Abstract
Evolution of mammalian skeletal structure can be rapid and the changes profound, as illustrated by the morphological diversity of the domestic dog. Here we use principal component analysis of skeletal variation in a population of Portuguese Water Dogs to reveal systems of traits defining skeletal structures. This analysis classifies phenotypic variation into independent components that can be used to dissect genetic networks regulating complex biological systems. We show that unlinked quantitative trait loci associated with these principal components individually promote both correlations within structures (e.g., within the skull or among the limb bones) and inverse correlations between structures (e.g., skull vs. limb bones). These quantitative trait loci are consistent with regulatory genes that inhibit growth of some bones while enhancing growth of others. These systems of traits could explain the skeletal differences between divergent breeds such as Greyhounds and Pit Bulls, and even some of the skeletal transformations that characterize the evolution of hominids.
Selection, acting on genetic variation, can rapidly change anatomical characters. The domestic dog is arguably the most morphologically diverse mammal in existence (1). Dachsunds, Great Danes, Chihuahuas, and Bulldogs are all descended from the gray wolf, and are the product of artificial selection that began approximately 100,000 to 135,000 years ago (2). The speed and coherence with which these functional adaptations have occurred suggests that selection may be acting on a few genetic loci that control multiple morphological structures. We used a population of Portuguese Water Dogs (PWD; Fig. 1) to analyze the genetic basis for canid morphological variation. This breed is a relatively recent isolate derived from only 31 founding ancestors (3, 4). Morphological variation segregating within this population, coupled with excellent pedigree records, has made possible the quantitative genetic study of skeletal traits of interest (4).
Figure 1.
Comparison of a young (a) with an adult (b) PWD. (c) Comparison of a Greyhound (Left) with a Pit Bull (Right). The adult PWD was shorn to display body shape.
Principal component (PC) analysis classifies phenotypic variation into independent systems of correlated traits (5). Individual dogs each have a value for every PC. Thus PCs are phenotypes subject to genetic analysis and quantitative trait loci (QTLs) can be identified that inform these phenotypes. As a result, the genetics of PCs can be used to dissect genetic networks that regulate complex biological systems. Despite the power of this approach, it is not as yet commonly used for identification of genetic loci that regulate complex systems.
We have used PCs in the genetic analysis of the PWD population to identify independent systems of skeletal genetic variation. These comprise systems of trait variation that characteristically distinguish two quite different breeds, Greyhounds and Pit Bulls (Fig. 1c). Greyhounds are characterized by long, gracile, powerful limbs; narrow, deep chests; and light heads with long, narrow snouts, delicate dentition, and minimal jaw musculature. In contrast, Pit Bulls are short in stature and length, with broad shoulders, and their heads are distinguished by short broad snouts with immense dentition and jaw musculature (6). We will use the comparison of these extremes to illustrate the variation we describe in PWD.
Materials and Methods
Materials.
Blood for DNA and x-rays were collected from PWD owners through the Georgie Project (http://www.georgieproject.com; Karen Miller, director). The 330 dogs enrolled here represent a cross-section of the entire PWD population. All of the dogs can trace their ancestry to 31 founders through ≈24 generations. Consanguinities ranged from 0 to 0.6 with a mean of 0.2. Metrics were corrected for sexual dimorphism by adjusting males and females to the same mean. There was no significant effect caused by age.
PC Analysis.
PC analysis partitions the total variation into unrelated (orthogonal) sets (eigenvectors, or PCs) containing correlated as well as inversely correlated fractional values of individual metrics, or traits (loadings) (5). We used xlstat (http://www.xlstat.com/) and the raw trait correlation matrix for our analysis.
Heritabilities.
Heritabilities were estimated by using a regression of the pedigree estimation of consanguinity between each pair of dogs (7) against the phenotypic similarity (8).
Associations Between Markers and Traits.
Associations between markers and traits were detected by using the correlation of allele sharing at a marker with phenoytpic similarity (9–11). Allele sharing values between pairs of dogs for each marker were first corrected for the mean across all pairs and for consanguinity between pairs. The residual sharing values were investigated for significant correlation with the phenotypic similarity. Significance was estimated by using permutation tests and bootstrap trials (12). A total of 1,000 bootstrap trials were used to estimate the standard deviation of the association statistic (12). The significance (i.e., probability of an association > 0) was estimated by using this standard deviation and assuming a normal distribution. Resulting P values were adjusted for the number of trials (e.g., 6,000 = 500 markers by 12 PCs). Separation between markers (lack of linkage) was established by the absence of correlation between marker sharing residuals.
Normalized Trait Residuals.
Each PC is defined by the contributions made to it by different traits (loadings). The residual trait values are the residuals from the regression of larger PCs onto each trait. Thus, values related to PC2 were the residuals from the regression of PC1 onto each trait, whereas values related to PC4 are the residuals from the regression of PC1, PC2, and PC3 onto each trait.
Correlating Additive Effects of QTLs with Trait Loadings.
For estimating the correlation between the additive effects of all four of the QTLs and the trait loadings for PC4, the additive effect of each marker was estimated by using a regression of allele count onto phenotype using a dummy variable for the marker allele with the highest variance in the population. This variable had a value indicating the allele count for each dog (e.g., 0, 1, or 2). All traits that were clustered according to the PC showed a similar sign for the additive effect (e.g., all traits with a negative loading had a negative additive effect). The fact that all four of the QTLs had a high correlation between the marker additive effects and the trait loadings for PC4 was highly significant (P < 10−4).
QTL Genotypes (Fig. 3).
Figure 3.
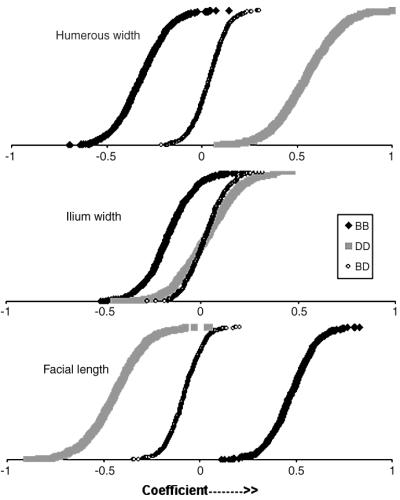
Genotypic separation of trait values contributing to PC4. Traits were residuals obtained by regressing PC1, PC2, and PC3 onto the trait metrics. These residuals were adjusted to a mean of zero and a standard deviation of 1.0. Each value in a distribution represents a weighting coefficient for a genotype of marker FH2356 obtained from a bootstrap trial. Distributions are given for the three traits humerus width, illium width, and facial length.
For the genotypic effects graphed in Fig. 3, we used dummy variables for each genotype such that each dog was assigned a value of 1 or 0 according to whether it did or did not have the genotype. These variables were used as predictors of the normalized trait residuals.
Results
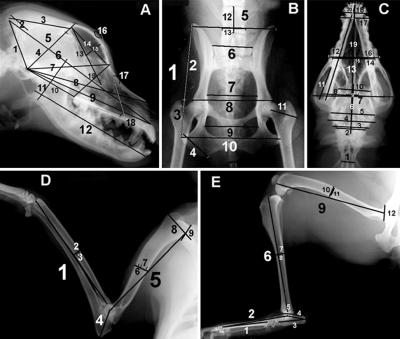
Fig. 2 presents an example of the set of five x-rays we have used for measurements of skeletal parameters. Sets of x-rays were obtained from 330 PWD, each at least 2 years old, and the metrics in Fig. 2 were measured. These measurements were used to prepare a PC analysis of skeletal variation. Ten independent components (eigenvectors) accounted for 75% of the skeletal variation. Of these, nine contained significant heritable (7, 8) components. The first four (Table 1) accounted for 61% of the variation of which about one-third (19%) was heritable. Although PC1 accounted for the largest portion of the variation, its heritability was relatively low, indicating that environmental factors (nutrition, exercise, etc.) played an important role in determining the variation. The heritabilities of two of the other three components were much higher and as a result, the heritable variation was about equally partitioned between the first component and the next three.
Figure 2.
Five x-ray views of a PWD. (A) Profile of skull. (B) Pelvis. (C) Ventral–dorsal view of skull. (D) Fore limb. (E) Hind limb. Trait measurements are numbered in each view. Trait numbers are referenced in Table 2.
Table 1.
PCs of skeletal measurements
| PCs | % Of total variation | Heritability | Heritable variation, % | Marker | QTLs LG | P |
|---|---|---|---|---|---|---|
| 1 | 43.6 | 0.23 ± 0.06 | 10.0 | FH2295 | CFA15 | 5.9 × 10−5 |
| FH2587 | CFA37 | 1.9 × 10−3 | ||||
| 2 | 8.1 | 0.55 ± 0.08 | 4.5 | DO5120 | – | 7 × 10−4 |
| FH3939 | CFA15 | 2.6 × 10−3 | ||||
| 3 | 4.6 | 0.24 ± 0.06 | 1.1 | FH3771 | CFA20 | 4.1 × 10−3 |
| 4 | 4.5 | 0.7 ± 0.06 | 3.2 | FH2356 | CFA18 | 6.7 × 10−12 |
| Σ = 60.7 | Σ = 18.8 | CO6405 | – | 1.2 × 10−6 | ||
| FH3278 | CFA5 | 1.6 × 10−4 | ||||
| FH2189 | CFA31 | 1.8 × 10−3 |
The properties of PCs 1, 2, 3, and 4 are presented. The percent of total variation, heritability (with 95% confidence interval), and percent of total variation are listed. The sum of the variation and heritable variation for the four components are presented at the bottom of each column. At the right are listed the properties of the markers associated with QTLs for the four principal components. PCR markers (13–15) were defined by primers listed on the web: www-recomgen.univ-rennes1.fr/Dogs/; www.fhcrc.org/science/dog_genome. Seven of the markers were placed on linkage groups (LG) of the canine genetic map. Dog autosomes are listed as CFA (Canis familiaris). The significance of each marker–QTL association is listed corrected for 6,000 trials (500 markers and 12 PCs).
Structure and Functional Implications of PC 1–4 (Table 2).
Table 2.
Trait characteristics for PCs 1, 2, 3, and 4
| PC 1
|
PC2
|
PC3
|
PC4
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fig. 2 | Trait | Load | Markers
|
Fig. 2 | Trait | Load | Markers
|
Fig. 2 | Trait | Load | Markers
|
Fig. 2 | Trait | Load | Markers
|
|||||
| FH2295 | FH2587 | DO5120 | FH3939 | FH3771 | FH2356 | CO6405 | FH3278 | FH2189 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| A6 | skullL | 0.13 | +++ | ++ | C19 | facialL | 0.24 | A3 | crestL | 0.28 | +++ | D3 | radius cavity | 0.35 | ++ | +++ | + | + | ||
| C4 | mastoidsp | 0.13 | +++ | ++ | C15 | mandible2sp | 0.21 | A4 | cranialht | 0.26 | D7 | hum. cavity | 0.29 | +++ | + | |||||
| D4 | olecranon | 0.13 | ++ | C12 | mandible1L | 0.21 | C20 | premaxillaL | 0.24 | D2 | radiusW | 0.29 | ++ | + | ++ | |||||
| C11 | zygomatic1L | 0.13 | ++ | ++ | C13 | skullbase | 0.18 | C10 | ant. cranialW | 0.24 | E11 | fem. cavity | 0.28 | ++ | ++ | |||||
| C13 | skullbase | 0.12 | +++ | ++ | C14 | mandible1sp | 0.17 | C6 | cranialL | 0.23 | E8 | tibia cavity | 0.23 | |||||||
| C7 | skullW | 0.12 | +++ | A17 | angle2 | 0.16 | +++ | A5 | skullht | 0.23 | ++ | D6 | humerusW | 0.22 | +++ | +++ | ||||
| A9 | prosthionL | 0.12 | ++ | ++ | C17 | up.caninesp | 0.15 | C5 | cranialW | 0.23 | E10 | femurW | 0.21 | + | + | |||||
| E9 | femur | 0.12 | ++ | +++ | C16 | snoutW | 0.12 | C8 | coronoidsp | 0.18 | D9 | glenoid | 0.13 | ++ | ++ | |||||
| D5 | humerusL | 0.12 | +++ | C18 | low.caninesp | 0.12 | C1 | axisW | 0.16 | E7 | tibiaW | 0.13 | ||||||||
| E2 | footL | 0.12 | +++ | ++ | A2 | crestht | 0.09 | A17 | angle2 | 0.15 | E12 | acetabulum | 0.13 | |||||||
| E6 | tibia | 0.12 | +++ | A19 | facialht | 0.09 | + | C9 | hamulussp | 0.14 | C1 | axisW | 0.12 | + | ||||||
| C12 | mandible1L | 0.12 | ++ | ++ | C11 | zygomatic1L | 0.08 | C16 | snoutW | 0.14 | B13 | lum. vertib.W | 0.12 | ++ | + | |||||
| C3 | jugularsp | 0.12 | ++ | + | A12 | mandibleL,v | 0.08 | ++ | C7 | skullW | 0.13 | C15 | mandible2sp | 0.09 | +++ | |||||
| B4 | ischialtub. | 0.12 | C3 | jugularsp | 0.08 | C18 | low.caninesp | 0.13 | E5 | anklejoint | 0.09 | |||||||||
| B2 | ilium | 0.12 | ++ | A15 | sinusht | 0.08 | ++ | C17 | up.caninesp | 0.13 | C3 | jugularsp | 0.09 | |||||||
| D1 | radius | 0.11 | +++ | C1 | axisW | 0.08 | C14 | mandible1sp | 0.10 | D8 | greatertub. | 0.08 | ++ | |||||||
| E7 | tibiaW | 0.11 | +++ | C9 | hamulussp | 0.05 | A7 | nasionL | 0.09 | B5 | illiumW | 0.08 | ||||||||
| A12 | mandibleL,v | 0.11 | +++ | +++ | C7 | skullW | 0.05 | ++ | C4 | mastoidsp | 0.08 | B6 | sacrumW | 0.08 | ++ | |||||
| D6 | humerusW | 0.11 | +++ | E6 | tibia | −0.03 | A2 | crestht | 0.07 | C2 | atlasW | 0.08 | + | |||||||
| A19 | facialht | 0.11 | ++ | + | C5 | cranialW | −0.03 | +++ | A1 | occipitalht | 0.07 | E3 | calcaneus | 0.05 | + | |||||
| A13 | s.o. | 0.11 | B4 | ischialtub. | −0.10 | D4 | olecranon | −0.05 | B1 | oscoxa | −0.04 | + | ||||||||
| A2 | crestht | 0.11 | ++ | + | C6 | cranialL | −0.11 | + | C15 | mandible2sp | −0.07 | C5 | cranialW | −0.05 | ++ | |||||
| E4 | anklein-lever | 0.11 | ++ | ++ | A16 | angle1 | −0.11 | B11 | trochanter | −0.08 | B2 | illum | −0.05 | |||||||
| A5 | skullht | 0.11 | +++ | B11 | trochanter | −0.12 | A16 | angle1 | −0.08 | C7 | skullW | −0.05 | ||||||||
| A10 | zygomaticL,v | 0.10 | B6 | sacrumW | −0.13 | D8 | greatertub. | −0.08 | A11 | coronoidht | −0.07 | ++ | ||||||||
| C19 | facialL | 0.10 | ++ | +++ | B13 | lum.vertib.W | −0.13 | E4 | anklein-lever | −0.09 | C9 | hamulussp | −0.09 | |||||||
| A1 | occipitalht | 0.10 | B5 | illiumW | −0.14 | E3 | calcaneus | −0.09 | E1 | metatarsal | −0.09 | |||||||||
| A14 | sinusL | 0.10 | B3 | ischium | −0.14 | C12 | mandible1L | −0.12 | A8 | internasalL | −0.11 | |||||||||
| C14 | mandible1sp | 0.10 | C20 | premaxillaL | −0.15 | A18 | angle3 | −0.14 | D1 | radius | −0.12 | |||||||||
| B3 | ischium | 0.10 | +++ | A18 | angle3 | −0.16 | D5 | humerus | −0.15 | A9 | prosthionL | −0.12 | ++ | + | ||||||
| C5 | cranialW | 0.10 | +++ | B12 | lum.vert.L | −0.16 | E1 | metatarsal | −0.15 | A12 | mandibleL,v | −0.14 | +++ | + | + | +++ | ||||
| B6 | sacrumW | 0.10 | ++ | B1 | oscoxa | −0.17 | D1 | radius | −0.16 | C12 | mandible1L | −0.17 | + | + | ||||||
| C18 | low.caninesp | 0.10 | + | B8 | hipW | −0.17 | E6 | tibia | −0.16 | C20 | premaxillaL | −0.20 | + | |||||||
| B9 | outletcavity | 0.10 | B7 | inletcavity | −0.22 | C19 | facialL | −0.21 | C19 | facialL | −0.23 | +++ | +++ | ++ | ||||||
Traits [nomenclature according to Evans (1)] are listed and referenced to figure 3 (e.g. SkullL(ength) = Fig. 3, A6). Subscripts: L, length; W, width; sp, span; ht, height; v, ventral. Only traits with significant loadings or significant associations with markers are listed. Loadings (load) are listed as well as the significance (+, ++, or +++) of the trait-marker association. Adjusted P values: +, 0.05 to 0.0001; ++, 0.0001 to E-10; +++, < E-10.
PC1.
Contributions, or loadings (5) of the individual traits, indicate that PC1 represented the overall size of the skeleton. All of the loadings were positively correlated, and most (85%) had similar values, (0.08 to 0.13). (A representative sample of traits with loadings of 0.1 or greater is presented in Table 2.)
PC2.
Metrics of the pelvis are inversely correlated with metrics of the head and neck. Specifically, individuals that have relatively small pelvic girdles and lumbar vertebrae tend to have relatively large posterior faces, small anterior faces, and large attachment sites for jaw and neck muscles. This finding implies that the size and strength of the pelvic and head-neck musculoskeletal systems are inversely related, as in the Greyhound and Pit Bull.
PC3.
Metrics of the length of the skull and limbs are inversely correlated with metrics of skull width and height, including those that define the volume of the cranium. Again, these tradeoffs are illustrated by the Greyhound's long, narrow head and long limbs compared with the Pit Bull's short limbs and broad robust head and neck (6).
PC4.
Skull and limb lengths are inversely correlated with metrics associated with the strength of the limb and axial skeletons. The more robust a bone is in cross-section, the greater its strength against failure, as in Pit Bulls. However, longer, thinner, bones are better adapted to the Greyhounds' speed.
Genetic Basis for the PCs.
DNA was isolated from each x-rayed dog and characterized by identification of the alleles of ≈500, largely tetranucleotide based, simple sequence repeat genetic markers (13). We used a modified form of the Haseman–Elston regression (9–11) to identify QTLs. Permutation tests were used to establish the significance of marker associations. In this manner, we were able to identify significant QTLs for each of the first four PCs (Table 1). Of the nine loci identified, seven were linked to markers already on the canine genetic map (ref. 14 and E.A.O. and T.D.L., unpublished data). Although the other two had not been mapped, they were not closely linked to each other or to the other seven. Moreover, the two on CFA15 also were well separated. Thus, all nine markers were associated with different QTLs.
FH2295 and FH2587 identify QTLs regulating PC1. We analyzed individual traits for association with these markers (Table 2). Most of the traits (91% of all traits including those not shown in Table 2) were associated with one or the other QTL. About half of these traits were associated with both QTLs, the remaining half were either associated with only one, or primarily with one rather than the other. The QTLs associated with FH2295 were significant for 91 of 100 traits measured, and QTLs associated with FH2587 were significant for 74 of 100. It seems clear that these two loci regulate systems controlling most of skeletal growth. The overlap in regulation could, in itself, give rise to variation in skeletal shape.
FH2295 is closely linked to IGF-1, the gene for insulin-like growth factor 1 (15), and also is syntenic to IGF-1sl2, a murine regulator of serum levels of IGF-1 (16). IGF-1 is known to regulate skeletal size in mice (17) and serum levels have been correlated with size of dogs (poodles; ref. 18). However, the QTL linked to FH2295 does not affect all traits, and in particular differentiates between growth axes of particular bones [e.g., affecting both the femur length and width (data for this lower loading not shown) but only the width of the tibia, humerus, and radius]. Such data suggest the existence of other, as yet unidentified genetic systems, with which this QTL may interact (e.g., receptors).
In Table 2, each of the markers associated with QTLs for PCs 2, 3, and 4 also was associated with several of the residual trait values (loadings) that define these PCs. In PCs 2, 3, and 4, inverse correlations occur between traits grouped by structure (skull, limbs, pelvis, and trunk) or by metric (length, width, height). The existence of separate systems (PCs 2, 3, and 4) that affect the skull while variously controlling the pelvis, limb length or width, respectively, allows flexibility in skull structure to be coupled with different body shapes. In particular, the markers associated with PC4 (FH2356, CO6405, FH3278, and FH2189) were associated with traits that had both positive and negative loadings (Table 2). These markers showed a highly significant association with characters of both limb width and skull length. To characterize this in greater detail, the additive effects for each of these putative QTLs were calculated by using a simple linear regression of allele count for the most frequent allele against the phenotype (e.g., the trait value with PC1, 2, and 3 removed). The high (>80%) correlation between the additive effects of all four of the QTLs and the trait loadings for PC4 was significant (P < 10−4) and indicated that each QTL has a coherent effect across all of the traits. In Fig. 3, we compare the contributions to PC4 of several traits, separated according to three genotypes of the two most prevalent alleles of the marker FH2356 (allele frequencies: B = 0.50; D = 0.39). Humerus width had a positive loading value (Table 2), and phenotypic values associated with DD are positive, whereas those associated with BB are negative. In contrast, facial length, a trait with a negative loading value, was positive when associated with BB and negative when associated with DD. Thus, the effects of these genotypes on the two traits were reversed, consistent with the loading values. There was no significant difference between genotypes for the trait, Illium width; a phenotype that also was not significantly associated with this marker. The data in Fig. 3 were not altered substantially when corrected for pedigree effects (using an additive model, data not shown). Our data indicate that diplotypes of the same QTL can exert an enhancing influence on some traits while inhibiting others, a hallmark of regulatory systems (e.g., ref. 19).
Discussion
We have used PC analysis to identify quantitative phenotypes that represent independent systems of trait variation. These phenotypes were then used to identify QTLs associated with suites of traits. Most probably, the QTLs that we have described represent regions of the genome containing regulatory genes. Such loci may have provided part of the diverse repertoire of genetic information on which selection has operated to produce the rapid evolution of various canine breeds. PC1 appears to represent variation in the overall size of the dog, whereas PCs 2, 3, and 4 represent other aspects of allometry discussed below.
Variation in the transition from the juvenile to the adult state (Fig. 1) could explain the complexes of traits that we see in PCs 2, 3 and 4. In mammals, the young require a functional morphology that often is not suited to adult life. The mammalian reproductive system of nursing altricial young requires a relatively limited, but critical, suite of motor skills in newborns to compete with siblings while nursing. This results in uniformity of newborn body shape among most mammalian species. In newborn mammals, the relatively short muzzle (20) is thought to facilitate suckling (21), and their relatively stout neck, trunk, and limbs likely facilitate displacement of competing siblings from nipples. Behaviors necessary in adults associated with functions such as feeding, locomotion, reproduction, and sociality require changes in shape and proportion of the skeletal system during postnatal growth in most or all mammalian species (22–24). Genetic components regulating the sets of inversely correlated characters of PCs 2, 3, and 4 could account for much of this transformation. For example, appropriate temporal activation of different genes associated with PCs 2, 3, and 4 could produce the short face and limbs as well as stout pelvic girdle and limb bones of puppies on the one hand, followed by development of the longer face and more gracile limbs of adults on the other. Additionally, genes of PC3 could initially produce the relatively large cranial capacity and short broad face of newborns followed by changes in other gene activities resulting in slow growth of the cranium but accelerated growth of the muzzle, giving rise to the adult form. Analysis of skeletal dimensions in domestic dogs has shown that allometry among adults of different breeds is nearly identical to the shapes encountered during the course of postnatal growth. For example, Wayne (25, 26) has suggested that the diversity of limb and cranial proportions among adult domestic dogs as well as the observed differences in limb proportions between dogs and wild canids “are somewhat predetermined” and reflect the diversity of proportions “evident during development of the domestic dog.” Thus, heterochrony (27) of the PWD genes responsible for PCs 2, 3, and 4 could result in the variation in skeletal shapes that we have seen in the PWD population.
The patterns of correlations and inverse correlations that comprise PCs 2, 3, and 4 raise the intriguing question of whether tradeoffs in genetic regulatory complexes have evolved in response to functional tradeoffs. For example, the functions of running and fighting are important to most mammals at some point in their life history, yet anatomical characters that improve locomotor ability often impair fighting performance and vice versa, influencing life histories traits such as the species' mating system and predator–prey relationships (28, 29). Thus, PCs 2, 3, and 4 may represent continuums of compromise between strong selection on different types of performance on the one hand and functional tradeoffs inherent in specialization for these behaviors on the other. These tradeoffs are illustrated by the Greyhound/Pit Bull comparison. PCs 2, 3, and 4 all could serve to differentiate these two functionally extreme breeds from each other. A dramatic feature of Greyhounds is the powerful build of their pelvic musculoskeletal system (6). The extensor muscles of the hip joint of Greyhounds, which originate on the pelvis, are on average 37% more massive than those in Pit Bulls (D.R.C., unpublished data). In contrast, Pit Bulls have shorter snouts and their posterior face and skull are massive compared with that of Greyhounds. Thus, the character complex of PC2 would, in part, explain the morphological differences between the Greyhound and Pit Bull. The characters of PC3 also appear to be consistent with these different demands of specialization. Pursuit requires long limbs for speed and a long skull and face for prey capture. In contrast, a fighting specialist would benefit from strong jaws and neck as well as the postural stability conferred by short stature. Finally, the characters in PC4 also serve to distinguish the breeds. Although thicker, stronger bones are useful to Pit Bulls, they are not to Greyhounds. As bone diameter increases, the weight of the bone also increases and, therefore, requires a greater amount of metabolic energy to accelerate and decelerate during each stride. Thus, a functional tradeoff exists between the strength of limb bones and the economy of running (30). Although PCs 2, 3, and 4 describe the differences observed in breeds such as the Pit Bull and Greyhound, it remains to be seen whether the QTLs involved are, indeed, responsible for the differences between these breeds. Improvements in the canine genetic map leading to syntenic relationships with the mouse and human genomes should lead to cloning and sequencing of the genes involved. This, in turn, should confirm or reject such a causal relationship.
The extent of canine variation, as well as the speed at which it evolved, appears closely matched by the great phenotypic variation that exists among human populations. Modern humans are difficult to define anatomically, but have existed for probably no more than 250,000 years (31), and much of the regional diversification of functional phenotypes appears to have begun 60,000 to 40,000 years ago (32). Some aspects of PCs 2, 3, and 4 appear to be present in characters that distinguish the two major taxa of hominids, Australopithecus and Homo. For example in PC2, the relatively stouter neck, head, and jaws combined with relatively diminutive lumbar and sacral vertebrae, and smaller premaxilla describe the configuration of australopithecines relative to Homo (31, 32). In PC3, the large cranial capacity and premaxilla, combined with a short posterior face (maxilla) are consistent with differences that distinguish Homo from australopithecines (31, 32). Finally, the large diameter limb bones, but short anterior face (premaxilla) and short limb bones of PC4 also are consistent with characters that distinguish australopithecines from Homo (31, 32). The evolution of the human skull has been suggested to represent the retention of juvenile characters (22, 33, 34). Relative to adults, newborn chimps have a greatly expanded cranium, a diminutive face, and an upright head posture on the neck, giving newborn chimps a remarkably human appearance. The structural sequence of Australopithecus africanus, early Homo erectus, and Homo sapiens exhibits a progressive retention of juvenile proportions by adults as the size of the cranium increases and the size of jaw decreases (31, 33). Perhaps regulatory complexes of genes, similar to some of those controlling PCs 2, 3, and 4, have played a role in hominid evolution. Whether this is the case will have to await the isolation and characterization of the genes involved.
We have suggested that changes in the complexes of QTLs that we have found to be associated with skeletal variation in PWD could, in part, explain the rapid evolution of different breeds of dogs described by Wayne (26) and might even explain some characteristics of hominids. PWD are a recent breed springing from a small but diverse founding population of working dogs (3, 4). As a consequence, skeletal variation has not gone to fixation. Other dog breeds, with population structures similar to PWD (4), could reveal additional, perhaps different, organizing principles.
Acknowledgments
We thank Karen Miller, whose vision inspired this project, for her continued interest and support. We thank Makiko Uemura, Pamela Mouser, and Kerry Matz for technical assistance, Deborah Broughton for help in obtaining blood and x-rays, and Sandra Johnson for assistance in studies of Greyhound functional morphology. We are grateful to Steven Dostie for supplying the pictures in Fig. 1. This research was supported by funds from the University of Utah, as well as gifts from the Judith Chiara Family Trust, the Ralston–Purina Co., and more than 100 PWD owners and breeders.
Abbreviations
- PWD
Portuguese Water Dogs
- QTL
quantitative trait loci
- PC
principal component
References
- 1.Evans H E. Miller's Anatomy of the Dog. Philadelphia: Saunders; 1993. [Google Scholar]
- 2.Vila C, Savolainen P, Maldonado J E, Amorim I R, Rice J E, Honeycutt R L, Crandall K A, Lundeberg J, Wayne R K. Science. 1997;276:1687–1689. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- 3.Molinari C. The Portuguese Water Dog. Portugal: ELO-Publicidade; 1993. pp. 1–156. [Google Scholar]
- 4.Chase K, Adler F R, Miller-Stebbings K, Lark K G. J Hered. 1999;90:43–51. doi: 10.1093/jhered/90.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Jackson J E. A User's Guide to Principal Components. New York: Wiley; 1991. [Google Scholar]
- 6.Clark A R, Brace A H. The International Encyclopedia of Dogs. New York: Howell Book House; 1995. [Google Scholar]
- 7.Falconer D S, Mackay T. Introduction to Quantitative Genetics. 4th Ed. New York: Longman; 1996. pp. 82–88. [Google Scholar]
- 8.Ritland K. Evolution (Lawrence, Kans) 1996;50:1062–1073. doi: 10.1111/j.1558-5646.1996.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 9.Haseman J K, Elston R C. Behav Genet. 1972;2:3–19. doi: 10.1007/BF01066731. [DOI] [PubMed] [Google Scholar]
- 10.Olson J M, Wijsman E M. Genet Epidem. 1993;10:87–102. doi: 10.1002/gepi.1370100202. [DOI] [PubMed] [Google Scholar]
- 11.Olson J M. Genet Epidem. 1994;11:41–49. doi: 10.1002/gepi.1370110105. [DOI] [PubMed] [Google Scholar]
- 12.Manly B F J. Randomization and Monte Carlo Methods in Biology. New York: Chapman and Hall; 1997. pp. 148–169. [Google Scholar]
- 13.Francisco L V, Langston A A, Mellersh C S, Neal C L, Ostrander E A. Mamm Genome. 1996;7:359–362. doi: 10.1007/s003359900104. [DOI] [PubMed] [Google Scholar]
- 14.Breen M, Jouquand S, Renier C, Mellersh C S, Hitte C, Holmes N G, Cheron A, Suter N, Vignaux F, Bristow A E, et al. Genome Res. 2001;11:1784–1795. doi: 10.1101/gr.189401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellersh C S, Hitte C, Richman M, Vignaux F, Priat C, Jouquand S, Werner P, Andre C, DeRose S, Patterson D F, et al. Mamm Genome. 2000;11:120–130. doi: 10.1007/s003350010024. [DOI] [PubMed] [Google Scholar]
- 16.Rosen C J, Churchill G A, Donahue L R, Shultz K L, Burgess J K, Powell D R, Ackert C, Beamer W G. Bone. 2000;27:521–528. doi: 10.1016/s8756-3282(00)00354-9. [DOI] [PubMed] [Google Scholar]
- 17.Baker J, Liu J P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 18.Eigenmann J E, Patterson D F, Froesch E R. Acta Endocrinol. 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- 19.Ducy P, Schinke T, Karsenty G. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 20.de Beer G R. The Development of the Vertebrate Skull. Chicago: Univ. of Chicago Press; 1985. [Google Scholar]
- 21.Emerson S B, Bramble D M. In: The Skull. Hanken J, Hall B K, editors. Vol. 3. Chicago: Univ. of Chicago Press; 1993. pp. 384–421. [Google Scholar]
- 22.Gould S J. Ontogeny and Phylogeny. Cambridge, MA: Belknap; 1977. [Google Scholar]
- 23.Shea B T. Am J Phys Anthro. 1981;56:179–201. doi: 10.1002/ajpa.1330560209. [DOI] [PubMed] [Google Scholar]
- 24.Carrier D R. Physiol Zool. 1996;69:467–488. [Google Scholar]
- 25.Alberch P, Gould S J, Oster G F, Wake D B. Paleobiology. 1979;5:296–317. [Google Scholar]
- 26.Wayne R K. Evolution (Lawrence, Kans) 1986;40:243–261. doi: 10.1111/j.1558-5646.1986.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Wayne R K. J Morph. 1986;187:301–319. doi: 10.1002/jmor.1051870304. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton W D. In: Sexual Selection and Reproductive Competition in Insects. Blum M S, Blum N A, editors. New York: Academic; 1979. pp. 167–220. [Google Scholar]
- 29.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 30.Currey J D, McAlexander R N. J Zool Lond. 1985;206:453–468. [Google Scholar]
- 31.Wolpoff H. Paleoanthropology. Boston: McGraw-Hill; 1999. [Google Scholar]
- 32.Klein R G. The Human Career, Human Biological and Cultural Origins. Chicago: Univ. of Chicago Press; 1999. [Google Scholar]
- 33.Bolk L. Das Problem der Menschwerdung. Jena, Germany: Gustav Fisher; 1926. [Google Scholar]
- 34.Shea B T. In: Size and Scaling in Primate Biology. Jungers W L, editor. New York: Plenum; 1985. pp. 175–205. [Google Scholar]