvested and made competent by using the lysozyme procedure as previously described (2). After electroporation with ∼1 μg of DNA, cells were incubated at 30°C in the presence of 0.5 μg of Em/ml for 1.5 h, followed by an additional 1.5 h in the presence of 10 μg of Em/ml. This procedure is designed to allow the induction of expression of Em resistance and to allow the integration plasmid to undergo a few rounds of replication, as described previously (20). The incubation temperature was increased to 37°C for 3 h to inactivate the RepA(ts) protein (supplied in trans from pVE6007) required for plasmid replication. Finally, the cells were plated on THB agar supplemented with Em and X-Gal at 37°C. Blue transformants, representing isolates in which the plasmid had been integrated by recombination, appeared within ∼24 h (typically zero to eight transformants per electroporation). For pCJK14 and pCJK2-1 integrants, replica plating to BHI agar supplemented with 1 mM 5-FU demonstrated that the integrants exhibited growth inhibition due to the presence of the P-upp cassette encoded in the backbone of the integrated plasmid.
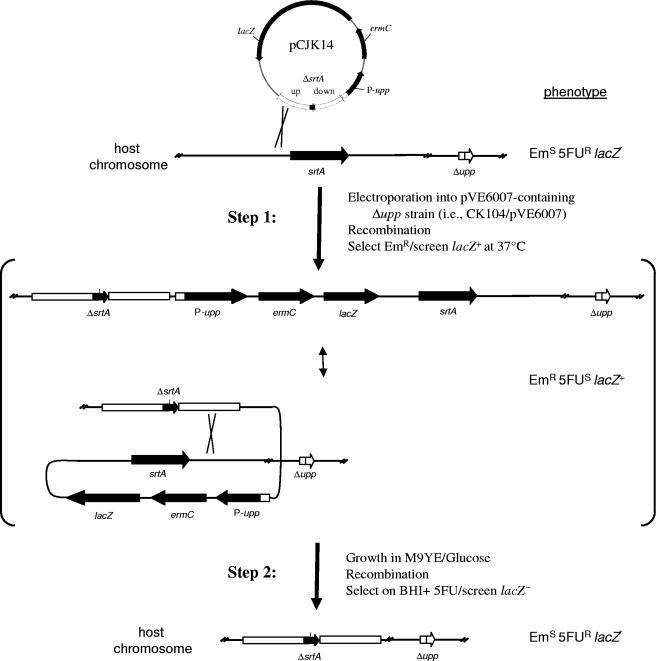
FIG. 3.
Markerless genetic exchange strategy used to create a deletion of srtA in E. faecalis. See Materials and Methods for a detailed description of the procedure. After electroporation of a Δupp host strain (i.e., CK104) carrying pVE6007 with pCJK14, cells were incubated at 30°C to allow for transient replication of the integration plasmid. The temperature was then raised to inactivate RepA(ts) (supplied by coresident pVE6007), preventing further replication of either the integration plasmid or pVE6007. A single recombination of pCJK14 occurred at a region of cloned chromosomal DNA (step 1), generating an Em-resistant recombinant that is sensitive to growth inhibition mediated by 5-FU due to constitutive expression of upp from the P-upp cassette. The srtA deletion is encoded by the pCJK14-borne allele. In step 2, nonselective growth in M9YE/Glucose allowed plasmid excision by recombination, leaving either the mutant allele (shown) or the wild-type allele on the chromosome. The excised plasmid cannot replicate and was lost by segregation. Recombinants from step 2 were selected by plating cells on BHI supplemented with 1 mM 5FU and screened to differentiate those carrying the wild-type srtA allele from those carrying the deletion. The phenotypes of the strains at each step are indicated on the right. Cloned chromosomal DNA fragments flanking srtA on the upstream (up) or downstream (down) sides are indicated as white rectangles juxtaposed to the ΔsrtA allele. An equivalent sequence of events can be drawn for plasmid integration by recombination at either the upstream or downstream fragment.
To isolate recombinants in which the integrated plasmid had been excised, integrants were cultured in M9YE supplemented with 0.5% glucose for approximately 20 generations at 37°C. A variety of growth media were investigated, including THB, BHI, M17, TSB, and M9YE/glucose, and growth in M9YE/glucose prior to plating on BHI-5-FU produced the best results. After the growth period in M9YE/glucose, serial dilutions of the cell suspension were plated on BHI agar supplemented with X-Gal and 1 mM 5-FU at 37°C. White colonies arose after ca. 16 to 20 h of incubation. Replica plating confirmed that all white, 5-FUr colonies were also sensitive to Em, as expected if the integrated plasmid had been excised and lost.
To identify recombinants carrying chromosomal deletions, white 5-FUr colonies were analyzed by using a colony PCR assay to determine whether they carried the wild-type or mutant allele at the target locus. Isolated colonies cultured on BHI agar plates supplemented with 1 mM 5-FU were introduced into PCRs as the source of template DNA. Reactions were incubated at 95°C for 10 min to disrupt the cells prior to thermal cycling, followed by standard thermal cycling for amplification with Taq polymerase. Primers for these amplifications annealed to flanking DNA sequences external to the duplicated (cloned) chromosomal regions. The primer pair used for analysis of the upp deletion was upp_oup/upp_odn, and that for analysis of the srtA deletion was srtA_oup/srtA_odn. After electrophoresis on agarose gels, the wild-type and deleted alleles could be differentiated on the basis of the size of the amplicon.
To identify recombinants carrying prgBΩ1638, white 5-FUr colonies were grown overnight in BHI supplemented with Tc. These cultures were diluted tenfold into BHI supplemented with 50 ng of synthetic cCF10 pheromone/ml (to induce synthesis of Asc10) and incubated at 37°C with shaking. After 1 to 2 h of incubation, visual inspection revealed the presence of cell aggregates in the aggregation-proficient cultures (20 isolates), whereas no such aggregates were visible for aggregation-defective cultures (12 isolates). Because the prgBΩ1638 insertion abolished aggregation when expressed with a heterologous system (43), nonaggregating recombinants were expected to harbor the prgBΩ1638 allele. To test this, the pCF10 prgB locus was amplified from one nonaggregating recombinant by PCR with the primers Brbs/PrgB10466 rev, and its nucleotide sequence was determined, confirming the presence of prgBΩ1638. No other mutations were found in the gene.
Immunoblot and aggregation analysis.
The surface extraction method of Galli et al. (11) was used with modifications. Bacterial strains were cultured overnight in THB (supplemented with Em when required for maintenance of pMSP3535 or derivatives) at 30°C. Cultures were diluted sixfold in THB, supplemented with cCF10 when indicated, and incubated at 30°C with occasional mixing for 2.3 h. Aggregation-proficient cells formed large aggregates that fell to the bottom of the culture tubes. For immunoblot analysis, EDTA was added to 2 mM with vortexing to disperse aggregates of E. faecalis cells, and the cell density was determined by measuring the A600 of the suspensions. Equivalent amounts of cells from each suspension were sedimented by centrifugation, and the culture supernatant was stored on ice until processed (see below). The cells were washed twice with ice-cold wash buffer (50 mM Tris, 25 mM EDTA) containing 1 M KCl, followed by one wash with ice-cold wash buffer (no KCl) and one wash with ice-cold protoplast buffer (wash buffer containing 23% sucrose). Cell pellets were resuspended in protoplast buffer containing lysozyme (10 mg/ml) and mutanolysin (250 U/ml) and incubated at 37°C for 30 min to hydrolyze the peptidoglycan layer. Protoplasts were sedimented by centrifugation and the resulting supernatant (constituting the cell-wall fraction) was retained. Sodium dodecyl sulfate loading buffer was added, and the samples were boiled for 5 min prior to electrophoresis. For preparation of the culture supernatant samples, culture supernatants remaining after initial sedimentation of bacteria (from above) were filtered with a 0.22-μm-pore-size filter to remove any remaining cells and subsequently precipitated with trichloroacetic acid (15%) on ice for 20 min. The resulting protein pellets were washed twice with acetone, resuspended in wash buffer, mixed with sodium dodecyl sulfate loading buffer, and boiled 5 min prior to electrophoresis. Immunoblotting was performed as described previously (44) with anti-Asc10 antiserum.
Determination of 5-FU MICs.
Strains to be tested were cultivated in M9YE supplemented with 0.5% glucose and, when required for plasmid maintenance, Em, at 37°C overnight, except for lactococci, which were cultivated in BHI at 30°C overnight. Serial dilutions of each cell suspension were made in phosphate-buffered saline, and ∼105 CFU were spotted onto BHI agar supplemented with various concentrations of 5-FU. Em and nisin were also included in the agar growth medium when required for plasmid maintenance and inducible expression of the upp gene. Growth was scored after 24 h at 37°C (30°C for lactococci), and the MIC was reported as the lowest 5-FU concentration at which no visible growth occurred.
Rapid evaluation of growth inhibition by 5-FU.
Strains to be tested were streaked from frozen stocks onto BHI agar and cultured overnight at 30°C. Individual colonies were selected and restreaked on BHI agar with or without supplementation by 1 mM 5-FU. Medium lacking 5-FU received an equivalent amount of DMSO (the solvent for 5-FU) as a control. The restreaked plates were incubated at 30°C for 16 h before photography with dark-field illumination. The organisms tested for growth inhibition included E. faecalis OG1RF, CK104, JH2 (17), V583 (29, 36), MMH594 (16, 38), and DS16 (41); Enterococcus faecium ATCC 51558 and ATCC 49224, as well as several isolates of E. faecium of various origins, obtained from the Minnesota Department of Health (MDH), including MDH 3-3051, MDH 3-1513, MDH 4-131, and MDH 3-1573; Enterococcus hirae ATCC 9790; Lactococcus lactis MG1363 (13) and LM2301 (42); and Staphylococcus aureus MN8 (37) and 8325-4 (28).
RESULTS
Deletion of upp in E. faecalis confers resistance to 5-FU.
Enterococci are relatively tolerant of many compounds that inhibit growth of other prokaryotic organisms. Consequently, it was unknown whether base analogs would be effective in a counterselection scheme for E. faecalis. To determine whether a PRTase could be used as the basis for a counterselection strategy, we surveyed a panel of base analogs for the ability to inhibit growth of E. faecalis when included in THB agar. Serial dilutions of an overnight culture of E. faecalis OG1RF were made in PBS, spotted onto THB agar plates containing the test compound, and incubated at 37°C. Of the compounds tested, only 5-FU was capable of inhibiting growth of OG1RF (not shown). The concentration of 5-FU required for growth inhibition of E. faecalis on agar plates was ∼100-fold higher than the previously reported concentration required for inhibition of B. subtilis (see Table 3) (9). Several independent strains of E. faecalis were also evaluated for growth inhibition by 5-FU, including the clinical isolates JH2 (17), V583 (29, 36), MMH594 (16, 38), and DS16 (41), and all were inhibited to a similar extent as OG1RF on BHI agar supplemented with 1mM 5-FU (not shown).
TABLE 3.
MIC determinations on BHI agar supplemented with various concentration of 5-FU
| Strain | 5-FU MIC (mM) |
|---|---|
| Enterococcus faecalis | |
| OG1RF/pCJK21 | 1 |
| CK104/pCJK21 | >8 |
| CK104/pCJK22 | 1 |
| Other species | |
| Enterococcus faecium 51558 | 0.1 |
| Enterococcus hirae 9790 | >8 |
| Staphylococcus aureus MN8 | 1 |
| Lactococcus lactis MG1363 | >8 |
Sensitivity of E. faecalis to 5-FU suggested that a upp-like PRTase might be acting on this base analog. The completely sequenced E. faecalis V583 genome (available at http://www.tigr.org) was searched by using the B. subtilis upp sequence (GenBank accession: NP_391570) as a query. A single high-scoring ORF was identified (E = 3.4 × 10−80), annotated in the TIGR database as EF2549, encoding a putative protein of 210 amino acids that is 70% identical to upp of B. subtilis. To determine whether EF2549 is an ortholog of B. subtilis upp that mediates sensitivity to 5-FU in E. faecalis, a mutant was generated in which the majority of EF2549 was replaced with an Em resistance gene (strain CK102), as described in Materials and Methods. To replace EF2549, the temperature-sensitive plasmid pCJK11 was constructed by cloning PCR amplicons encoding sequences that flank EF2549 on either side of an Em resistance determinant (see Fig. 1), creating a upp::erm cassette. This cassette was subcloned into the temperature-sensitive plasmid pAM401ts (46), and the resulting plasmid (pCJK11) was introduced into E. faecalis OG1RF at permissive temperature. Subsequent growth at nonpermissive temperature allowed the isolation of a recombinant in which the upp::erm cassette had been transferred to the chromosome. Initial results from an evaluation of this upp::erm mutant suggested that it was resistant to growth inhibition when streaked on BHI agar supplemented with 1 mM 5-FU (not shown). However, as noted above, one significant advantage of markerless exchange is the ability to create unmarked mutations in the chromosome. Therefore, for a more careful analysis of the growth phenotype and for potential use in a markerless exchange scheme, a mutant of OG1RF that carries an in-frame deletion of 83% of the EF2549 ORF (strain CK104) was constructed, as described in Materials and Methods. Briefly, the PCR amplicons from above (encoding segments of chromosomal DNA flanking EF2549, in addition to the first 15 or the last 20 codons of EF2549) were sequentially cloned into a plasmid unable to replicate in E. faecalis (pCJK8; Fig. 1), such that an in-frame deletion of most of EF2549 was created. This plasmid was introduced by electroporation into OG1RF, and single-crossover recombinants were selected as Em-resistant and blue colonies on THB agar containing X-Gal. Subsequent excision of the integrated plasmid by a second recombination event was expected to yield both wild-type and ΔEF2549 recombinants. Based on preliminary analysis of the CK102 growth phenotype, loss of EF2549 function was expected to result in resistance to 5-FU. Therefore, the pCJK8-integrant was cultivated in the absence of Em, and aliquots were plated on BHI agar containing 1 mM 5-FU. White colonies that arose were replica plated and found to be sensitive to Em, as expected if the integrated plasmid had been excised and lost. The genomic DNA of several white, 5-FUr, Ems colonies was analyzed by PCR to determine whether these isolates carried the in-frame deletion of EF2549, as desired. Three of three colonies tested in this way were confirmed to carry the EF2549 deletion.
The EF2549 deletion mutant exhibited a doubling time that was indistinguishable from OG1RF when cultured in BHI (not shown). To evaluate the effect of the EF2549 deletion on sensitivity to 5-FU, MICs were determined for cells of the mutant and its isogenic parent on BHI agar supplemented with various concentrations of 5-FU. Whereas the MIC for the parental strain under our conditions was 1 mM 5-FU, the EF2549 deletion mutant exhibited an MIC of >8 mM (Table 3), indicating that EF2549 mediates sensitivity to 5-FU. To confirm that the EF2549 deletion and not any other unknown mutation was responsible for the resistant phenotype, complementation analysis was performed by cloning EF2549 into a derivative of the nisin-inducible expression vector pMSP3535 to create pCJK22. Expression of EF2549 from pCJK22 in the deletion mutant restored sensitivity to 5-FU (Table 3), verifying that deletion of EF2549 confers resistance to 5-FU in E.faecalis. Coupled with sequence similarity, these results indicate that EF2549 is an ortholog of B. subtilis upp and, henceforth, EF2549 will be referred to as upp in accordance with that nomenclature. Furthermore, these results demonstrate that a counterselection strategy using 5-FU and the upp deletion mutant can provide the basis for a markerless genetic exchange system in E. faecalis.
The composition of the growth medium was found to be a critical determinant of the 5-FU resistant phenotype in E.faecalis. For example, when cultured on THB, M9YE, or TSB, the upp deletion mutant did not exhibit detectable 5-FU resistance. Only when cultured on BHI or M17 was the mutant clearly resistant to the toxic base analog. We did not investigate the underlying basis for these differences; however, it is well known that nutritional content of the growth medium affects 5-FU resistance of upp mutants of other species of bacteria. For example, upp mutants of both B. subtilis and E. coli are sensitized to 5-FU when supplied with purine ribonucleosides, because 5-FU can be converted to UMP by the sequential action of uridine phosphorylase and uridine kinase under these conditions (27). Thus, the medium-dependent phenotype observed with the E. faecalis upp mutant may be a consequence of differences in nutrient content of the various media tested. Although the basis for the phenotypic medium dependence of 5-FU resistance is unknown in E. faecalis, it carries important practical implications for the use of 5-FU in a counterselection scheme.
Construction of an integration plasmid for markerless exchange.
A previously described allelic exchange approach for lactic acid bacteria, the pORI system (20, 21), was chosen as the basis for construction of our markerless exchange system. The pORI system features a pWVO1-based plasmid (e.g., pORI280) that cannot replicate independently of the chromosome unless the host strain supplies the repA gene product in trans. Thus, if chromosomal DNA is cloned into a pORI plasmid (using a repA+ helper strain of E. coli for plasmid propagation) and the recombinant plasmid is introduced into a repA− host, recombination can occur at the duplicated host chromosomal sequence, leading to integration of the plasmid. These recombinants can be selected by using the antibiotic resistance marker present in the plasmid backbone. The plasmid backbone also carries a constitutively expressed lacZ gene, permitting colonies with an integrated plasmid to be easily identified by virtue of their blue color on growth media containing X-Gal. However, the pORI system lacks any counterselectable marker. Therefore, identification of the desired secondary recombinants that have excised the plasmid is laborious.
To modify the pORI system for use with our 5-FU counterselection strategy, a constitutively expressed allele of upp needed to be introduced into the pORI plasmid backbone. Because the promoter controlling the expression of E. faecalis upp in its natural chromosomal context was unknown, a constitutive promoter from plasmid pFW11 (31) (the promoter that drives expression of the plasmid-encoded spectinomycin resistance gene) was fused upstream of the E. faecalis upp ORF in a recombinant construct. An overlap-extension PCR strategy was used to create this synthetic P-upp cassette, which was subsequently cloned into pORI280 to create the integration plasmid pCJK2 (Fig. 2). Many of the unique restriction sites found in pORI280 remain in pCJK2 and can be used to clone target DNA into the integration plasmid. When such a recombinant plasmid is introduced into E. faecalis CK104, recombination will not occur at the upp locus (due to the upp deletion of the host) and therefore will only occur at the duplicated target sequence that has been cloned into the plasmid. These recombinants will be resistant to Em, blue on plates containing X-Gal, and sensitive to inhibition by 5-FU. To select for derivatives that have undergone a second recombination event excising the integrated plasmid (with the concomitant loss of the upp expression cassette), these recombinants are cultured and subsequently plated on BHI agar containing 5-FU.
Construction of a ΔsrtA mutant of E. faecalis.
To evaluate the effectiveness of the 5-FU counterselection strategy during markerless genetic exchange, we chose to make an in-frame deletion of the srtA gene, which is predicted to encode the major sortase of E. faecalis. Sortases are membrane-associated enzymes that act on a subset of extracellular proteins destined for attachment to the cell wall of gram-positive organisms (25). Sortases catalyze two reactions: (i) cleavage of the protein precursor at a conserved motif found near the C terminus and (ii) covalent cross-linking of these substrate proteins to cell wall precursor molecules. Subsequent incorporation of the modified cell wall precursors into mature peptidoglycan results in the covalent attachment of the sortase protein substrates into the cell wall. Comfort and Clubb recently used a bioinformatic approach to identify EF3056 of the completed E.faecalis genome as the probable srtA homolog (5).
To construct a mutant bearing an in-frame deletion of srtA, DNA segments flanking the srtA gene were first amplified by PCR. These amplicons also contained the codons for the first three and the last five residues of the srtA gene product, which were retained in the deletion construct in an effort to avoid any unanticipated effects on the expression of adjacent genes. The amplicons were sequentially cloned into the integration plasmid pCJK2, such that an in-frame deletion allele of srtA was created, yielding pCJK14 (Fig. 2). More than 96% of the 245-amino-acid ORF was deleted in this construct. This plasmid was introduced by electroporation into CK104 cells with selection for Em resistance, yielding blue colonies on medium containing X-Gal (see Materials and Methods for details and Fig. 3 for an overview of the procedure). To test for sensitivity to 5-FU, these pCJK14-integrants were replica plated onto BHI agar supplemented with 1 mM 5-FU. In contrast to the parental strain, the integrants exhibited a severe growth defect, indicating that the P-upp expression cassette was sufficient to restore 5-FU toxicity after single-copy integration into the chromosome. One recombinant was selected and grown for approximately 20 generations in the absence of Em in M9YE supplemented with 0.5% glucose. Serial dilutions of the resulting cell suspension were plated on BHI agar supplemented with 1 mM 5-FU and X-Gal at 37°C, yielding almost exclusively white colonies at a frequency of approximately 5.8 × 10−4. White colonies were replica plated and found to be sensitive to Em, as expected if the integrated plasmid had been excised from the chromosome. The srtA locus of 29 white isolates was evaluated by using a colony PCR method to determine whether these isolates carried wild-type srtA or the srtA deletion, revealing 21 recombinants that retained the wild-type srtA locus and 8 recombinants that carried the srtA deletion. One ΔsrtA recombinant (strain CK107) selected for further analysis was cultured in BHI and exhibited a doubling time indistinguishable from the parental strain CK104 (not shown).
The E. faecalis ΔsrtA mutant secretes Asc10 into the culture supernatant and is impaired at Asc10-mediated aggregation.
SrtA cleaves protein substrates at a conserved sequence motif (LPXTG) found near the C terminus of the precursor (25). Thus, by searching for such a motif in secreted proteins, it is possible to identify potential substrates for SrtA. The surface protein Asc10 (encoded by the prgB gene) of the E. faecalis conjugative plasmid pCF10 contains an LPKTG motif near its C terminus (19, 26), suggesting that SrtA is responsible for anchoring Asc10 to the cell wall. Surface-bound Asc10 (also known as aggregation substance) mediates the formation of visible aggregates of E. faecalis cells after induction of its synthesis by the peptide pheromone, cCF10. Waters and Dunny (43) found that a C-terminal deletion mutant of Asc10 lacking the coding sequence for the LPKTG anchor motif did not mediate aggregation and was secreted into the culture medium, indicating that covalent cell wall anchoring is required for Asc10 to successfully mediate aggregation. To test the hypothesis that SrtA is responsible for anchoring Asc10 to the cell wall, pCF10 was transferred to the ΔsrtA mutant by conjugation and Asc10 expression was induced, by the addition of cCF10, in the presence or absence of SrtA expression from a complementing plasmid-borne allele of srtA. Samples of cell-wall-bound protein were compared to culture supernatants by using immunoblot analysis with antiserum specific for Asc10. The results showed that cell-wall-bound wild-type Asc10 is observed only after induction with cCF10 (Fig. 4, lanes 1 and 3), and that Asc10 synthesis and cell-wall localization was not affected by the Δupp mutation (Fig. 4, compare lanes 3 and 4 with lanes 7 and 8). However, unlike for the wild-type strain, a substantial amount of wild-type Asc10 was found in the culture supernatant of the ΔsrtA mutant (Fig. 4, lane 10). These results suggest that SrtA is responsible for anchoring Asc10 to the cell wall and, in the absence of SrtA, Asc10 is secreted into the culture medium. Complementation of the ΔsrtA mutant with a plasmid-borne allele of srtA restored the wild-type phenotype, i.e., Asc10 could no longer be observed in the culture supernatant and was found exclusively in the cell wall fraction (Fig. 4, lanes 11 and 12), indicating that no mutation other than the srtA deletion was causing the phenotypic defect. Although a substantial amount of Asc10 was secreted into the culture supernatant of the ΔsrtA mutant, some Asc10 remained associated with the cell wall (Fig. 4, lane 9). This residual Asc10 might be covalently attached to the cell wall or, alternatively, the proline-rich domain near the C-terminal end of Asc10 might be embedded in the cell wall, resulting in cell wall retention of a fraction of the exported Asc10 without covalent linkage to the peptidoglycan. A mutant Asc10 that lacks the proline-rich domain and the LPKTG anchor motif (prgBΩ3599) was found exclusively in the culture supernatant under the conditions tested here (Fig. 4, lanes 13 and 14).
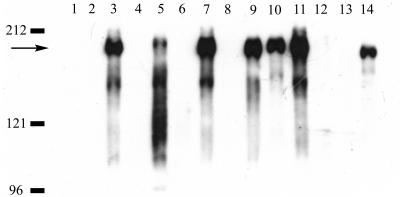
FIG. 4.
Immunoblot analysis of Asc10 localization. Bacterial cultures were grown in the presence of nisin as described in Materials and Methods and treated to prepare cell wall fractions (lanes 1, 3, 5, 7, 9, 11, and 13) and culture supernatant fractions (lanes 2, 4, 6, 8, 10, 12, and 14). Cell wall protein corresponding to 20 μl of bacterial culture (after adjusting to equivalent optical density) was loaded for the cell wall samples; supernatant protein corresponding to 10 μl of culture supernatant (after removal of bacteria) was loaded for culture supernatant samples. Samples were prepared from the following strains: lanes 1 and 2, OG1RF/pCF10+pMSP3535 (no cCF10); lanes 3 and 4, OG1RF/pCF10+pMSP3535 supplemented with cCF10 pheromone inducer; lanes 5 and 6, CK104/pCF10-1 supplemented with cCF10; lanes 7 and 8, CK104/pCF10+pMSP3535 supplemented with cCF10; lanes 9 and 10, CK107/pCF10+pMSP3535 supplemented with cCF10; lanes 11 and 12, CK107/pCF10+pCJK15 supplemented with cCF10; lanes 13 and 14, CK104/pCWΩ3599. Molecular mass standards (in kilodaltons) are indicated at the left. The arrow indicates the position of full-length Asc10.
Given that a significant amount of Asc10 was secreted into the culture medium of the ΔsrtA mutant, we hypothesized that this mutant strain would not be able to aggregate properly upon production of Asc10. To test this, Asc10 expression was induced with cCF10 in pCF10-containing cells in the presence or absence of SrtA expression from a complementing plasmid-borne allele of srtA. The cultures were monitored for aggregation, which is manifest by clearing of the culture medium as the aggregated cells fall to the bottom of the tube after a brief period of standing. Introduction of the Δupp mutation into the parental strain OG1RF did not affect the ability of pCF10-containing cells to aggregate (Fig. 5, tubes 2 and 3), but the subsequent deletion of srtA resulted in substantially impaired aggregation that could be rescued by complementation with a plasmid-borne allele of srtA (Fig. 5, tubes 4 and 5). Under some conditions, slight accumulation of the ΔsrtA mutant into small aggregates could be observed, but this was never sufficient to clear the culture medium. When the anchorless mutant prgBΩ3599 was expressed in CK104, no aggregates of any kind were detected (not shown), a finding consistent with the absence of prgBΩ3599 in the cell wall fraction (Fig. 4). Therefore, it seems likely that the residual low-level of aggregation observed after expression of Asc10 in the ΔsrtA mutant can be attributed to the Asc10 that remains associated with the cell wall in this mutant. Regardless, the results show that SrtA is required for efficient aggregation mediated by Asc10, presumably a reflection of the requirement for efficient anchoring of Asc10 to the cell wall.
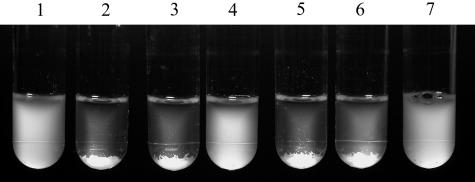
FIG. 5.
Asc10-mediated aggregation of E. faecalis cells. Bacterial cultures were grown in the presence of nisin as described in Materials and Methods, held stationary for ∼15 min, and photographed. Synthetic pheromone inducer was added at 25 ng/ml, where indicated. Tube 1, OG1RF/pCF10+pMSP3535 (no cCF10); tube 2, OG1RF/pCF10+pMSP3535 supplemented with cCF10; tube 3, CK104/pCF10+pMSP3535 supplemented with cCF10; tube 4, CK107/pCF10+pMSP3535 supplemented with cCF10; tube 5, CK107/pCF10+pCJK15 supplemented with cCF10; tube 6, CK104/pCF10 supplemented with cCF10; tube 7, CK104/pCF10-1 supplemented with cCF10.
Markerless exchange into pCF10.
Much effort has been devoted to study of the biology of pCF10, including the mechanism of pheromone-responsive conjugative transfer and the role of the plasmid-encoded virulence factor, Asc10, in the pathogenesis of enterococcal infections. Because pCF10 is a large plasmid (67 kb) that cannot replicate outside the enterococci, sophisticated manipulation of pCF10 genes in their natural plasmid-encoded context has not been achieved thus far. We hypothesized that our markerless exchange system could be used to address this deficiency.
With a heterologous expression system, Waters and Dunny (43) previously identified an in-frame insertion mutant allele of Asc10 that could not mediate aggregation despite being expressed on the cell surface (prgBΩ1638). Using our markerless exchange system, the wild-type allele of prgB on pCF10 was exchanged with the prgBΩ1638 allele, using a procedure analogous to that described above for the construction of the ΔsrtA mutant. Briefly, the prgBΩ1638 allele was removed from plasmid pCWΩ1638 (43) and cloned into the integration plasmid pCJK2 to create pCJK2-1 (Fig. 2), which was subsequently introduced into CK104 cells (carrying pCF10) by electroporation. Blue, Em-resistant colonies (pCJK2-1 integrants) were isolated, cultured in the absence of Em, and plated on BHI agar supplemented with 5-FU and X-Gal. Because the prgBΩ1638 allele was previously found to impair the ability to aggregate, isolated white colonies were initially screened using an aggregation assay to identify recombinants that could not aggregate in response to pheromone inducer, yielding 12 nonaggregating mutants of 32 white colonies tested. One representative nonaggregating isolate was chosen, and the nucleotide sequence of its prgB allele was determined, confirming the presence of the prgBΩ1638 insertion in pCF10. To verify that cell-wall-associated Asc10 was produced from this allele, cell-wall-bound protein was analyzed by immunoblot, as described above. The results showed that full-length prgBΩ1638 is produced and localized to the cell wall (Fig. 4, lanes 5 and 6). Compared to wild-type Asc10, there appears to be less full-length prgBΩ1638 protein and more degradation products present in the preparations, suggesting that the prgBΩ1638 gene product is somewhat less stable than the wild-type protein, as has been observed previously (43). Regardless, the prgBΩ1638 insertion completely abolishes aggregation when expressed from the natural prgB locus of pCF10 (Fig. 5, tube 7) despite the presence of full-length cell-wall-bound protein, a phenotype that is consistent with previous findings obtained using a heterologous expression system (43).
Other gram-positive cocci are inhibited by 5-FU.
A 5-FU counterselection strategy would be useful for the genetic manipulation of other gram-positive organisms. Even in organisms for which there currently exists a well-defined genetic system, the ability to transfer unmarked mutations to the chromosome would be advantageous. To evaluate the feasibility of a upp-based counterselection strategy in other gram-positive bacteria, GenBank was searched for homologs of E. faecalis upp using the BLAST algorithm at the National Center for Biotechnology Information. High-scoring homologs (≥70% amino acid sequence identity) of upp were found to be widely distributed among gram-positive bacteria, including various species of Streptococcus, Staphylococcus, Lactococcus, Lactobacillus, Listeria, Clostridium, and others, suggesting that a 5-FU counterselection approach was possible for these species. To evaluate the feasibility of this approach for other gram-positive bacteria, MICs for several species were determined on BHI agar supplemented with various concentrations of 5-FU. Susceptibility to 5-FU varied widely between species (see Table 3 for MICs): strains of E.faecium generally exhibited lower MICs than E. faecalis, strains of S. aureus exhibited MICs similar to E. faecalis, and E. hirae 9790 and strains of L.lactis exhibited significantly higher MICs. However, even though growth of the latter strains was not completely inhibited at any 5-FU concentration tested, a rapid test for 5-FU susceptibility revealed that 5-FU clearly impaired their growth at concentrations of 1 mM or higher (Fig. 6), suggesting that a counterselection strategy based on growth inhibition mediated by 5-FU might be possible if more inhibitory growth conditions could be identified. Thus, it seems likely that a counterselection scheme based on 5-FU-mediated growth inhibition can be used in a wide variety of gram-positive bacteria, although for certain species implementation of this strategy may require modification of the growth conditions to identify the optimal parameters for inhibition of the wild-type strain.
FIG. 6.
Other gram-positive bacteria are inhibited by 5-FU. A representative collection of gram-positive species was streaked on BHI agar supplemented with DMSO (included as a solvent control, top plate) or DMSO and 1 mM 5-FU (bottom plate) and then incubated at 30°C for 16 h. A full list of organisms evaluated for 5-FU sensitivity is found in Materials and Methods.
DISCUSSION
Sophisticated genetic analysis of the important opportunistic pathogen E. faecalis has been hindered by a lack of advanced genetic tools. In the present study, we describe the development of a system for markerless genetic exchange in E. faecalis that begins to address this deficiency. Markerless exchange is a broadly applicable technology, facilitating the analysis of a wide range of mutations, including insertions, deletions, and point mutations. A significant benefit of using markerless exchange is that mutations can be analyzed in the natural chromosomal context of the wild-type gene, an optimal situation for careful genetic studies. Furthermore, mutants carrying unmarked lesions can now easily be constructed using this technology. Because mutant strains do not retain an antibiotic resistance marker, an unlimited number of mutations can be sequentially combined in a single strain without encountering conflicts of multiple resistance markers or without disrupting the ability to subsequently introduce plasmids into the mutant (e.g., for complementation analysis). In addition, when mutating genes found in operons, markerless exchange technology allows the construction of in-frame deletions, an advantage that helps to avoid polar effects on expression of downstream genes.
Introduction of a counterselectable marker for E. faecalis makes markerless exchange feasible for routine use. The cumbersome and inefficient process of screening thousands of individual colonies to identify the rare recombinants in which the merodiploid state has been resolved is no longer necessary. Our markerless exchange system circumvents this task by providing a positive selection for the desired recombinants. Of course, mutants resistant to 5-FU can arise by other mechanisms, for example, by spontaneous point mutation of the upp ORF in the plasmid-encoded P-upp cassette, but such mutants can easily be eliminated from further consideration because they will remain resistant to Em and will yield blue colonies on media containing X-Gal. In practice we have found that such mutations occur at a substantially lower frequency than excision of the integrated plasmid by recombination and therefore do not present a problem for isolation of the desired recombinants.
We applied the 5-FU markerless exchange system to the construction of E. faecalis mutants deficient in pheromone-induced aggregation. We first created an in-frame deletion of the chromosomally encoded srtA gene, which is one of three genes found in the E. faecalis V583 genome that is predicted to encode a sortase-like gene product (5). Based on bioinformatic analysis, the srtA gene product was predicted to catalyze cross-linking of surface proteins containing an LPXTG motif to the enterococcal cell wall (5). Asc10, the pCF10-encoded surface protein that mediates aggregation, contains such a motif near its C terminus, leading to the hypothesis that SrtA was responsible for anchoring of Asc10 to the cell wall. Our results showed that the srtA gene product does indeed contribute to cell wall anchoring of Asc10, since the ΔsrtA mutant secretes Asc10 into the culture supernatant in substantial quantities (Fig. 4), whereas no Asc10 can be detected in the supernatant of the srtA+ parental strain. However, a portion of the Asc10 remained associated with the cell wall in the ΔsrtA mutant. One possible explanation for this observation is that one of the two remaining sortases identified in the E. faecalis genome (5) is also capable of cross-linking Asc10 to peptidoglycan precursors, although with reduced efficiency relative to SrtA. A second possibility is that the proline-rich domain near the C-terminal end of Asc10 remains embedded in the cell well after protein export and leads to the retention of a fraction of secreted Asc10 in the cell wall. In either case, because the ΔsrtA mutant exhibits a severe defect in pheromone-induced aggregation (Fig. 5), the fraction of Asc10 that remains associated with the cell wall of the ΔsrtA mutant is insufficient to mediate effective aggregation. This might be because the cell-wall-associated Asc10 is functionally defective or because the Asc10 observed in the cell wall fraction is actually only transiently associated with the cell wall as it passes through en route to the extracellular medium and is therefore unable to productively mediate cell-cell interactions. We note that, to a first approximation, the amount of Asc10 present in the cell wall fraction of the srtA mutant is similar to that in the cell wall fraction of its wild-type parent (Fig. 4). This result is surprising, given that Asc10 cannot be efficiently anchored to the cell wall in the srtA mutant. However, the amount of Asc10 found in the extracellular medium of the srtA mutant is roughly twice that found in the cell wall fraction (because the Asc10 in lane 10 of Fig. 4 is derived from half the culture equivalent used to prepare the cell wall fraction in lane 9), indicating that the total amount of Asc10 secreted across the cytoplasmic membrane by the srtA mutant is significantly greater than that secreted by the wild type. Thus, we speculate that a mechanism of feedback control exists to regulate the amount of production or secretion of Asc10 by wild-type E. faecalis and that this feedback control is impaired in the srtA mutant, resulting in overproduction or secretion of Asc10. According to this model, overproduction and secretion of Asc10 from the srtA mutant could result in incidentally higher levels of Asc10 in the cell wall fraction as it transits to the extracellular medium. In any case, the obvious differences in Asc10 accumulation in the extracellular medium of the srtA mutant and its wild-type parent clearly indicate a role for SrtA in anchoring of Asc10 to the cell wall.
Using an inducible shuttle-plasmid system for heterologous expression of Asc10, Waters and Dunny (43) previously identified an insertion mutant of Asc10 that could not mediate aggregation despite being expressed on the enterococcal surface (prgBΩ1638). We applied 5-FU markerless exchange to transfer this mutant allele of Asc10 into the Asc10-encoding prgB locus of pCF10, thereby replacing the wild-type prgB allele. Replacement of the wild-type prgB allele in pCF10 with prgBΩ1638 allowed the effect of the Asc10 mutation to be evaluated when expressed from its natural plasmid-encoded context. The mutant Asc10 was apparently more susceptible to proteolysis than wild-type Asc10, leading to a laddering pattern of degradation fragments after electrophoresis (Fig. 4). However, a portion of the mutant Asc10 existed in full-length form and was associated with the cell wall after pheromone induction (Fig. 4) but was completely unable to mediate aggregation (Fig. 5). These results are essentially identical to those of Waters and Dunny (43). Thus, these results confirm that the heterologous Asc10 expression system previously used to identify mutants of Asc10 accurately reflects Asc10 expression from its natural context in pCF10, at least for prgBΩ1638. The 5-FU markerless exchange system therefore allows genetic manipulation of large conjugative plasmids, such as pCF10, that cannot be easily modified by standard techniques of molecular cloning.
Our results suggest that a counterselection strategy based on upp-mediated sensitivity to 5-FU will be generally applicable to other gram-positive cocci. Homologs of upp were identified in the completed genome sequences of many gram-positive bacteria, and growth of several representative species was inhibited on BHI agar plates supplemented with 5-FU (Table 3 and Fig. 6). Some species were more sensitive to growth inhibition than others under the conditions tested. For example, multiple strains of E. faecium exhibited almost no detectable growth under the conditions tested, whereas strains of L. lactis exhibited impaired, but detectable, colony formation. For species that were not completely inhibited under the conditions tested, we speculate that a systematic survey of alternative growth conditions will be able to identify parameters under which growth of the wild-type is sufficiently inhibited to permit 5-FU counterselection. However, we have made no effort to identify such conditions for any species other than E. faecalis.
In summary, we have developed a generally applicable markerless genetic exchange system for use in E. faecalis and demonstrated its use by creating mutants in two independent replicons, the E. faecalis chromosome and the enterococcal conjugative plasmid pCF10. Introduction of a counterselectable marker based on upp-mediated sensitivity to the base analog 5-FU permits selection, rather than screening, to be used for identification of desired recombinants, thereby facilitating routine mutant construction. Consistent with that notion, we have recently used this system to generate numerous additional mutants of E. faecalis, carrying mutations in both chromosomal and pCF10 loci, by allelic exchange (unpublished data). These include some genetic knockouts that we were previously unable to construct using other methods. Thus, this markerless exchange system represents an important advance, enabling refined genetic analysis that is required to develop a thorough understanding of the biology of this important opportunistic pathogen.
Acknowledgments
We are grateful to Tim Leonard for assistance with photography. We thank Patrick Schlievert, Kristen Pederson, and Joanne Bartkus for providing strains.
This study was supported by award HL51987 from the NIH to G.M.D. C.J.K. was supported by training grant HD07381-12 and by NRSA fellowship F32-AI56684 from the NIH.
REFERENCES
- 1.Arbeloa, A., H. Segal, J. E. Hugonnet, N. Josseaume, L. Dubost, J. P. Brouard, L. Gutmann, D. Mengin-Lecreulx, and M. Arthur. 2004. Role of class A penicillin-binding proteins in PBP5-mediated beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 186:1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, T., B. Kozlowicz, and G. M. Dunny. 2002. Two targets in pCF10 DNA for PrgX binding: their role in production of Qa and prgX mRNA and in regulation of pheromone-inducible conjugation. J. Mol. Biol. 315:995-1007. [DOI] [PubMed] [Google Scholar]
- 3.Bitan-Banin, G., R. Ortenberg, and M. Mevarech. 2003. Development of a gene knockout system for the halophilic archaeon Haloferax volcanii by use of the pyrE gene. J. Bacteriol. 185:772-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan, E. M., T. Bae, M. Kleerebezem, and G. M. Dunny. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183-190. [DOI] [PubMed] [Google Scholar]
- 4a.Chandler, J. R., A. R. Flynn, E. M. Bryan, and G. M. Dunny. 2005. Specific control of endogenous cCF10 pheromone by a conserved domain of the pCF10-encoded regulatory protein PrgY in Enterococcus faecalis. J. Bacteriol. 187:4830-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comfort, D., and R. T. Clubb. 2004. A comparative genome analysis identifies distinct sorting pathways in gram-positive bacteria. Infect. Immun. 72:2710-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunny, G., C. Funk, and J. Adsit. 1981. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid 6:270-278. [DOI] [PubMed] [Google Scholar]
- 7.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunny, G. M., and D. B. Clewell. 1975. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J. Bacteriol. 124:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabret, C., S. D. Ehrlich, and P. Noirot. 2002. A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol. Microbiol. 46:25-36. [DOI] [PubMed] [Google Scholar]
- 10.Fukagawa, T., N. Hayward, J. Yang, C. Azzalin, D. Griffin, A. F. Stewart, and W. Brown. 1999. The chicken HPRT gene: a counter selectable marker for the DT40 cell line. Nucleic Acids Res. 27:1966-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galli, D., F. Lottspeich, and R. Wirth. 1990. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 4:895-904. [DOI] [PubMed] [Google Scholar]
- 12.Garsin, D. A., J. Urbach, J. C. Huguet-Tapia, J. E. Peters, and F. M. Ausubel. 2004. Construction of an Enterococcus faecalis Tn917-mediated-gene-disruption library offers insight into Tn917 insertion patterns. J. Bacteriol. 186:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold, O. G., H. V. Jordan, and J. van Houte. 1975. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch. Oral Biol. 20:473-477. [DOI] [PubMed] [Google Scholar]
- 15.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 16.Huycke, M. M., C. A. Spiegel, and M. S. Gilmore. 1991. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kak, V., and J. W. Chow. 2002. Acquired antibiotic resistances in enterococci, p. 355-383. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 19.Kao, S. M., S. B. Olmsted, A. S. Viksnins, J. C. Gallo, and G. M. Dunny. 1991. Molecular and genetic analysis of a region of plasmid pCF10 containing positive control genes and structural genes encoding surface proteins involved in pheromone-inducible conjugation in Enterococcus faecalis. J. Bacteriol. 173:7650-7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leenhouts, K., G. Buist, A. Bolhuis, A. ten Berge, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabeled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217-224. [DOI] [PubMed] [Google Scholar]
- 22.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloy, S. R., and W. D. Nunn. 1981. Selection for loss of tetracycline resistance by Escherichia coli. J. Bacteriol. 145:1110-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalyzed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama, J., R. E. Ruhfel, G. M. Dunny, A. Isogai, and A. Suzuki. 1994. The prgQ gene of the Enterococcus faecalis tetracycline resistance plasmid pCF10 encodes a peptide inhibitor, iCF10. J. Bacteriol. 176:7405-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhard, J. 1983. Utilization of preformed pyrimidine bases and nucleosides, p. 95-148. In A. Munch-Petersen (ed.), Metabolism of nucleotides, nucleosides, and nucleobases in microorganisms. Academic Press, Inc., New York, N.Y.
- 28.Novick, R. P., I. Edelman, and S. Lofdahl. 1986. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J. Mol. Biol. 192:209-220. [DOI] [PubMed] [Google Scholar]
- 29.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 30.Peck, R. F., S. Dassarma, and M. P. Krebs. 2000. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol. Microbiol. 35:667-676. [DOI] [PubMed] [Google Scholar]
- 31.Podbielski, A., B. Spellerberg, M. Woischnik, B. Pohl, and R. Lutticken. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137-147. [DOI] [PubMed] [Google Scholar]
- 32.Pritchett, M. A., J. K. Zhang, and W. W. Metcalf. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 70:1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin, X., K. V. Singh, Y. Xu, G. M. Weinstock, and B. E. Murray. 1998. Effect of disruption of a gene encoding an autolysin of Enterococcus faecalis OG1RF. Antimicrob. Agents Chemother. 42:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 35.Ried, J. L., and A. Collmer. 1987. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239-246. [DOI] [PubMed] [Google Scholar]
- 36.Sahm, D. F., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, J. Solliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33: 1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 38.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spring, K. J., J. S. Mattick, and R. H. Don. 1994. Escherichia coli gpt as a positive and negative selectable marker in embryonal stem cells. Biochim. Biophys. Acta 1218:158-162. [DOI] [PubMed] [Google Scholar]
- 40.Tannock, G. W., and G. Cook. 2002. Enterococci as members of the intestinal microflora of humans, p. 101-132. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 41.Tomich, P. K., F. Y. An, S. P. Damle, and D. B. Clewell. 1979. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob. Agents Chemother. 15:828-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh, P. M., and L. L. McKay. 1981. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J. Bacteriol. 146:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waters, C. M., and G. M. Dunny. 2001. Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance. J. Bacteriol. 183:5659-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waters, C. M., H. Hirt, J. K. McCormick, P. M. Schlievert, C. L. Wells, and G. M. Dunny. 2004. An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid. Mol. Microbiol. 52: 1159-1171. [DOI] [PubMed] [Google Scholar]
- 45.Weaver, K. E., and D. B. Clewell. 1988. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J. Bacteriol. 170:4343-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver, K. E., K. D. Walz, and M. S. Heine. 1998. Isolation of a derivative of Escherichia coli-Enterococcus faecalis shuttle vector pAM401 temperature sensitive for maintenance in E. faecalis and its use in evaluating the mechanism of pAD1 par-dependent plasmid stabilization. Plasmid 40:225-232. [DOI] [PubMed] [Google Scholar]