Abstract
Malaria is a complex infectious disease in which the host/parasite interaction is strongly influenced by host genetic factors. The consequences of plasmodial infections range from asymptomatic to severe complications like the neurological syndrome cerebral malaria induced by Plasmodium falciparum in humans and Plasmodium berghei ANKA in rodents. Mice infected with P. berghei ANKA show marked differences in disease manifestation and either die from experimental cerebral malaria (ECM) or from hemolytic anemia caused by hyperparasitemia (HP). A majority of laboratory mouse strains so far investigated are susceptible to ECM; however, a number of wild-derived inbred strains show resistance. To evaluate the genetic basis of this difference, we crossed a uniquely ECM-resistant, wild-derived inbred strain (WLA) with an ECM susceptible laboratory strain (C57BL/6J). All of the (WLA × C57BL/6J) F1 and 97% of the F2 progeny displayed ECM resistance similar to the WLA strain. To screen for loci contributing to ECM resistance, we analyzed a cohort of mice backcrossed to the C57BL/6J parental strain. A genome wide screening of this cohort provided significant linkage of ECM resistance to marker loci in two genetic regions on chromosome 1 (χ2 = 18.98, P = 1.3 × 10−5) and on chromosome 11 (χ2 = 16.51, P = 4.8 × 10−5), being designated Berr1 and Berr2, respectively. These data provide the first evidence of loci associated with resistance to murine cerebral malaria, which may have important implications for the search for genetic factors controlling cerebral malaria in humans.
Malaria is one of the most serious parasitic diseases of humans worldwide, being endemic in many tropical countries and claiming the life of over 2–3 million people every year (www.who.ch). Plasmodium falciparum is responsible for one of the most severe form of the disease, the neurological syndrome cerebral malaria (CM) (1, 2). CM is a complex pathological process influenced by multiple factors including the parasite genotype and the immune status and genetic background of the host (3–7).
In the search for host genetic factors controlling malaria infections, several mutations have been reported to have a protective effect against severe malaria, as observed in sickle cell anemia, Melanesian Ovalocytosis, glucose-6-phosphate dehydrogenase, and thalassaemias deficiencies (8, 9). Certain HLA haplotypes (5, 10) and an allelic variant of the tumor necrosis factor (11–13) have also been associated with disease severity. Despite some progress, better understanding of the genetics controlling host resistance to malaria is essential to disclose the complex host/parasite interactions.
Even if murine models do not reproduce exactly the human disease, they are of great importance in the study of malaria infections by allowing the control of several factors, such as environment stimuli and parasite diversity, that cannot be controlled in human studies (14, 15). Genetic analysis of such models may also be essential to identify candidate genes contributing to the control of the human disease. Thus, genetic analysis of susceptibility to infection by Plasmodium chabaudi in F2 and backcross cohorts has been used to map several loci associated with survival to infection on chromosome 9 (Char1) (16), control of peak parasitemia on chromosome 8 (Char2) (17), and control of parasite clearance on chromosome 17 (Char3) (18). A previously uncharacterized malaria susceptibility locus (Char4) on chromosome 3 controlling peak parasitemia was identified by using recombinant congenic mouse strains (19). Resistance to peak parasitemia and death induced by Plasmodium yoelii infection was recently mapped to a distal region on chromosome 9 (Pymr), overlapping with the Char1 region, probably representing one locus controlling susceptibility to rodent malaria (20).
Genetics of mouse malaria has mainly focused on resistance/susceptibility to parasitemia induced by the strains P. chabaudi and P. yoelii. To study the genetic factors controlling CM, an experimental model is provided by the lethal strain P. berghei ANKA. All inbred mouse strains tested so far are susceptible to the infection with this parasite strain, and either die from ECM (experimental CM) or with hemolytic anemia caused by hyperparasitemia (HP) (21–23). The fact that the same parasite can induce different disease manifestations in different mouse strains illustrates the importance of the genetic background of the host for the course of the infection. It is well established that C57BL/6J mice infected with P. berghei ANKA are highly susceptible to ECM. The mice die 5–12 days after parasite inoculation with severe neurological symptoms and moderate levels of parasitemia (10–20%). Relative resistance to ECM has been reported for some laboratory mouse strains, and more recently we reported that certain wild-derived inbred mouse strains display more pronounced resistance to ECM (21).
This phenotypic heterogeneity has recently been used to screen for genetic factors controlling resistance to experimental severe malaria, a phenotype based on the survival of mice infected with P. berghei ANKA. Thus, using a F2 generation from a cross between two laboratory strains, the susceptible C57BL/6J and a relatively resistant strain DBA/2, a locus on chromosome 18 was reported to be associated with resistance to experimental severe malaria (24).
To exploit the pronounced ECM resistance observed in wild-derived inbred mouse strains as well as the unique naturally occurring genetic variability they represent (25), we have used a cross between the susceptible strain C57BL/6J and the highly resistant, wild-derived inbred strain WLA to elucidate the genetic mechanism involved in the control of susceptibility/resistance to ECM induced by P. berghei ANKA clone 1.49L. We used 190 (WLA × C57BL/6J) F1 × C57BL/6J backcross mice and 132 microsatellite markers covering the genome, and found two resistance loci on chromosome 1 (Berr1) and chromosome 11 (Berr2).
Materials and Methods
Animals.
The C57BL/6J Rj mice were purchased from Elevage Janvier (Le Genest St Isle, France). The inbred strain WLA/Pas (Mus musculus domesticus) was obtained from a wild pair originally caught in France that was bred by brother–sister mating for over 60 generations at the Institut Pasteur. All of the mice used in this study were kept in conventional facilities at the Institut Pasteur.
Infection Protocol.
P. berghei ANKA clone 1.49L, kindly provided by D. Walliker (Institute of Genetics, Edinburgh, U.K.) was maintained by passage on C57BL/6J mice. This clone was selected for its ability to induce ECM (26). All of the mice in this study were infected with the same stabilate by i.p. injection of 106 parasitized red blood cells at 8 weeks of age. Parasitemia progression was determined using flow cytometry analysis (27). Neurological symptoms and death were recorded every day. ECM was diagnosed by clinical signs including ataxia, paralysis (mono, hemi, para, or tetraplegia), deviation of the head, convulsions, and coma, followed by death. Infected mice that died with neurological symptoms were classified as CM susceptible.
Genotyping.
Genomic DNA was prepared from mouse tails before infection using standard techniques. The 190 backcross mice were genotyped by using conventional PCR protocols for 131 microsatellite markers obtained from the Whitehead/MIT Center for Genome Research collection (www.genome.wi.mit.edu/cgi-bin/mouse/index). The TGFb2 primers (left primer 5′-CAGCAGTCTCAGATTGGAAGG-3′ and right primer 5′-ACATGGTGGGGATCACTCAT-3′) were synthesized in accordance with the GenBank database sequence (accession no. AF118263) and detect a polymorphism in the promoter region of the TGFb2 at the position 830 bp. All genotypes were detected by conventional protocols (28).
Statistical Analysis.
Genetic association of ECM resistance was analyzed by χ2 test on contingency tables. Evidence for significant linkage (1 degree of freedom) was considered at P < 0.0001.
Genetic interaction analysis was performed essentially as described by Cordell et al. (29). Briefly, the relative penetrances were calculated as ratio resistant/total animals for each genotype category. The mathematical models used for the additive, multiplicative and heterogeneity interaction are described by Cordell et al. (29). The models were fitted to the data by maximizing the model likelihood with the respect to the parameters to be estimated. The goodness of fit of each model was tested by the χ2 of twice the difference between the natural logarithms of the maximized likelihood and of the observed data likelihood.
Results
ECM is defined by the appearance of neurological signs, typically ataxia, paralyses, deviation of the head, and coma, with death occurring between day 5 and 12 after infection. Death by ECM occurs with low levels of parasitemia, not higher than 25% parasitized red blood cells in the strains of mice studied.
We have previously reported that the wild-derived inbred mouse strain WLA display a unique resistance to ECM contrasting to susceptible strains like C57BL/6J, but also to other relatively resistant strains like DBA/2 (21). After infection with P. berghei ANKA clone 1.49L, all WLA mice tested so far do not show any neurological symptoms but invariably die 11–25 days later (Fig. 1) with high levels of parasitized red blood cells reaching peaks of 50–90% (21). This phenotype defines the ECM resistance trait. All (WLA × C57BL/6J) F1 animals were found to be resistant to ECM similar to the WLA parental strain (Fig. 1) (21). In the F2 progeny, resistance to ECM was observed in 97% individuals. These data indicate that resistance to ECM is a dominant trait and is under polygenic control. Interestingly, a fraction (16%) of the ECM resistant F2 animals also displayed resistance to HP. This represents a previously undescribed phenotype in relation to P. berghei ANKA infections.
Figure 1.
Genetic resistance to ECM after infection with P. berghei ANKA clone 1.49L is a dominant trait. After infection, the mice that died between days 5 and 12 developed a neurological syndrome, and were designated ECM susceptible (ECMS). The remaining animals resist ECM but died at a later stage with HP and hemolytic anemia, being designated HP susceptible (HPS). In the F2 progeny, some individuals were found to resist to both ECM and HP. We tested 49 females and 15 males C57BL/6J, 11 females and 10 males WLA, 13 females and 18 males (WLA × C57BL/6J) F1, 99 females and 91 males (WLA × C57BL/6J) F1 × C57BL/6J and 79 females and 96 males (WLA × C57BL/6J) F2.
In this report we focused on the ECM phenotype to separate the genetic analysis of CM from other causes of death by malaria infection. To facilitate the identification of genetic factors controlling susceptibility/resistance to ECM, we chose to use a (WLA × C57BL/6J) F1 × C57BL/6J backcross for genetic mapping. In a cohort of 190 such mice, 96 (51.5%) were qualitatively scored as ECM susceptible when showing neurological symptoms typical of CM. All ECM susceptible mice died within 12 days after infection (Fig. 1). The remaining mice were scored as ECM resistant, and they succumbed with evident signs of anemia at a later stage (Fig. 1). We next performed a whole-genome scan in the 190 backcross mice using 132 microsatellite markers covering the genome with an average interval of 11 centimorgans (cM). The markers used were identified on the course of a polymorphism survey for WLA and 9 other wild-derived inbred mouse strains (25). Because no obvious gender difference was observed in the parental strains or in the backcross cohort studied here (Fig. 1), we included all individuals in this analysis independent of sex. The association of the phenotype with the genotype at each locus was evaluated by performing χ2 tests on contingency tables and taking a P value < 0.0001 as evidence for significant genetic linkage (30). Segregation analysis revealed that two chromosomal regions located on chromosomes 1 and 11 (Figs. 2 and 3) showed significant linkage to ECM resistance. An additional locus with suggestive linkage was also observed on chromosome 14. No evidence for linkage was found to any other chromosomal regions.
Figure 2.
Analysis of the association between ECM resistance and markers on chromosome 1. Markers shown were use to genotype 190 backcross mice. χ2 tests of association (1 degree of freedom) were performed for the indicate markers and the obtain χ2 and P values are shown. Marker orders and absolute positions along the chromosomes were based on the mouse genome database (www.jax.org) and are indicated (in cM).
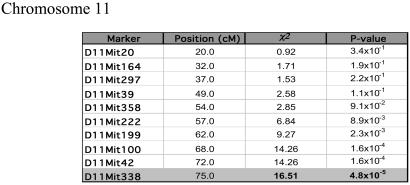
Figure 3.
Analysis of the association between ECM resistance and markers on chromosome 11. The results are present as described in Fig. 2.
On chromosome 1 significant linkage was observed over a 20 cM region between marker loci D1Mit203 and D1Mit462, reaching the highest significance over D1Mit221 (χ2 = 18.98; P = 1.3 × 10−5) (Fig. 2). Once the Tgfb2 (transforming growth factor β2) gene was found to map 0.5 cM centromeric apart from the D1Mit221 marker, we also genotyped the backcross progeny for this gene. The linkage observed for the Tgfb2 gene was equal to the marker with the highest significance value.
To our knowledge, no loci related to resistance to plasmodia infections have previously been identified in this region of chromosome 1. We have named this locus in the genetic control of malaria infection Berr1 (Berghei resistance locus 1).
Evidence for significant linkage on chromosome 11 spans a 7-cM region, reaching the maximum value at D11Mit338 (χ2 = 16.51; P = 4.8 × 10−5) (Fig. 3). Also in this case this represents a new locus controlling malaria infections being designated Berr2. Additional markers provided evidence for suggestive linkage of ECM resistance to a region on chromosome 14 where D14Mit113 had the highest association (χ2 = 10.19; P = 1.4 × 10−3). The WLA alleles at Berr1 and Berr2 are well over-represented among the animals resistant to ECM indicating that these alleles mediate ECM resistance at these loci (Tables 1 and 2) with an estimated relative penetrance of 0.65 and 0.64, respectively.
Table 1.
Contingency Table for D1Mit221
ECMR, experimental cerebral malaria resistance; ECMS, experimental cerebral malaria susceptibility.
h, heterozygous; b, C57BL/6J homozygous.
Table 2.
Contingency Table for D11Mi338
ECMR, experimental cerebral malaria resistance; ECMS, experimental cerebral malaria susceptibility.
h, heterozygous; b, C57BL/6J homozygous.
To investigate whether resistance to ECM in the backcross progeny was contributed by the joint effect of these loci, we preformed two-locus interaction analysis. The relative penetrance of the combined genotypes of markers D1MIT221 and D11MIT338 in the backcross progeny suggested that the WLA allele at both these loci have a joint effect in the penetrance of the resistance phenotype (Table 3). We modeled mathematically the joint effect of these loci on the linear, log odds of the penetrance, and on the liability scale using several genetic models of interaction (29). The likelihood of fitted models (additive, multiplicative, and heterogeneity) was then compared with the likelihood of the observed data. When scaling the ECM resistance phenotype for log odds or for liability, we rejected the additive, multiplicative, and heterogeneity genetic models because the likelihood of the fitted models was significantly different from the observed data (P < 0.05) (29). Modeling the joint effect of the Berr1 and Berr2 loci on the penetrance scale, however, did not rule out any of the fitted models of genetic interaction.
Table 3.
Relative penetrance of the ECM resistance phenotype given the combined genotypes at D1MIT221 and D11MIT338
h, heterozygous; b, C57BL/6J homozygous.
Relative penetrance was calculated as a ratio ECMR/total animals within each genotype category.
Discussion
Malaria is a severe infectious disease that is one of the most serious health problems worldwide. Because the interaction between the parasite and the host is extremely complicated, a better understanding of the genetics of host response stands to be essential. Some progress has been made both in the human and murine field to discovery loci encoding host resistance to malaria. However, the genetic component of malaria resistance/susceptibility is very complex, and additional approaches should be of use to disclose this problem.
Mouse genetic mapping mainly rely on the use of common inbred laboratory strains, which are genetically closely related (31). The limited degree of allelic variation, therefore, constitutes a restriction for genetic analysis. The use of wild derived inbred strains may overcome this because they constitute a repertoire of new and natural genetic variability and provide a larger spectrum of phenotypic differences that may not be found in conventional strains (25, 32, 33). Previous examples have proven the usefulness of wild-derived inbred strain in the search of loci contributing to complex genetic traits (34–36).
To exploit this approach and aiming to identify loci associated with resistance to ECM induced by P. berghei ANKA clone 1.49L infection, we used a cross between the susceptible strain C57BL/6J and the highly resistant, wild-derived, inbred strain WLA (21). We used this strategy, and found two new resistance loci, which has not been possible to identify using conventional inbred strains.
In the (WLA × C57BL/6J) F1 × C57BL/6J backcross mice, around 50% of the progeny were ECM resistant, both in males and females. By genotyping 190 backcross individuals with 132 microsatellite markers, the resistance loci were identified on chromosomes 1 (χ2 = 18.98, P = 1.3 × 10−5) and 11 (χ2 = 16.51, P = 4.8 × 10−5), and were designated Berr1 and Berr2, respectively. The WLA alleles at both these loci confer resistance to ECM, because it is over-represented among the resistant individual (in 67.0% for Berr1 and 64.9% for Berr2 in 94 ECM resistant mice). In fact, these results differ markedly from those of a study that used the DBA/2 laboratory strain and reported that a locus on chromosome 18 was found to confer resistance to death by a severe form of malaria induced by infection with P. berghei ANKA (24). Although is not clear whether this locus controls resistance to CM, the recent research on the genetics of murine malaria shows that the diversity of resistance loci reported by different laboratories may reflect the use of different parasite strains, mouse strains, and disease phenotypic manifestations.
To investigate the mode of interaction between Berr1 and Berr2, we used the statistical modeling of the joint effect of the two loci on the ECM resistance phenotype. Additive, multiplicative, and heterogeneity models of genetic interaction were fitted on the linear, log odds, and liability scales of penetrance (29). The results do not indicate a likely model for the interaction of Berr1 and Berr2, although they suggest that the joint action of these loci is best observed at the level of the raw penetrance scale. It is important to note that the use of a backcross progeny may limit the power to discriminate between models because the modeling is limited to four genotype categories and because the penetrance within each genotype category may be influenced by the genetic background variation (29). The poor segregation of the ECM phenotype in F2 generation, where nine combined-genotype categories are present, also suggests a strong interaction among the genetic factors conferring resistance to CM. In fact, the backcross results here presented predict that eight of the nine F2 genotype categories at Berr1 and Berr2 could confer resistance to ECM. The development of congenic strains will provide a tool both to verify the mapping results and to produce double-congenic mice that allow enhanced power to analyze genetic interactions.
The regions identified as containing genes that contribute to ECM resistance are of a size that makes it rather futile to speculate on potential candidate genes. In the case of the Berr1 locus, however, we note that the Tgfb2 (transforming growth factor β2) stands out because it was reported that levels of Tgfb are inversely correlated with the severity of malaria infections in mice infested either with P. chabaudi or P berghei (37–39). Furthermore, we observed that Tgfb2 maps in the region with the highest association to ECM resistance.
To further increase the resolution of the genetic mapping of Berr1 and Berr2, it will be necessary to establish congenic lines on the C57BL/6J background harboring the respective chromosomal segments from the WLA parental strain.
The detailed analysis of each of the loci identified here will allow further dissection of the phenotype, and will contribute to the disclosing of the biological mechanisms involved in the resistance to CM, and will be useful in the further analysis of the genetic basis of CM in humans.
Acknowledgments
We are very grateful to Isabelle Lanctin for breeding WLA/Pas strain and Danièle Voegtlé for technical assistance. We thank H. Cordell for help and calculation methods to the two loci interaction analysis. This work was conducted in the frame of the Centre National de la Recherche Scientifique Laboratoires Européens Associés “Génétique et dévelopement de la tolérance naturelle” and in part supported by the Instituto de Cooperación Científica y Tecnológica Internacional and French Embassy in Lisbon. It was supported by the “Program de recherche fondamentale en microbiologie, maladies infectieuses et parasitaires” from the French Ministry of Research and in part (S.B. and S.P.) by Institut National de la Santé et de la Recherche Médicale U511. The work at Instituto Gulbenkian de Ciência was supported by the Fundação para a Ciência e Tecnologia Grant 36392/99. S.C. is supported by a Fundação para a Ciência e Tecnologia fellowship. S.B. is a recipient of a fellowship from the Fondation pour la Recherche Medicale and from the Ministère de la Recherche. D.H., S.P., and P.-A.C. were recipients of Visiting Scientist Fellowships from the Fundação para a Ciência e Tecnologia (Portugal).
Abbreviations
- CM
cerebral malaria
- ECM
experimental CM
- HP
hyperpasitemia
- Chr
chromosome
- cM
centimorgans
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Turner G. Brain Pathol. 1997;7:569–582. doi: 10.1111/j.1750-3639.1997.tb01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh K, English M, Crawley J, Peshu N. Ann Tropical Med Parasitol. 1996;90:395–402. doi: 10.1080/00034983.1996.11813068. [DOI] [PubMed] [Google Scholar]
- 3.Day K P, Marsh K. Immunol Today. 1991;12:A68–71. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 4.Newbold C I, Craig A G, Kyes S, Berendt A R, Snow R N, Peshce N, Marsh K. Ann Tropical Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 5.Hill A V. Proc Assoc Am Physicians. 1999;111:272–277. doi: 10.1046/j.1525-1381.1999.99234.x. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert S C. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 7.Mazier D, Nitcheu J, Idrissa-Boubou M. Parasite Immunol. 2000;22:613–623. doi: 10.1046/j.1365-3024.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill A V. Parasitology. 1996;112:S75–S84. doi: 10.1017/s003118200007668x. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski D. Curr Opin Genet Dev. 2000;10:320–324. doi: 10.1016/s0959-437x(00)00087-3. [DOI] [PubMed] [Google Scholar]
- 10.Hill A V. Nature (London) 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 11.McGuire W, Knight J C, Hill A V, Allsopp C E, Greenwood B M, Kwiatkowski D. J Infect Dis. 1999;179:287–290. doi: 10.1086/314533. [DOI] [PubMed] [Google Scholar]
- 12.McGuire W, Hill A V, Allsopp C E, Greenwood B M, Kwiatkowski D. Nature (London) 1994;371:508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 13.Knight J C, Udalova I, Hill A V, Greenwood B M, Peshu N, Marsh K, Kwiatkowski D. Nat Genet. 1999;22:145–150. doi: 10.1038/9649. [DOI] [PubMed] [Google Scholar]
- 14.Medana I M, Chaudhri G, Chan-Ling T, Hunt N H. Immunol Cell Biol. 2001;79:101–120. doi: 10.1046/j.1440-1711.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Robinson A W. Parasitol Today. 1995;11:407–409. doi: 10.1016/0169-4758(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 16.Foote S J, Burt R A, Baldwin T M, Presente A, Roberts A W, Laural Y L, Lew A M, Marshall V M. Nat Genet. 1997;17:380–381. doi: 10.1038/ng1297-380. [DOI] [PubMed] [Google Scholar]
- 17.Fortin A, Belouchi A, Tam M F, Cardon L, Skamene E, Stevenson M M, Gros P. Nat Genet. 1997;17:382–383. doi: 10.1038/ng1297-382. [DOI] [PubMed] [Google Scholar]
- 18.Burt R A, Baldwin T M, Marshall V M, Foote S J. Immunogenetics. 1999;50:278–285. doi: 10.1007/s002510050603. [DOI] [PubMed] [Google Scholar]
- 19.Fortin A, Cardon L R, Tam M, Skamene E, Stevenson M M, Gros P. Proc Natl Acad Sci USA. 2001;98:10793–10798. doi: 10.1073/pnas.191288998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno T, Ishih A, Kohara Y, Yonekawa H, Terada M, Nishimura M. Immunogenetics. 2001;53:736–740. doi: 10.1007/s00251-001-0390-z. [DOI] [PubMed] [Google Scholar]
- 21.Bagot S, BouBou M I, Campmo S, Behrschmidt C, Gorgette O, Guénet J-L, Penha-Goncalves C, Mazier D, Pied S, Cazenave P-A. Infect Immun. 2002;70:2049–2056. doi: 10.1128/IAI.70.4.2049-2056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg J, Kendrick L P. J Parasitol. 1957;43:413–419. [PubMed] [Google Scholar]
- 23.Greenberg J, Nadel E M, Coatney G R. J Infect Dis. 1954;95:114–116. doi: 10.1093/infdis/95.1.114. [DOI] [PubMed] [Google Scholar]
- 24.Nagayasu E, Nagakura K, Akaki M, Tamiya G, Makino S, Nakano Y, Kimura M, Aikawa M. Infect Immun. 2002;70:512–516. doi: 10.1128/IAI.70.2.512-516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campino S, Behrsmidt C, Bagot S, Guénet J-L, Cazenave P-A, Holmberg D, Penha-Gonçalves C. Genomics. 2002;79:618–620. doi: 10.1006/geno.2002.6570. [DOI] [PubMed] [Google Scholar]
- 26.Amani V, Boubou M I, Pied S, Marussig M, Walliker D, Mazier D, Renia L. Infect Immun. 1998;66:4093–4099. doi: 10.1128/iai.66.9.4093-4099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janse C J, Van Vianen P H. Methods Cell Biol. 1994;42:295–318. doi: 10.1016/s0091-679x(08)61081-x. [DOI] [PubMed] [Google Scholar]
- 28.Penha-Goncalves C, Leijon K, Persson L, Holmberg D. Genomics. 1995;28:398–404. doi: 10.1006/geno.1995.1167. [DOI] [PubMed] [Google Scholar]
- 29.Cordell H J, Todd J A, Hill N J, Lord C J, Lyons P A, Peterson L B, Wicker L S, Clayton D G. Genetics. 2001;158:357–367. doi: 10.1093/genetics/158.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lander E, Kruglyak L. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 31.Beck J A, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig J T, Festing M F, Fisher E M. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 32.Bonhomme F, Guénet J-L. In: Genetic Variants and Strains of the Laboratory Mouse. Lyon M F, Rastan S, Brown S, editors. Oxford: Oxford Univ. Press; 1996. pp. 1577–1596. [Google Scholar]
- 33.Guenet J L. Pathol Biol. 1998;46:685–688. [PubMed] [Google Scholar]
- 34.Melanitou E, Joly F, Lathrop M, Boitard C, Avner P. Genome Res. 1998;8:608–620. doi: 10.1101/gr.8.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell M K, Mu J L, Higgins D C, Elango R, Whitmore H, Harris S, Paigen B. Mamm Genome. 2001;12:495–500. doi: 10.1007/s00335001-0006-9. [DOI] [PubMed] [Google Scholar]
- 36.Lyu M S, Koak C A. J Virol. 1996;70:830–833. doi: 10.1128/jvi.70.2.830-833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omer F M, Riley E M. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omer F M, Kurtzhals J A, Riley E M. Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 39.de Kossodo S, Grau G E. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]