Abstract
This study addresses how humic substance (HS) chemical composition and photoreactivity affect bacterial growth, respiration, and growth efficiency (BGE) in lake water. Aqueous solutions of HSs from diverse aquatic environments representing different dissolved organic matter sources (autochthonous and allochthonous) were exposed to artificial solar UV radiation. These solutions were added to lake water passed through a 0.7-μm-pore-size filter (containing grazer-free lake bacteria) followed by dark incubation for 5, 43, and 65 h. For the 5-h incubation, several irradiated HSs inhibited bacterial carbon production (BCP) and this inhibition was highly correlated with H2O2 photoproduction. The H2O2 decayed in the dark, and after 43 h, nearly all irradiated HSs enhanced BCP (average 39% increase relative to nonirradiated controls, standard error = 7.5%, n = 16). UV exposure of HSs also increased bacterial respiration (by ∼18%, standard error = 5%, n = 4), but less than BCP, resulting in an average increase in BGE of 32% (standard error = 10%, n = 4). Photoenhancement of BCP did not correlate to HS bulk properties (i.e., elemental and chemical composition). However, when the photoenhancement of BCP was normalized to absorbance, several trends with HS origin and extraction method emerged. Absorbance-normalized hydrophilic acid and humic acid samples showed greater enhancement of BCP than hydrophobic acid and fulvic acid samples. Furthermore, absorbance-normalized autochthonous samples showed ∼10-fold greater enhancement of BCP than allochthonous-dominated samples, indicating that the former are more efficient photoproducers of biological substrates.
Photochemical degradation of dissolved organic matter (DOM) can play an important role in carbon cycling in natural waters, either directly by the photochemical production of volatile carbon species or indirectly through the production of CO2 by sequential photochemical/biological oxidation (45). Approximately 10 to 50% of the dissolved organic carbon (DOC) in various water types can be photomineralized directly to CO2 and CO (10, 43, 48), and comparable percentages (as bioavailable photolysis products) can be taken up and remineralized to CO2 by microheterotrophs (44, 48). Previous studies have indicated that the humic fraction of DOM is mainly responsible for the absorbance of UV light and for the photoproduction of labile substrates that can be subsequently utilized by bacteria (12, 28, 38, 44, 57). The photochemical breakdown of humic substances (HSs) provides a source of substrates for bacteria by at least four pathways (37): (i) by increasing the bioavailability of molecules that are bound to humic substances (e.g., amino acids, carbohydrates, and aromatic compounds) (5, 33); (ii) through photolytic formation of low-molecular-weight (LMW) substrates such as organic acids, carbonyl compounds, and hydrocarbons (17, 18, 36, 46, 51, 62); (iii) by modifying the high-molecular-weight (HMW) fraction of humic substances rendering it more labile to microbial attack (28, 38, 57); and (iv) by increasing the pool of limiting inorganic nutrients (20).
Even though several studies have demonstrated an enhancement of microbial production after amending unfiltered water samples with irradiated filtered water, in other cases, inhibition was seen (8, 9, 13, 26, 34, 47). It has been suggested that these contrasting results were mainly due to the relative importance of photoproduction versus photodestruction of substrates in different waters containing different types of DOM (e.g., biologically labile DOM and older refractory DOM) (47, 48). However, the situation appears to be more complex (45). Factors such as the photoproduction of inhibitory substances like reactive oxygen species (26, 40, 53), photochemically induced enzyme deactivation (54), carbon limitation (27), and shifts in bacterial growth efficiency (BGE) in response to substrate addition (24, 63) may also be important but have not been addressed in many of these past studies (45).
While a number of studies support the supposition that photolysis of humic substances increases its bioavailability, it is still not known how the chemical composition and source of different humic substances affect their photoreactivity with respect to the production of substrates. Humic substances account for about 50 to 90% of the DOM pool (6). They originate from two main sources, in situ microbial production and terrestrial plants, and are usually referred to as autochthonous and allochthonous (terrestrial) humic substances, accordingly. The latter are prevalent in freshwaters, receiving their main organic input through soil leaching and surface runoff. Humic substances of terrestrial origin contain high levels of lignin and lignin degradation products, which are derived from the decomposition of vascular land plants and give terrestrial humic substances their pronounced aromatic character (41). In contrast, autochthonous humic substances are predominantly produced in situ from algal decay and bacterial activity. Autochthonous humic substances dominate the DOM pool in the oceans, eutrophic lakes, and lakes receiving limited terrestrial organic input and are highly aliphatic with lower aromatic character (3, 41, 42). Humic substance photoreactivities and optical properties are comparable to natural DOM at similar carbon and chromophore concentrations (35, 60), making them suitable surrogates for natural samples in photochemical experiments. In addition, their amenability to 13C liquid nuclear magnetic resonance spectroscopy (13C NMR) provides a level of chemical characterization (aromatic and other carbon group concentrations) presently unattainable for unconcentrated natural samples.
In this study, we determined how the photoreactivity and chemical nature of diverse aquatic humic substances added to lake water affected bacterial carbon utilization, as measured by bacterial carbon production (BCP), respiration, BGE, bacterial abundance, and bacterial production on a per cell basis. In order to make this comparison possible, a natural mixed bacterial community from a single lake water sample was used in all incubations.
MATERIALS AND METHODS
Chemicals.
All chemicals used in the experiments were from Merck, Germany (Pro-Analysis grade), except the chemicals used for the measurements of hydrogen peroxide (see below). Solutions were prepared using deionized water (Milli-Q water) from a four-bowl low-carbon system (Millipore Corp., Milford, MA). All glassware used to prepare stock solutions and for the experiments was previously acid (10% HCl) and Milli-Q rinsed.
Lake water sampling.
Approximately 20 liters of surface water (0 to 0.5 m) was collected in an acid-rinsed polyethylene container in April 1999 from Lake Fiolen, which is an oligotrophic lake situated in a boreal forest in southern Sweden (57°07′N, 14°34′E). Lake Fiolen has an average hydraulic residence time of 4 years, a Secchi depth of 5 to 6 m, and pH of 6.5 (30). At the time of sampling, Lake Fiolen had a DOC content of 5.7 mg C liter−1 and a water temperature of 18°C. After sampling, the water was vacuum filtered through a Gelman 142-mm attaching and effacing (A/E) filter (0.7 μm) in order to remove the bacterial grazers (9, 26). The filtered water was stored in an acid-rinsed borosilicate glass bottle (Schott Duran Glass) for 72 h in the dark at 15°C so that the bacterial community could recover. Just prior to incubation with irradiated or dark-treated humic substances, the bacterial abundance in the lake water was 7.35 × 105 cells ml−1 and no flagellates were observed as determined by counts using an epifluorescence microscope.
Description of humic substance samples and extraction procedures.
Sixteen different aquatic humic substances were isolated from diverse environments (Table 1). Samples were characterized by elemental 13C NMR and UV-visible analyses. Details of the 13C NMR and UV-visible characteristics of the humic isolates and their correlation to photochemical reactivity (including CO and H2O2 photoproduction) are presented elsewhere (K. Mopper, A. M. Anesio, W. Granéli, G. Aiken, and D. J. Kieber, unpublished data, Stubbins, A., K. Mopper, C. Law, R. C. Upstill-Goddard, G. Uhe, and G. Aiken, submitted for publication).
TABLE 1.
Elemental composition, absorbance coefficient at 300 nm, percentage of aromaticity and aliphatic content of humic substance isolates (ash-free values)a
| Humus sample (no.) | Sample | Sourceb | Type | %C | %H | %O | %N | A (m−1) | %Ar | %Al | DIC (μg liter−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Williams Lake | M | HPOA | 53.9 | 6.0 | 37.4 | 1.9 | 6.9 | 10.2 | 64.4 | 92 |
| 2 | Williams Lake | M | HPIA | ND | ND | ND | ND | 6.1 | 8.9 | 64.4 | 94 |
| 3 | Shingobee Inlet | M | HPOA | ND | ND | ND | ND | 17.6 | ND | ND | 123 |
| 4 | Shingobee Inlet | M | HPIA | ND | ND | ND | ND | 9.0 | ND | ND | 136 |
| 5 | IHSS Reference Suwannee River | AL | HA | 53.4 | 3.9 | 40.9 | 1.1 | 40.6 | 42.0 | 35.0 | 182 |
| 6 | IHSS Reference Suwannee River | AL | FA | 53.5 | 4.3 | 41.0 | 0.7 | 23.5 | 28.0 | 47.0 | 153 |
| 7 | Ogeechee River | AL | HA | 52.7 | 5.6 | 40.0 | 2.0 | 15.8 | 40.8 | 42.4 | 51 |
| 8 | Ogeechee River | AL | FA | 53.0 | 4.8 | 38.8 | 1.1 | 24.5 | 26.6 | 50.0 | 184 |
| 9 | Lake Fryxell | AU | HPOA | 54.9 | 5.5 | 34.9 | 3.3 | 10.0 | 15.2 | 65.3 | 98 |
| 10 | Pacific Ocean | AU | HPOA | 57.5 | 6.1 | 34.5 | 1.5 | 2.9 | 7.3 | 71.5 | 20 |
| 11 | Everglades F1 | M | HPOA | 51.9 | 4.3 | 40.3 | 1.8 | 22.8 | 25.4 | 44.3 | 141 |
| 12 | Everglades F1 | M | HPIA | 47.7 | 4.1 | 44.0 | 2.5 | 11.6 | 16.3 | 51.4 | 116 |
| 13 | Everglades 2BS | M | HPOA | 51.7 | 4.5 | 40.4 | 1.9 | 16.5 | 21.3 | 50.3 | 109 |
| 14 | Everglades 2BS | M | HPIA | 47.2 | 4.0 | 44.2 | 3.0 | 9.0 | 13.5 | 55.9 | 93 |
| 15 | Everglades U3 | M | HPOA | 54.7 | 4.8 | 37.5 | 1.9 | 20.8 | 24 | 47.5 | 143 |
| 16 | S10E Hillsborough Canal | M | HPOA | 54.5 | 4.9 | 37.4 | 1.9 | 21.9 | 27.5 | 50.6 | 147 |
DIC is the amount produced after 100 mg liter−1 of 0.2-μm-filtered HSs were exposed to artificial solar radiation for 24 h. %Ar, aromaticity; %Al, aliphatic content (%Al-I + %Al-II + %Al-III); ND, not determined.
AU is predominantly autochthonous, AL is predominantly allochthonous, and M is considered a mixed source.
HSs are operationally defined by their isolation procedures as hydrophilic organic acids (HPIA) and hydrophobic organic acids (HPOA) by using Amberlite XAD resins according to Aiken et al. (2). Briefly, a sample of natural water passed through a 0.2-μm-pore-size filter was acidified to pH 2 with HCl and passed sequentially through columns containing XAD-8 and XAD-4 resin. The HPOA, which contained both humic (HA) and fulvic (FA) acids, was retained on the XAD-8 column, while the HPIA fraction was retained on the XAD-4 resin. Each column was back eluted with 0.1 N NaOH. All samples were desalted using H+-saturated AG-MP 50 cation exchange resin, lyophilized, and stored in a desiccator. For some samples, the HPOA fraction was further separated into HMW HA and LMW FA fractions by acidifying the XAD-8 eluate to a pH of <1 with HCl. The HA precipitate was removed by centrifugation and lyophilized. The supernatant containing FA was then desalted, hydrogen saturated, lyophilized, and stored in a desiccator.
The HS isolates were distributed between three broad types of aquatic-dissolved organic matter (depending on the dominant source of the DOM) as autochthonous HS (in situ microbial sources, including algae and bacteria), allochthonous HS (higher plant and soil derived), and a mixture of both (Table 1). The predominantly allochthonous samples included the Suwannee River FA and HA reference materials (riverine FA and HA obtained from the International Humic Substances Society) and Ogeechee River FA and HA extracted from samples taken from the Ogeechee River at a site near Grange, Georgia (1). The predominantly autochthonous-derived samples included the Lake Fryxell HPOA sample, which was extracted from water from Lake Fryxell in the lower Taylor Valley (one of the McMurdo Dry Valleys) near the Ross Sea, Antarctica (3, 42), and the Pacific Ocean FA sample, which was extracted from water collected in the eastern equatorial Pacific Ocean near Hawaii from a depth of about 200 m (41). The other samples represent mixed sources, including Williams Lake, a seepage lake in Minnesota, the Shingobee River, Minnesota, and samples from the Florida Everglades. The organic matter in Williams Lake is primarily from autochthonous sources, with the addition of organic matter from groundwater that recharges the lake (4). The Shingobee River, part of the hydrologic flow path downstream of Williams Lake, contains organic matter from local wetlands and soils (4), in addition to organic matter derived from lakes, including Williams Lake. The Everglades samples are from a subtropical wetland environment containing peat soils, cattail and saw grass vegetation, and significant microbial activity. Samples from the Everglades displayed characteristics of both allochthonous-derived and autochthonous-derived humic substances. The Everglades samples were collected from various locations in the northern Everglades west of West Palm Beach. These included S10E Hillsborough Canal HPOA (26°27′32"N, 80°26′54"E); the U3 (26°17′15"N, 80°24′41"E) HPOA and F1 (26°21′35"N, 80°22′14"E) isolates extracted from water taken from sites located in Water Conservation Area 2A; and Everglades 2BS (26°09′00"N, 80°22′30"E) samples from Water Conservation Area 2B.
Humic substance irradiation.
Stock solutions of each humic substance sample (100 mg liter−1) in Milli-Q water were vacuum filtered through a 0.2-μm-pore-size VacuCap filter (Gelman Scientific). The filter-sterilized solutions were poured into quartz tubes (diameter, 22 mm; length, 145 mm; volume, 37 ml) which had been previously washed in dilute aqueous HCl (1:10), rinsed in Milli-Q water, and heat sterilized (140°C overnight) and stoppered without headspace. The quartz tubes were exposed to artificial UV radiation for 24 h at ambient room temperature. DOC changes were within the measuring error. Replicate samples were kept in the dark as controls. The UV radiation source consisted of a fan-cooled bank of eight fluorescent tubes (UVA-340; Q-Panel Co.) emitting UVB (∼2 W m−2), UVA (23 W m−2), and negligible photosynthetically available radiation (<5 W m−2), as determined using a model IL 1400A radiometer with broadband sensors (International Light Corp.). Using the same radiometer, measurements of solar UVB and UVA were 2.2 and 27 W m−2, respectively, at noon during clear summer conditions in Lund, Sweden. Thus, the UV intensity under the lamps closely simulated the natural UV solar spectrum. The light field at different locations where the samples were irradiated was found to be uniform in time and space, with a variation of less than ±5% at any one location in the field.
Experimental design for measuring bacterial response to irradiated humic additions.
The humic solutions (irradiated and dark controls) were added immediately after irradiation to lake water passed through a 0.7-μm-pore-size filter which was then decanted into triplicate Pyrex glass bottles (65 ml) with ground glass stoppers. In order to minimize dilution effects on the natural bacterial community, irradiated or nonirradiated humic solutions were added to only 10% of the total volume of the lake water, representing 8 to 10 mg added humic C liter−1 (final concentration). Triplicate samples of the lake water passed through a 0.7-μm-pore-size filter were also mixed with Milli-Q water (also 10% of total volume) as a control. Inorganic nutrients were added to all samples using aqueous solutions of KH2PO4 and NH4NO3 (added to a final concentration of 1 and 10 μM of P or N, respectively) to ensure that the bacteria were not limited by P and N. Humic-amended lake water samples and controls were incubated at the in situ lake water temperature (18 ± 0.1°C) in the dark.
Two different experiments were run in parallel. In the first experiment, we followed the bacterial abundance, carbon production, and carbon production per cell at 0, 5, 43, and 65 h in the lake water samples with added irradiated and dark-incubated humic substances of all 16 humic samples and in the lake water controls (with no added humic substances). In the second experiment, bacterial respiration rates were determined, in addition to bacterial abundance and production rates, in order to examine temporal changes in the bacterial growth efficiency in lake water amended with humic substances from the Shingobee Inlet, Pacific Ocean, and Everglades (i.e., samples 3, 10, 11, and 12) (Table 1). Separate sets of triplicate bacterial respiration samples were analyzed for each incubation time point (0, 43, and 65 h). Respiration was not determined for the 5-h samples because the method (i.e., dissolved inorganic carbon [DIC] production) was not sensitive enough to detect respiration over this short time frame. Bacterial abundance samples (6 ml) were collected for all time points and preserved in 2% borate-buffered formalin (pH ∼8) at 4°C (8). Hydrogen peroxide was determined in all samples after incubation for 0, 5, and 43 h. Hydrogen peroxide was not determined for the 65-h incubation samples, as it was below detection for the 43-h samples.
For both experiments, the results for the 65-h incubation were similar to the 43-h incubation; therefore, for clarity, results are presented in the figures and tables as the averages between 43 and 65 h.
Analytical methods.
Samples (∼6 ml) for DOC were taken from the tubes and transferred into acid-rinsed, precombusted (500°C overnight) glass vials with Teflon-lined screw caps. All samples were analyzed immediately after the conclusion of the experiments. DOC was analyzed by the Pt-catalyzed high-temperature combustion method using a Shimadzu TOC-5000 total carbon analyzer equipped with an ASI-5000 autosampler. Inorganic carbon was purged for 5 min from acidified samples (pH of ∼2, HCl). For each DOC analysis, at least three replicate injections were made, resulting in a coefficient of variation (CV) of less than 2%.
Hydrogen peroxide was quantified by the method outlined by Tranvik and Kokalj (58). The reagent was prepared by mixing 25 μl of N-acetyl-3,7-dihydroxyphenoxanine (1 mg in 1 ml of dimethyl sulfoxide), 1 ml of Milli-Q water, and 2 ml of horseradish peroxidase (Sigma type VI; 50 U ml−1 in a 0.25 M Tris buffer, pH 7.2). For each analysis, 30 μl of the reagent was added to 1 ml of lake water samples containing 10% of the irradiated or nonirradiated HS solution. After 2 min, the fluorescence was measured in a 1-cm cuvette by using a Shimadzu RF-1501 spectrofluorometer, with excitation at 570 nm and emission at 585 nm (10-nm bandwidth). Calibration was performed by multiple standard additions to the samples at each time point.
Quantitative 13C NMR data were obtained from 100 mg of HS samples dissolved in 1 ml H2O-D2O (1:1), adjusted to pH 7 in 10-mm tubes, and analyzed on a Varian model 300 spectrometer at 75.429 MHz using inverse gated decoupling with an 8-s delay (3). Quantification of the 13C NMR spectra was done by integrating the area under the bands (64). Elemental analyses of the humic samples were carried out by Huffman Laboratories (Golden, CO) according to the method of Huffman and Stuber (31).
The bacterial abundance was determined with a FACSort (Becton Dickinson) flow cytometer according to the method of Del Giorgio et al. (23). Cells were stained with SYTO 13 (Molecular Probes) at a final concentration of 2.5 μM. Fluoresbrite carboxylate microspheres (diameter, 1.58 μm) were used as a reference. BCP was measured by radiolabeled leucine incorporation as described by Smith and Azam (56). Aliquots of 1.7-ml duplicates were incubated with 50 nM l-leucine [8 nM 131 Ci mmol−1 (4,5-3H) l-leucine (Amersham) and 42 nM cold l-leucine]. Triplicate trichloroacetic acid (TCA)-killed controls were incubated for every humic substance type. After 60 min, incubations were stopped by adding 5% TCA by volume (except in the killed controls, for which TCA was added in the beginning of the incubations). All samples and controls were centrifuged at 16,000 × g for 10 min and aspirated. The remaining pellet was washed with 5% TCA and finally 80% ethanol. The ethanol was allowed to evaporate prior to the addition of 0.5 ml of scintillation cocktail (Ecoscint A). Counts were recorded using a Beckman LS 6500 scintillation counter. Bacterial protein production was calculated assuming a twofold isotopic dilution and converted to BCP by multiplying by a factor of 0.86 (55).
The bacterial respiration was determined by measuring the increase in DIC after the addition of irradiated or dark-treated humic substances to the lake water passed through a 0.7-μm-pore-size filter. The DIC was quantified with a Shimadzu TOC-5000 total carbon analyzer following the procedures of Granéli et al. (30). The water was pulled from the bottom of each tube via Teflon tubing directly into the carbon analyzer in order to avoid exchange of inorganic carbon between the sample and the atmosphere. At least three measurements were made per tube, resulting in a CV of less than 2% (30). We calculated the BGE as BCP/(BCP + BR), where BR is the bacterial respiration.
RESULTS AND DISCUSSION
Effects of H2O2 and initial HS bioavailability on bacterial carbon production.
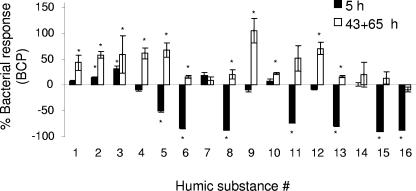
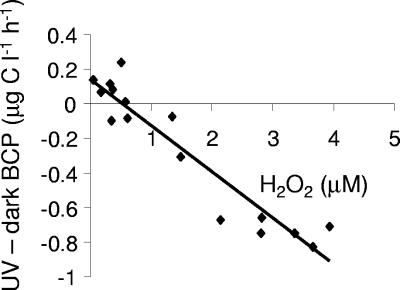
Irradiated HSs added to lake water had both short-term effects (i.e., 5-h incubation after addition of HSs) and long-term effects (i.e., average between 43- and 65-h incubations after addition of HSs) on BCP. The BCP was significantly lower (∼40 to 100%) in about half of the irradiated humic samples (samples 5, 6, 8, 11, 13, 15, and 16) (Fig. 1) 5 h after their addition to the lake water relative to the BCP observed in lake water with nonirradiated humic substance additions. The other samples showed either a slight enhancement or no effect on the BCP relative to the controls. As shown in Fig. 2, this suppression in BCP was highly correlated with the concentration of photochemically formed H2O2 that was initially present in the amended lake water samples (ca. 0.1 to 4 μM H2O2). Our study suggests that H2O2 concentrations of about 2 to 3 μM were inhibitory, which is in agreement with Farjalla et al. (26), who attributed inhibitory effects on microbial growth to 4 to 8 μM H2O2 formed during UV exposure of aquatic macrophyte leachates. At the same time, H2O2 inhibition levels noted in our study are higher by approximately 1 order of magnitude than those reported by Xenopoulos and Bird (65). They observed that the addition of only 0.1 μM hydrogen peroxide to humic lake water inhibited the BCP by as much as 40%. In contrast, Arana et al. (11) found that 2,000 to 3,000 μM hydrogen peroxide was needed to produce significant cell dormancy and loss of culturability in Escherichia coli and I. Obernosterer (personal communication) found that inhibition of bacterial production was not observed in lake cultures until mid-μM levels of H2O2 were present. The large differences that have been reported with respect to the threshold for inhibition are presumably due to differences in the sensitivities of different microorganisms or microbial communities to hydrogen peroxide. Given this large range (over 4 orders of magnitude), the potential negative impact of even low concentrations of H2O2 in these types of photochemical experiments may have important implications in bacterial community composition and should not be ignored in future studies. However, it should also be considered that the negative effect of humic irradiation on the BCP, although highly correlated to hydrogen peroxide, may be partly due to the photochemical production of other reactive oxygen species (53) as well as unknown, HS-derived inhibitory substances (29, 40, 58).
FIG. 1.
Bacterial carbon production response {i.e., [(BCPUV − BCPdark)/BCPdark] × 100} after 5 h and 43 to 65 h of the addition of irradiated or nonirradiated humic substances. Results are given as the means ± standard errors (number of samples = 4). *, statistically significant differences between the dark and UV treatments (t test, P < 0.05). See Table 1 for humic substance number.
FIG. 2.
Change in bacterial carbon production (i.e., percentage of increment in BCP from the addition of irradiated HS relative to nonirradiated control) 5 h after the humic samples were added to unfiltered lake water plotted as a function of the concentration of the hydrogen peroxide produced during humic substance irradiation (y = −0.27x + 0.14, r2 = 0.90).
In contrast to the negative responses in BCP observed in the 5-h incubation, after incubation for 43 to 65 h, BCP in treatments with irradiated HSs significantly surpassed BCP in dark-treated controls in 11 out of 16 cases (t test, P < 0.05) (Fig. 1). The enhancement in BCP observed in the irradiated humic samples ranged from 0 to 105% (average, 39%; standard deviation = 30%, n = 16) for the 43- to 65-h incubations. Hydrogen peroxide did not affect BCP rates for the longer incubations because H2O2 was not detected in the 43-h incubation of lake water with added irradiated humic substances. This loss of H2O2 during the bacterial incubations is consistent with the 1- to 8-h half-life of hydrogen peroxide observed in high-DOM environments (22, 32, 58). However, since observed differences in BCP in our study represent net differences, it is possible that some inhibition of BCP occurred even in the longer incubations due to the possible photoproduction of long-lived inhibitory substances, e.g., phenolic compounds (29, 40, 48, 58) and other effects (54). Sample-to-sample variability in the BCP may have also partly resulted from changes in the bacterial community composition during the 43-h incubation.
The magnitude of the increase in the BCP in the irradiated humic samples incubated for 43 to 65 h (relative to the nonirradiated controls) was affected not only by the initial hydrogen peroxide concentration but also by the initial bioavailability of the added HS. The initial bioavailability was estimated as BCP in lake water with nonirradiated HS minus BCP in lake water without the addition of HS (i.e., with the addition of Milli-Q water, 10% of final volume). The latter had a mean BCP of 1.81 ± 0.32 μg C liter−1 for the 43- to 65-h incubations in the dark. The addition of the nonirradiated humic substances increased BCP significantly relative to the addition of Milli-Q in only 6 of 16 cases for the 43- to 65-h incubations (t test, P < 0.05). The remaining 10 cases showed no significant change in BCP (t test, P > 0.05). Single linear regressions between the enhancement of BCP and either initial hydrogen peroxide concentration (r2 = 0.23) or initial bioavailability (r2 = 0.16) were not statistically significant (Pearson, P > 0.05, n = 16). However, together, the initial H2O2 concentration and initial bioavailability accounted for about 60% of the variance in the 43- to 65-h BCP response to the irradiation of humic substances, as revealed by multiple linear regression analysis (P < 0.01). This analysis showed that the degree of enhancement of BCP was lower for those samples for which either initial bioavailability of the humic substance or the initial hydrogen peroxide concentration was high.
Bacterial abundance, carbon production on a per cell basis, respiration, and BGE.
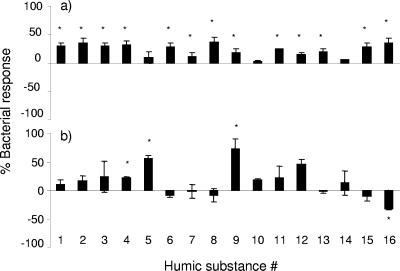
The observed enhancements of bacterial carbon production can be due to either an increase in bacterial abundance (cell number) or an increase in the rate of BCP on a per cell basis or both. Our results showed that BCP per cell was significantly enhanced in lake water with irradiated humic substances in only 4 of the 16 HS samples. On the other hand, the bacterial abundance increased in 13 of 16 samples for the 43- to 65-h incubations following irradiation (Fig. 3). In the five cases where the BCP did not increase in the light-treated HS samples (relative to controls), the bacterial response was variable. For example, the addition of irradiated HS no. 16 had no effect on BCP, but a positive effect on bacterial abundance and a negative effect on BCP on a per cell basis relative to the dark-treated control. These findings clearly illustrate that conclusions regarding the effect of DOM photodegradation on bacterial growth can depend strongly on the method used to estimate bacterial growth (9, 45).
FIG. 3.
(a) Bacterial abundance and (b) bacterial production response on a per cell basis {as percentages, i.e., [(BCPUV − BCPdark)/BCPdark] × 100}, after the addition of irradiated or nonirradiated humic substances to lake water. Results are given as means (43 to 65 h) ± standard errors (number of samples = 4). *, statistically significant differences between dark and UV treatments (t test, P < 0.05).
BGE, an indicator of the use of DOM for bacterial growth, can provide additional insights into photochemically enhanced BCP and into the total carbon flow through bacteria (24, 50). To determine the effect of DOM photolysis on BGE, we compared both BCP and respiration in lake water with added irradiated and nonirradiated HSs for four different HS samples. The DIC production due to respiration was linearly related to time for the four HSs examined (data not shown). Bacterial respiration and BCP were on average 18% and 71% (respectively) higher in lake water with irradiated humic substance than nonirradiated controls (Table 2). These enhancements yielded an average increase of 32% in the BGE for the four HS samples. If we had not measured the respiration and had simply assumed that it did not change during the incubation, an average increase in 45% in BGE would have been estimated. Thus, in agreement with Pullin et al. (50), the impact of photodegradation products on microbial activity cannot be estimated from changes in BCP alone, as has been often done in the past (45). Changes in both BCP and respiration (or BGE) must be considered, especially since the latter is usually a larger fraction of the total carbon taken up (Table 2) (24).
TABLE 2.
Effect of irradiation on bacterial production, respiration, BGE, and carbon utilization (i.e., BCP plus bacterial respiration) in lake water with irradiated and nonirradiated humus incubated for 43 h
| Humic isolate no. | Treatment | Avg ± SE value
|
|||
|---|---|---|---|---|---|
| Respiration (μg C liter−1 h−1) | BCP (μg C liter−1 h−1) | BGEa (%) | C utilizationa (μg C liter−1 h−1) | ||
| 3 | Dark | 7.3 ± 0.1b | 2.0 ± 0.4 | 20.6 ± 2.3b | 9.3 ± 0.3b |
| UV | 9.7 ± 0.4 | 3.5 ± 0.7 | 26.2 ± 1.8 | 13.3 ± 0.4 | |
| 10 | Dark | 8.8 ± 0.2b | 4.1 ± 0.3b | 31.8 ± 0.9 | 13.0 ± 0.2b |
| UV | 9.8 ± 0.1 | 5.1 ± 0.4 | 33.8 ± 1.1 | 14.9 ± 0.2 | |
| 11 | Dark | 8.0 ± 0.2b | 1.7 ± 0.2b | 17.6 ± 0.9b | 9.7 ± 0.1b |
| UV | 8.9 ± 0.3 | 3.3 ± 0.5 | 26.7 ± 1.0 | 12.2 ± 0.2 | |
| 12 | Dark | 7.4 ± 0.1b | 1.9 ± 0.2b | 20.1 ± 0.8b | 9.2 ± 0.1b |
| UV | 8.6 ± 0.1 | 3.6 ± 0.7 | 28.4 ± 2.0 | 12.2 ± 0.5 | |
| Avg | Dark | 7.9 ± 0.4c | 2.4 ± 0.6c | 22.6 ± 3.2c | 10.3 ± 0.9c |
| UV | 9.3 ± 0.3 | 3.8 ± 0.4 | 28.8 ± 1.7 | 13.1 ± 0.7 | |
| Avg enhancement (% relative to dark) | 18 ± 5c | 71 ± 16c | 32 ± 10c | 29 ± 6c | |
BGE and carbon utilization are averages of all possible combinations between the independent measurements of respiration and production (i.e., n = 3 for bacterial production and respiration and n = 9 for BGE and carbon utilization). The average percent enhancement was calculated as [(irradiated−dark)/dark] × 100 ± standard error (n = 4). The numerical identification of the humic isolates is given in Table 1.
Indicates statistically significant differences between dark and UV treatments (t test for BCP and respiration and U test for BGE and carbon utilization, P < 0.05).
Indicates statically significant differences in average values and enhancement (paired t test, P < 0.05).
DOM photoproducts are more oxidized than dark-treated controls (50, 66), and these more oxidized products appear to be incorporated less efficiently by bacteria (24, 48), which should lead to a decrease in BGE. However, we observed a statistically significant increase in the BGE after irradiation in three out of four samples (U test, P < 0.05) (Table 2). The effect of photochemically transformed DOM on BGE is complex due to a number of competing processes including photoproduction of inhibitory substances (see above), DOM mineralization, substrate formation versus destruction, shifts in the microbial population, and changes in the nutritional value of DOM by photooxidation (45). For example, Farjalla et al. (26) exposed sterile aquatic macrophyte leachates that contained high concentrations of readily utilizable LMW substrates (e.g., carboxylic acids) to UV radiation. They found that BGE in the irradiated leachates was significantly lower than in nonirradiated controls, indicating that the more oxidized photoproducts were less efficiently utilized by bacteria, causing a decrease in BGE. In contrast, DOM with low concentrations of utilizable LMW organic substrates, e.g., the humic substances used in our study or humic-rich marsh waters, which have low nutritive value (50), will be more efficiently utilized by bacteria after irradiation (i.e., BGE increases). In support of these trends, Anesio et al. (9) found a significant, negative correlation between BGE in dark-treated incubations and BGE after UV exposure for different leachates from aquatic macrophytes. Tranvik and Bertilsson (59) observed similar contrasting effects in bacterial production in a study of 32 lakes. Thus, we attribute the enhancement in BGE upon extended irradiation of our HS isolates (Table 2) to an overall increase in the nutritional value of the DOM pool.
Impact of HS and properties on BCP.
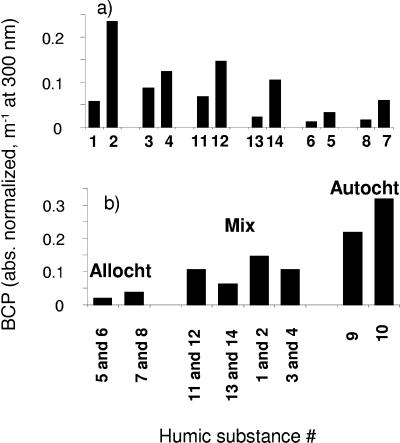
In order to determine if HS and properties (i.e., UV absorbance and chemical) affected the observed photochemical enhancements of BCP observed at 43 to 65 h (Fig. 1), principle component and linear correlation analyses were performed. Surprisingly, factors including humic substance absorbance (at 300 nm), absorbance bleaching (at 300 nm), and aromatic or aliphatic carbon content (from 13C NMR) were poorly correlated (i.e., r2 = 0.04 to <0.4) with the enhanced BCP measured after addition of irradiated HSs to lake water (43 to 65 h of incubation). However, when photochemically enhanced BCP was normalized to (i.e., divided by) the initial absorbance coefficient (m−1) at 300 nm of the lake sample containing added HSs, several trends emerged with respect to HS origin (allochthonous, autochthonous, or mixed) and humic substance isolation fraction (HPOA, HPIA, FA, and HA isolates) (see Materials and Methods for details regarding the different humic substance classifications). The basis for normalizing BCP to the absorbance coefficient (as opposed to carbon content; discussed below) is that numerous studies have shown that UV absorbance is strongly correlated with DOM photoreactivity (for examples, see references 46, 49, and 60), which, in turn, controls the photoproduction of microbially available substrates (37) and consequently, photochemically enhanced BCP (45). Thus, normalizing the latter to the UV absorbance coefficient allows us to estimate and compare the efficiency of HSs to photoproduce microbially utilizable substrates.
Comparing the different HS fractions on an absorbance coefficient-normalized basis (at 300 nm), photolyzed HPIA (hydrophilic acids) samples stimulated BCP ∼2 to 5 times more than the corresponding HPOA samples in all pairs, while absorbance coefficient-normalized humic acids were three- to sixfold more stimulating than the corresponding fulvic acids samples (Fig. 4a). The results that we observed at 300 nm were also observed at other UV wavelengths (i.e., 250 to 350 nm) (data not shown). These results imply that the hydrophilic acid and humic acid fractions of DOM are more efficient at producing substrates (i.e., more photochemically productive or reactive per unit absorbance) than the hydrophobic acid and fulvic acid fractions. This difference in photoreactivity is in agreement with Bertilsson and Bergh (15), who found that HPIA extracts had higher quantum yields for the photoproduction of LMW organic acids (i.e., microbially available substrates) and for the photodestruction of DOC than the corresponding HPOA extracts. Our results are also in general agreement with those of Bano et al. (12), who found that irradiation of the non-XAD-8-retained component (presumably mainly hydrophilic acids) of marsh water had a significantly greater stimulatory effect on BCP than the XAD-8-retained component (i.e., hydrophobic acids).
FIG. 4.
(a) Comparison of absorbance-normalized BCP (i.e., increment in BCP from the addition of irradiated HS relative to nonirradiated control, divided by the absorbance coefficient at 300 nm) after the 43-h incubation for different humic substance extraction types. (b) Comparison of the average absorbance-coefficient-normalized BCP for the 43-h incubation, among the different HS sample sources [i.e., (HPOA + HPIA)/2 and (HA + FA)/2]. Abbreviations: Allocht (predominantly allochthonous), Mix (mixed source), Autocht (predominantly autochthonous). See Table 1 for more details about the HSs.
Comparing the different sources of the HSs, the general order of BCP stimulation (absorbance coefficient normalized) was autochthonous > mixed > allochthonous as shown in Fig. 4b, which compares average BCP enhancement signals [i.e., (HPOA + HPIA)/2 and (HA + FA)/2, for the different HS sample types]. From these plots, it is apparent that absorbance coefficient-normalized autochthonous (i.e., microbial-derived)] HSs are significantly more efficient at producing biologically labile substrates (i.e., more photochemically reactive per unit of absorbance) than the allochthonous-dominated HSs. The above trends in HS source and type were further supported by principle component analyses (with and without Varimax rotation), with principle component 1 accounting for over 70% of the variance in the absorbance (or photobleaching or percent aromaticity)-normalized data.
In contrast to the UV absorbance-coefficient-normalized results, when our photochemically enhanced BCP results are instead normalized to carbon content, no statistically significant trends are observed (data not shown). It should be pointed out that only a fraction of the total carbon is responsible for the absorption of UV radiation (19, 25) and that it is this fraction that controls the photoproduction of microbially available substrates from DOM. Thus, normalizing the latter to UV absorbance provides a means for evaluating the efficiency of HSs (or DOM) for photoproducing utilizable substrate, which cannot be obtained by normalizing to total carbon.
The apparent greater photochemical reactivity of the absorbance-normalized hydrophilic and humic acid samples and the absorbance-normalized autochthonous-dominated HS samples may be related to their chemical composition, in particular, aromaticity. The percentage of aromatic carbon (from 13C NMR) for the 16 HS samples was found to be strongly correlated with initial absorbance between 250 and 350 nm (r2 = 0.91 to 0.94) (A. Stubbins, K. Mopper, C. Law, R. C. Upstill-Goddard, G. Uhe, and G. Aiken, submitted). In addition, when aromaticity was normalized to the UV absorbance coefficient, most HPOA and FA samples had lower aromatic carbon contents (per unit absorbance) than the corresponding HPIA and HA samples, while the allochthonous-dominated (i.e., higher plant derived) HS samples exhibited lower aromatic content per unit of absorbance than the autochthonous-derived (i.e., microbially/algally derived) HS samples (Table 1). Since the photochemical reactivity and photodegradation of a sample appears to be mainly dependent on its aromaticity (45, 52, 66; A. Stubbins, K. Mopper, C. Law, R. C. Upstill-Goddard, G. Uhe, and G. Aiken, submitted), these results may explain why, on a per unit absorbance basis, the HPIA, HA, and autochthonous-derived HS samples are more photochemically reactive (i.e., more efficient at stimulating BCP) than the corresponding HPOA, FA, and allochthonous HS counterparts. Furthermore, fulvic acids are thought to be diagenetically more altered, i.e., of lower molecular weight and more oxidized (i.e., more carboxylated) than humic acids (14, 39), which may render them less available for uptake and less productive of biologically labile substrates during photodegradation (7). However, it should be noted that in many freshwaters, allochthonous HSs are quantitatively more important than autochthonous HSs, and therefore, most of photoreactions and production of bacterial substrates upon irradiation of freshwaters are still probably due to the allochthonous fraction.
Summary and conclusions.
Past studies have shown that photodegradation of DOM can be a source of biologically available substrates, especially in carbon-limited surface waters. This photoproduction of substrates can result in enhanced microbial activity and accelerated carbon cycling in natural waters. Since HSs are usually the main UV-absorbing component in the DOM pool, HS photodegradation has often been assumed to be mainly responsible for this photoproduction.
Some long-lived photoproducts that are formed during UV irradiation of DOM can stimulate bacterial growth. In past studies, this stimulatory effect was usually measured by a reinoculation approach, whereby filtered, sterilized DOM or DOM isolates (e.g., HSs) were exposed to radiation and then reinoculated with a natural bacterial community (e.g., 16, 17, 21, 28, 38). However, short-term effects of DOM phototransformations on the natural bacterial community cannot be properly evaluated by this approach because bacteria reach their stationary phase on a time scale of days. The experimental approach used in this study (i.e., adding the irradiated or nonirradiated humic substances to lake water with minimum dilution and containing a mature microbial community rather than reinoculate irradiated, filtered lake water with a natural bacterial community) allowed us to evaluate the short-term effects of irradiated HSs on bacterial growth. Using this approach, we found that the HS phototransformations had a negative effect on bacterial growth for a number of HS samples in a short-term incubation. Furthermore, this negative effect was positively correlated to the production of hydrogen peroxide. The experimental approach used in this study indicated that the production of inhibitory substances during HS irradiation can strongly impact microbially mediated processes in lakes and should be considered in future studies, as those substances can be transported in the water column and affect bacteria negatively even in layers below the influence of direct solar radiation (32). Future studies should examine the nature, stability, and relative importance of photochemically produced short- and long-lived inhibitory substances, including H2O2 and phenolic compounds. These studies should also address how duration and UV intensity affect the production of inhibitory substances, as well as determine the temporal response (and recovery) of the bacterial community, including shifts in community structure induced by these substances.
In long-term incubations, irradiation of aquatic humic substances from a variety of environments resulted in increased carbon mineralization (i.e., organic carbon converted into inorganic carbon), both directly through abiotic photooxidation (Table 1) and indirectly through enhanced bacterial respiration (Table 2). The total enhancement of bacterial carbon utilization (BCP plus respiration) due to UV irradiation was about 30% relative to nonirradiated samples after the 43-h incubation (Table 2). This increase was proportionally higher for BCP than respiration, which suggests that more bacterial biomass is available for transfer through the food web as a result of the UV-induced degradation of humic substances. The increase in both BCP and bacterial respiration resulted in an enhancement of BGE (average, ∼32%) (Table 2). It is not known whether this photochemically induced change in BGE was due to shifts in community structure or to changes in the types of substrates available for uptake or both; however, it is clear that photodegradation of humic substances can be an important component in carbon cycling in aquatic ecosystems, particularly in carbon-limited and humic rich systems. Thus, the impact of photodegradation products on bacterial activity cannot be estimated from changes in the BCP alone, as has been often done in the past. Changes in both BCP and respiration or BGE are needed, especially since respiration usually represents a much larger fraction of the carbon taken up, and, depending on the magnitude of the respiration change, can even cause a decrease in BGE (45).
Although irradiation of aquatic humic substances generally enhanced BCP and respiration relative to nonirradiated controls, the rates of enhancement were poorly correlated to chemical and physical properties of the humic substances. However, when BCP rates were normalized to the initial absorbance of the samples, irradiated hydrophilic acid and humic acid samples were found to enhance BCP to a greater degree than the corresponding hydrophobic acid and fulvic acid samples. Likewise, absorbance coefficient-normalized microbially derived HS samples enhanced BCP to a greater degree than terrestrially dominated samples. These enhancements were presumably due to the higher photoproduction of substrates by those samples, which are probably related to their higher aromaticity, as evidenced by their higher UV absorbance (61). We conclude from these results that there are distinct differences in photochemical HS reactivity related to the source and chemical nature of the HS extracts, in particular, their aromatic content, which appears to control the production of microbially utilizable substrates during DOM photolysis.
Acknowledgments
Financial support to A.M.A. was given by the Brazilian Research Council (CNPq) and by the Swedish Foundation for International Cooperation in Research and Higher Education (STINT). Financial support for this study was provided in part by grants from the U.S. National Science Foundation Division of Ocean Sciences (0096426, 0196220, 0241946, and 0327446 to K.M. and 0096413 to D.J.K.), from a Hanse Institute for Advanced Study Fellowship (to K.M.), and from a grant from the Swedish Natural Science Research Council (B-AA/BU 04969-321 to W.G.). A grant (to W.G.) from the Nils G. Sträng Foundation supported K.M. while in Sweden.
Lars-Olof Björn kindly helped with the measurements of spectral irradiance of the lamps. We also thank three anonymous reviewers for constructive comments.
REFERENCES
- 1.Aiken, G. R., and R. Malcolm. 1987. Molecular weight of aquatic fulvic acids by vapor pressure osmometry. Geochim. Cosmochim. Acta 51:2177-2184. [Google Scholar]
- 2.Aiken, G. R., D. McKnight, K. Thorn, and E. Thurman. 1992. Isolation of hydrophilic organic acids from water using non-ionic macroporous resins. Org. Geochem. 18:567-573. [Google Scholar]
- 3.Aiken, G. R., D. McKnight, R. Harnish, and R. Wershaw. 1996. Geochemistry of aquatic humic substances in the Lake Fryxell Basin, Antarctica. Biogeochemistry 34:157-188. [Google Scholar]
- 4.Aiken, G. R., and D. McKnight. 1997. The influence of hydrological factors on the nature of organic matter in the Williams and Shingobee lake systems, p. 71-76. In T. C. Winter (ed.), Interdisciplinary research initiative: hydrological and biogeochemical research in the Shingobee River headwaters area, north-central Minnesota. Document 96-4215. U.S. Geological Survey Water Supply, Denver, Colo.
- 5.Amador, J. A., M. Alexander, and R. G. Zika. 1991. Degradation of aromatic compounds bound to humic acid by the combined action of sunlight and microorganisms. Environ. Toxicol. Chem. 10:475-482. [Google Scholar]
- 6.Amador, J. A., P. J. Milne, C. A. Moore, and R. G. Zika. 1990. Extraction of chromophoric humic substances from seawater. Mar. Chem. 29:1-17. [Google Scholar]
- 7.Amon, R. M. W., and R. Benner. 1996. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41:41-51. [Google Scholar]
- 8.Anesio, A. M., C. M. T. Denward, L. J. Tranvik, and W. Granéli. 1999. Decreased bacterial growth on vascular plant detritus due to photochemical modification. Aquat. Microb. Ecol. 17:159-165. [Google Scholar]
- 9.Anesio, A. M., J. Theil-Nielsen, and W. Granéli. 2000. Bacterial growth on photochemically transformed leachates from aquatic and terrestrial primary producers. Microb. Ecol. 40:200-208. [DOI] [PubMed] [Google Scholar]
- 10.Anesio, A. M., and W. Granéli. 2003. Increased photoreactivity of DOC by acidification: implications for the carbon cycle in humic lakes. Limnol. Oceanogr. 48:735-744. [Google Scholar]
- 11.Arana, I., A. Muela, J. Iriberri, L. Egea, and I. Barcina. 1992. Role of hydrogen peroxide in loss of culturability mediated by visible light in Escherichia coli in a freshwater ecosystem. Appl. Environ. Microbiol. 58:3903-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bano, N., M. A. Moran, and R. E. Hodson. 1998. Photochemical formation of labile organic matter from two components of dissolved organic carbon in a freshwater wetland. Aquat. Microb. Ecol. 16:95-102. [Google Scholar]
- 13.Benner, R., and B. Biddanda. 1998. Photochemical transformations of surface and deep marine dissolved organic matter: effects on bacterial growth. Limnol. Oceanogr. 43:1373-1378. [Google Scholar]
- 14.Benner, R. 2002. Chemical composition and reactivity, p. 59-90. In D. A. Hansell and C. A. Carlson (ed.), Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, Calif.
- 15.Bertilsson, S., and S. Bergh. 1999. Photochemical reactivity of XAD-4 and XAD-8 adsorbable dissolved organic compounds from humic waters. Chemosphere 39:2289-2300. [Google Scholar]
- 16.Bertilsson, S., R. Stepanauskas, R. Cuadros-Hansson, W. Granéli, J. Wikner, and L. Tranvik. 1999. Photochemically induced changes in bioavailable carbon and nitrogen pools in a boreal watershed. Aquat. Microb. Ecol. 19:47-56. [Google Scholar]
- 17.Bertilsson, S., and L. J. Tranvik. 1998. Photochemically produced carboxylic acids as substrates for freshwater bacterioplankton. Limnol. Oceanogr. 43:885-895. [Google Scholar]
- 18.Bertilsson, S., and L. J. Tranvik. 2000. Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 45:753-762. [Google Scholar]
- 19.Blough, N. V., and R. Del Vecchio. 2002. Chromophoric DOM in the coastal environment, p. 509-546. In D. A. Hansell and C. A. Carlson (ed.), Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, Calif.
- 20.Bushaw-Newton, K. L., and M. A. Moran. 1999. Photochemical formation of biologically available nitrogen from dissolved humic substances in coastal marine systems. Aquat. Microb. Ecol. 18:285-292. [Google Scholar]
- 21.Chrost, R. J., and M. A. Faust. 1999. Consequences of solar radiation on bacterial secondary production and growth rates in subtropical coastal water (Atlantic Coral Reef off Belize, Central America). Aquat. Microb. Ecol. 20:39-48. [Google Scholar]
- 22.Cooper, W. J., and R. G. Zepp. 1990. Hydrogen peroxide decay in waters with suspended soils: evidence for biologically mediated processes. Can. J. Fish. Aquat. Sci. 47:888-893. [Google Scholar]
- 23.Del Giorgio, P., D. F. Bird, Y. T. Prairie, and D. Planas. 1996. Flow cytometric determination of bacterial abundance in lake plankton with the green nucleic acid stain SYTO 13. Limnol. Oceanogr. 41:783-789. [Google Scholar]
- 24.Del Giorgio, P., and J. J. Cole. 2000. Bacterial energetics and growth efficiency, p. 289-325. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley, New York, N.Y.
- 25.Del Vecchio, R., and N. V. Blough. 2002. Photobleaching of chromophoric dissolved organic matter in natural waters: kinetics and modeling. Mar. Chem. 78:231-253. [Google Scholar]
- 26.Farjalla, V. F., A. M. Anesio, S. Bertilsson, and W. Granéli. 2001. Photochemical reactivity of aquatic macrophyte leachates: abiotic transformations and bacterial response. Aquat. Microb. Ecol. 24:187-195. [Google Scholar]
- 27.Fuhrman, J. A., T. D. Sleeter, C. A. Carlson, and L. M. Proctor. 1989. Dominance of bacterial biomass in the Sargasso Sea and its ecological implications. Mar. Ecol. Prog. Ser. 57:207-217. [Google Scholar]
- 28.Geller, A. 1986. Comparison of mechanisms enhancing biodegradability of refractory lake-water constituents. Limnol. Oceanogr. 31:755-764. [Google Scholar]
- 29.Gjessing, E. T., and T. Källqvist. 1991. Algicidal and chemical effect of u.v.-radiation of water containing humic substances. Water Res. 25:491-494. [Google Scholar]
- 30.Granéli, W., M. J. Lindell, and L. J. Tranvik. 1996. Photo-oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnol. Oceanogr. 41:698-706. [Google Scholar]
- 31.Huffman, E. W. D., and H. Stuber. 1985. Analytical methodology for elemental analysis of humic substances. p.433-455, In G. R. Aiken, D. M. McKnight, R. L. Wershaw, and P. McCarthy (ed.), Humic substances in soil, sediment and water: geochemistry, isolation, and characterization. John Wiley and Sons, New York, N.Y.
- 32.Häkkinen, P. J., A. M. Anesio, and W. Granéli. 2004. Hydrogen peroxide distribution, production and decay in boreal lakes. Can. J. Fish. Aquat. Sci. 61:1520-1527. [Google Scholar]
- 33.Jørgensen, N. O. G., L. Tranvik, H. Edling, W. Granéli, and M. Lindell. 1998. Effects of sunlight on occurrence and bacterial turnover of specific carbon and nitrogen compounds in lake water. FEMS Microbiol. Ecol. 25:217-227. [Google Scholar]
- 34.Kaiser, E., and B. Sulzberger. 2004. Phototransformation of riverine dissolved organic matter (DOM) in the presence of abundant iron: effect on DOM bioavailability. Limnol. Oceanogr. 49:540-554. [Google Scholar]
- 35.Kalbitz, K., S. Geyer, and W. Geyer. 2000. A comparative characterization of dissolved organic matter by means of original aqueous samples and isolated humic substances. Chemosphere 40:1305-1312. [DOI] [PubMed] [Google Scholar]
- 36.Kieber, D., J. McDaniel, and K. Mopper. 1989. Photochemical source of biological substrates in seawater: implications for carbon cycling. Nature 341:637-639. [Google Scholar]
- 37.Kieber, D. J. 2000. Photochemical production of biological substrates, p. 130-148. In S. J. de Mora, S. J. S. Demers, and M. Vernet (ed.), The effects of UV radiation in the marine environment. Cambridge University Press, Cambridge, United Kingdom.
- 38.Lindell, M. J., W. Granéli, and L. J. Tranvik. 1995. Enhanced bacterial growth in response to photochemical transformation of dissolved organic matter. Limnol. Oceanogr. 40:195-199. [Google Scholar]
- 39.Loh, A. N., J. E. Bauer, and E. R. M. Druffel. 2004. Variable ageing and storage of dissolved organic components in the open ocean. Nature 430:877-881. [DOI] [PubMed] [Google Scholar]
- 40.Lund, V., and D. Hongve. 1994. Ultraviolet irradiated water containing humic substances inhibits bacterial metabolism. Water Res. 28:1111-1116. [Google Scholar]
- 41.Malcolm, R. 1990. The uniqueness of humic substances in each of soil, stream, and marine environments. Anal. Chim. Acta 232:19-30. [Google Scholar]
- 42.McKnight, D., G. Aiken, and R. Smith. 1991. Aquatic fulvic acids in microbially based ecosystems: results from two desert lakes in Antarctica. Limnol. Oceanogr. 36:998-1006. [Google Scholar]
- 43.Miller, W. L., and R. G. Zepp. 1995. Photochemical production of dissolved inorganic carbon from terrestrial organic matter: significance to the oceanic organic carbon cycle. Geophys. Res. Lett. 22:417-420. [Google Scholar]
- 44.Miller, W. L., and M. A. Moran. 1997. Interaction of photochemical and microbial processes in the degradation of refractory dissolved organic matter from a coastal marine environment. Limnol. Oceanogr. 42:1317-1324. [Google Scholar]
- 45.Mopper, K., and D. J. Kieber. 2002. Photochemistry and the cycling of carbon, sulfur, nitrogen and phosphorus, p. 455-489. In D. Hansell and C. Carlson (ed.), Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, Calif.
- 46.Mopper, K., X. Zhou, R. Kieber, D. Kieber, R. Sikorski, and R. Jones. 1991. Photochemical degradation of dissolved organic carbon and its impact on the ocean carbon cycle. Nature 353:60-62. [Google Scholar]
- 47.Obernosterer, I., B. Reitner, and G. J. Herndl. 1999. Contrasting effects of solar radiation on dissolved organic matter and its bioavailability to marine bacterioplankton. Limnol. Oceanogr. 44:1645-1654. [Google Scholar]
- 48.Obernosterer, I., and R. Benner. 2004. Competition between biological and photochemical processes in the mineralization of dissolved organic carbon. Limnol. Oceanogr. 49:117-124. [Google Scholar]
- 49.Pos, W. H., D. D. Riemer, and R. G. Zika. 1998. Carbonyl sulfide (OCS) and carbon monoxide (CO) in natural waters: evidence of a coupled production pathway. Mar. Chem. 62:89-101. [Google Scholar]
- 50.Pullin, M. J., S. Bertilsson, J. V. Goldstone, and B. M. Voelker. 2004. Effects of sunlight and hydroxyl radical on dissolved organic matter: bacterial growth efficiency and production of carboxylic acids and other substrates. Limnol. Oceanogr. 49:2011-2022. [Google Scholar]
- 51.Riemer, D. D., P. J. Milne, R. G. Zika, and W. H. Pos. 2000. Photoproduction of nonmethane hydrocarbons (NMHCs) in seawater. Mar. Chem. 71:177-198. [Google Scholar]
- 52.Schmitt-Kopplin, P., N. Hertkorn, H. R. Schulten, and A. Kettrup. 1998. Structural changes in a dissolved soil humic acid during photochemical degradation processes under O2 and N2 atmosphere. Environ. Sci. Technol. 32:2531-2541. [Google Scholar]
- 53.Scully, N. M., W. J. Cooper, and L. J. Tranvik. 2003. Photochemical effects on microbial activity in natural waters: the interaction of reactive oxygen species and dissolved organic matter. FEMS Microbiol. Ecol. 46:353-357. [DOI] [PubMed] [Google Scholar]
- 54.Scully, N. M., L. J. Tranvik, and W. J. Cooper. 2003. Photochemical effects on the interaction of enzymes and dissolved organic matter in natural waters. Limnol. Oceanogr. 48:1818-1824. [Google Scholar]
- 55.Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 52:201-213. [Google Scholar]
- 56.Smith, D. C., and F. Azam. 1992. A simple economical method for measuring bacterial protein synthesis rate in seawater using 3H-leucine. Mar. Microbiol. Food Webs. 6:107-114. [Google Scholar]
- 57.Strome, D. J., and M. C. Miller. 1978. Photolytic changes in dissolved humic substances. Int. Ver. Theor. Angew. Limnol. Verh. 20:1248-1254. [Google Scholar]
- 58.Tranvik, L., and S. Kokalj. 1998. Decreased biodegradability of dissolved organic carbon of phytoplankton origin due to interactive effects of UV radiation and humic matter. Aquat. Microb. Ecol. 14:301-307. [Google Scholar]
- 59.Tranvik, L. J., and S. Bertilsson. 2001. Contrasting effects of solar UV radiation on dissolved organic sources for bacterial growth. Ecol. Lett. 4:458-463. [Google Scholar]
- 60.Valentine, R. L., and R. G. Zepp. 1993. Formation of carbon monoxide from the photodegradation of terrestrial dissolved organic carbon in natural waters. Environ. Sci. Technol. 27:409-412. [Google Scholar]
- 61.Weishaar, J. L., G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii, and K. Mopper. 2003. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 37:4702-4708. [DOI] [PubMed] [Google Scholar]
- 62.Wetzel, R. G., P. G. Hatcher, and T. S. Bianchi. 1995. Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter to simple substrates for rapid bacterial metabolism. Limnol. Oceanogr. 40:1369-1380. [Google Scholar]
- 63.Williams, P. J. I. B. 2000. Heterotrophic bacteria and the dynamics of dissolved organic matter, p. 153-200. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley, New York, N.Y.
- 64.Wilson, M. A. 1981. Applications of nuclear magnetic resonance spectroscopy to the study of the structure of soil organic matter. J. Soil Sci. 32:167-186. [Google Scholar]
- 65.Xenopoulos, M. A., and D. F. Bird. 1997. Effects of acute exposure to hydrogen peroxide on the production of phytoplankton and bacterioplankton in a mesohumic lake. Photochem. Photobiol. 66:471-478. [Google Scholar]
- 66.Xie, H., O. C. Zafiriou, W. J. Cai, R. G. Zepp, and Y. Wang. 2004. Photooxidation and its effects on the carboxyl content of dissolved organic matter in two coastal rivers in the Southeastern United States. Environ. Sci. Technol. 38:4113-4119. [DOI] [PubMed] [Google Scholar]