Abstract
Saccadic chronostasis refers to the subjective temporal lengthening of the first visual stimulus perceived after an eye movement, and is most commonly experienced as the “stopped clock” illusion. Other temporal illusions arising in the context of movement (e.g. “intentional binding”) appear to depend upon the volitional nature of the preceding motor act. Here we assess chronostasis across different saccade types, ranging from highly volitional (self-timed saccades, anti saccades) to highly reflexive (peripherally-cued saccades, express saccades). Chronostasis was similar in magnitude across all these conditions, despite wide variations in their neural bases. The illusion must therefore be triggered by a “lowest common denominator” signal common to all the conditions tested and their respective neural circuits. Specifically, it is suggested that chronostasis is triggered by a low-level signal arising in response to efferent signals generated in the superior colliculus.
Introduction
When subjects glance at a silently ticking clock, they often initially think that the clock has stopped; then, after a short pause, the second hand begins to move again. Recently, an experimental paradigm has been introduced permitting the quantification of this subjective lengthening of the post-saccadic stimulus, and the effect has been termed “chronostasis” (Yarrow, Haggard, Heal, Brown, & Rothwell, 2001). Observers fixated a cross on one side of a monitor then made a saccade to a target “0” on the other side. Eye movement triggered a change of digit to a “1” which remained on screen for 400-1600 ms. Subsequent digits (“2”,”3”) remained on the screen for 1 s each, culminating in the appearance of a “4”. Subjects indicated whether the time they saw the “1” was longer or shorter than that for the subsequent digits, allowing matched estimates to be derived. In general, subjects overestimated the time they had seen the saccadic target by about 120 ms.
Saccadic chronostasis depends upon eye movement. It is found following a saccade, but not during static viewing or when the counter is moved towards fixation while the eye remains still. The effect also depends upon the size of the preceding eye movement, with illusion size increasing approximately linearly with the duration of a saccade. The illusion can be disrupted by some but not all changes in the visual scene occurring mid saccade. Specifically, when the counter is noticeably displaced the illusion disappears (Yarrow et al., 2001). These data suggest the following explanation of the effect. During a saccade, retinal blur and mechanisms of active suppression degrade visual input (Ross, Morrone, Goldberg, & Burr, 2001) leaving a “gap” in perception, yet we have continuous awareness of the state of objects in the world. The brain simply assumes that the information in the post-saccadic image has remained constant throughout the saccade, providing the continuity we experience. Hence, post-saccadic events are antedated to just before saccadic onset. This antedating is a specific construction of the brain: when sensory evidence suggests that this assumption is incorrect (as when the target is perceived to have jumped) antedating does not occur and chronostasis is not observed.
Saccadic chronostasis findings have recently been supplemented by reports of similar illusions arising after reaching movements (Yarrow & Rothwell, 2003) or in the context of shifts of auditory attention (Hodinott-Hill, Thilo, Cowey, & Walsh, 2002). Two theories have been proposed to explain chronostasis across these various experimental situations. Hodinott-Hill et al suggested that the critical factor may be arousal, which is known to influence time estimation (Treisman, Faulkner, Naish, & Brogan, 1990; Wearden, Edwards, Fakhri, & Percival, 1998). Arousal might increase the speed of a hypothetical pacemaker-accumulator internal clock (Treisman, 1963) and lead to overestimation of time immediately after a movement. By contrast, Yarrow and Rothwell have argued that the illusion arises when movement produces uncertainty about the onset of a sensory event. They suggested that in saccadic chronostasis the initial response of neurones with receptive fields that shift in the temporal vicinity of a movement (Duhamel, Colby, & Goldberg, 1992; Walker, Fitzgibbon, & Goldberg, 1995; Umeno & Goldberg, 1997) may be used as a time marker for the onset of perceptual properties that are only established later (Yarrow et al., 2003). The idea that a specific neural event might subsequently be used as a time marker for temporal judgements has a clear precedent. It was used by Libet, Wright, Feinstein, and Pearl (1979) to explain why trains of direct electrical stimulation of a duration just long enough to elicit tactile sensation (approx. 200 ms) appear delayed relative to stimulation of the skin if applied to somatosensory cortex, but not when applied to the medial lemniscus (see Pockett, 2002, for a critique).
The arousal account cannot give a satisfactory explanation for a number of experimental results, such as the illusion’s dependency on saccade size and the spatial continuity of the saccade target. However, the alternative receptive-field-shift account also faces difficulties. The pronounced variability across cells in the timing of receptive field shifts makes this event unsuitable as a time marker (Kusunoki & Goldberg, 2003). In reformulating this account in the discussion, we will suggest that receptive field shifts (and other processes relating more directly to duration estimation) are triggered by a specific efference copy signal that may be generated elsewhere in the brain. Receptive field shifts may then underlie conscious visual perception at the time chronostasis occurs; subjective experience might effectively reflect an average of the temporally smeared representation provided by a large number of neurones.
The present experiments do not address the arousal and receptive field shift hypotheses directly, but were instead designed to assess the neural/cognitive level at which the signal that triggers the saccadic chronostasis illusion arises. In the original saccadic experiments, chronostasis was elicited using a self-timed saccade. However, a number of different kinds of saccade have been identified (e.g. Deubel, 1996) differing primarily along a dimension which might be termed the intentional-reactive axis. To assess whether chronostasis arises at the level of 1) volitional or 2) low-level oculomotor processes, we therefore compared the illusion’s magnitude for various types of saccade. This comparison can offer data to refine both the arousal and receptive field shift accounts, but will not necessarily support one account over the other. We provide a more direct test of these alternative hypotheses elsewhere (Yarrow, Haggard and Rothwell, submitted).
Experiment 1: Pro/anti saccades.
Chronostasis is not the only temporal illusion that has recently been reported in the context of movement. Haggard, Clark, and Kalogeras (2002) made use of the Libet clock paradigm to investigate the relationship between the perceived time of various combinations of movements and briefly presented auditory stimuli. Presenting a tone after a movement caused the perceived times of occurrence for both events to be drawn closer together, as if they had been temporally bound. This finding shows a striking resemblance to chronostasis, where a post-movement event is antedated to the point of movement initiation. A second result was that this binding effect disappeared (and was even reversed) in the absence of volition, when transcranial magnetic stimulation (TMS) was applied over the contralateral motor cortex to elicit a movement. This led the authors to term their effect “intentional binding,” considering it to depend upon the volitional nature of the motor act.
In light of these experiments, it seems natural to ask whether chronostasis also depends upon volition. Varying the self-timed/cued nature of a saccade approximates the manipulation of volition provided by comparing self-timed actions with movements induced by TMS. If chronostasis has a volitional origin, we might reasonably predict that chronostasis will be reduced for cued saccades relative to volitional saccades. In Experiment 1 chronostasis was therefore measured following cued saccades made in response to a sudden and unpredictable peripheral onset (pro saccades). In addition, anti-saccades (saccades made in a direction opposite to a sudden peripheral onset) were considered. This task requires active suppression of a dominant response, yielding a high volitional component and slow reaction times (Hallett, 1978). In anti saccades, the intention to move should occur at a normal latency after the instructive stimulus. However, this intention is translated into an actual eye movement only after a much longer delay than in pro saccades because of the substantial additional time taken to inhibit the prepotent, reflexive response to saccade towards the target instead of away from it. It follows that the temporal interval between the time of intention and the time of movement onset will be greater in the anti-saccade condition than in the pro-saccade condition; the anti-saccade condition increases the separation between these events. A comparison between pro and anti saccades allows us to investigate whether the chronostasis effect is tied to the intention or to the movement. Specifically, if we assume that the signal driving chronostasis were to arise early in motor preparation (i.e. before the processes that produce a reaction time deficit for anti-saccades, such as the re-specification of saccade direction) greater chronostasis is anticipated in the anti-saccade than the pro-saccade condition. Effect size should be enhanced in this condition by an amount roughly commensurate with the observed increase in reaction time.
Experiment 2: Express saccades.
Whichever account of chronostasis is favoured, information about the eye movement itself must be transmitted to the neural structures responsible for temporal perception in order to obtain the observed modulation of subjective time. We therefore consider where in the motor system such a signal arises. The oculo-motor plant receives its primary input from the brainstem burst generators of the reticular formation (Moschovakis, Scudder, & Highstein, 1996). Different types of saccade selectively recruit additional higher cortical areas during saccade generation (Scudder, Kaneko, & Fuchs, 2002). For example, typical peripherally-cued saccades, arising with a latency of around 180 ms, may have a different neural basis from express saccades, made with a latency of 130 ms or less and elicited using a gap paradigm (Fischer & Ramsperger, 1984). So far, chronostasis has only been demonstrated for self-timed saccades. If an effect of similar magnitude arises for other saccades, then the information used to adjust perceptual experience must arise in areas common to both types of saccade. Hence while all saccades involve low-level dedicated areas such as the superior colliculus and the brainstem (Hopp & Fuchs, 2002) certain kinds of saccade additionally recruit areas in frontal and parietal cortex. If these cortical structures contribute signals necessary for chronostasis effects, then no illusion would be predicted following saccades that do not engage such regions. Therefore, in experiment 2, a comparison was made between express saccades, peripherally-cued saccades and self-timed saccades to assess the degree of cortical involvement in saccade generation required to produce chronostasis.
Results
Experiment 1.
In separate blocks, subjects made speeded pro or anti saccades out from central fixation in response to a peripheral open box cue (Fig 1). Following correctly targeted saccades, they judged the duration of a variable (400–1600 ms) circular stimulus, appearing during the saccade and therefore only perceived at refixation, relative to a reference (1000 ms) stimulus presented just afterwards at the same location (forced choice longer/shorter). These judgements were used to derive a subjective duration estimate in each condition. This is defined as the time for which the variable-duration stimulus needed to be present to be judged as of equal duration to the reference stimulus. In a constant fixation control condition, subjects fixated a peripheral cross which disappeared briefly prior to presentation of the same sequence of a variable-duration stimulus followed by a reference stimulus.
Figure 1.
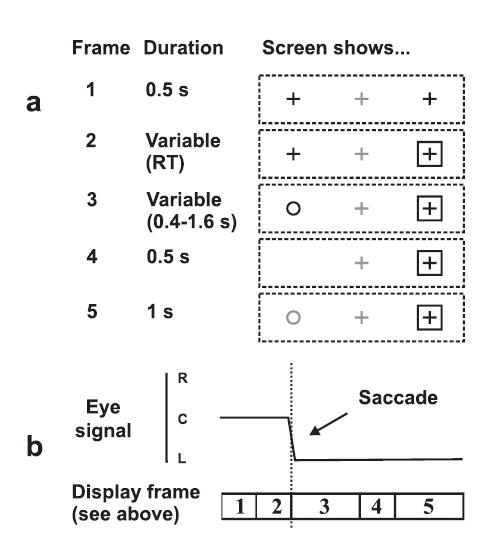
Schematic of procedure for pro/anti-saccade experiment. (a) Sequence of visual stimuli. (b) Eye position. A trial from an anti-saccade block is shown (saccade made away from the box-shaped “go” cue). Eye movement triggered the appearance of a circle (frame 3) and subjects were required to compare its duration with that of a reference stimulus (frame 5). In pro-saccade trials, the circle replaced the cross within the box-shaped cue. Stimuli shown in grey were actually displayed in red.
Eye movement measures were similar across conditions. Saccades took an average of 58.4 ms in the pro-saccade condition and 61.2 ms in the anti-saccade condition. Mean reaction times, however, differed, being 274 ms in the pro-saccade condition and 335 ms for anti-saccades, a predicted and highly significant difference of 61 ms (t = 6.52, df = 25, p < 0.001).
Figure 3(a) shows mean corrected time estimates in all three conditions. The estimated duration of 1063 ms in the constant fixation control condition was just above, but did not differ significantly from, the target value of 1000 ms. Estimates were substantially reduced in both the pro- and anti-saccade conditions, by an identical 90 ms relative to control. ANOVA showed a significant difference across conditions (f = 5.448, df = 2, 50, p = 0.007) with pairwise follow ups showing significant differences between control and pro-saccade (p = 0.007) and control and anti-saccade (p = 0.015) conditions.
Figure 3.
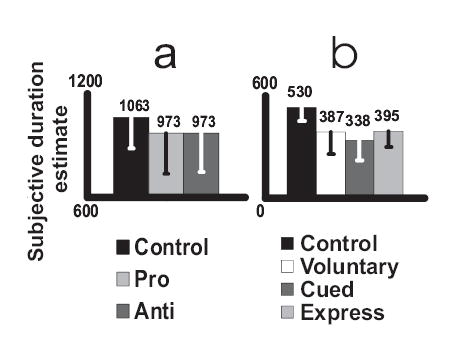
Mean subjective duration estimates following different kinds of saccade for (a) pro/anti saccade and (b) express saccade experiments. The control conditions show values derived from constant fixation trials. Error bars show standard deviations.
Experiment 2.
Because express saccades are typically elicited in the absence of a structured visual background (e.g. Hopp et al., 2002) Experiment 2 took place in darkness using red/green light emitting diodes (LEDs) as stimuli. A red target stimulus switched to become green mid-saccade to provide the intervals used in duration judgements. Variance in time judgements increases with the duration of the interval that is being judged (Allan, 1998). Therefore the duration of the reference stimulus was reduced from 1000 ms (Exp. 1) to 500 ms, with the variable stimulus taking a value between 100 and 900 ms. Subjects completed three saccade conditions (self-timed saccades, peripherally-cued saccades and express saccades; see Fig 2) and a constant fixation control. Self-timed saccades lasted, on average, 77 ms. Cued saccades were made with an average latency of 197 ms, and lasted for an average of 72 ms. Saccades elicited in a gap paradigm (Saslow, 1967) yielded an average latency of 150 ms. Only those trials in which saccadic RT was between 70 and 130 ms (mean 37% of acceptable trials) were classified as express saccades and used to derive subjective duration estimates in the express condition. Saccades in such trials lasted an average of 79 ms.
Figure 2.
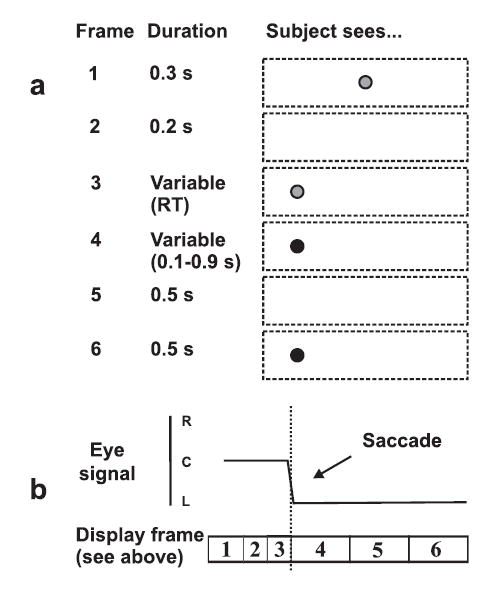
Schematic of procedure for express saccade experiment. (a) Sequence of visual stimuli. (b) Eye position. A trial from a gap (express saccade) block is shown. Red stimuli are shown in grey, green stimuli in black. Eye movement towards the appearance of a peripheral red LED caused it to change colour to green. Subjects were required to compare the duration of this fixated green stimulus (frame 4) to that of a reference stimulus (frame 6). In cued saccade blocks, no gap was inserted between central fixation and peripheral onset (i.e. frame 1 lasted 500 ms and frame 2 was removed). In self-timed saccade blocks, both central and peripheral LEDs were initially displayed, and subjects made a saccade in their own time eliciting the sequence shown in frames 4 to 6.
Mean subjective duration estimates are shown in Figure 3(b). Once again, the control estimate was a little above the veridical value of 500 ms but this difference was not significant. As in the pro/anti saccade experiment, estimates were substantially reduced in the various saccade conditions, with effect sizes of between 135 and 192 ms relative to control. ANOVA showed a significant difference across conditions (f = 24.306, corrected df = 3, 30, p < 0.001) with pairwise follow ups showing significant differences between the control and each of the saccadic conditions (all p < 0.001) while the saccadic conditions did not differ significantly from one another.
Discussion
The two experiments reported here suggest that the size of the chronostasis effect is approximately similar in all types of saccadic eye movements tested (self timed, cued, anti, pro, express) despite wide variations in underlying neural mechanisms. We will argue below that this is compatible with an efference copy signal arising late in saccade generation, perhaps in the superior colliculus, that influences other areas responsible for visual awareness and timing operations. Firstly, however, we will deal with two subsidiary features of the data: a trend towards biased estimates in the constant fixation control conditions, and the different effect magnitudes found in Experiments 1 and 2.
In our experiments, subjective duration estimates were established in both constant fixation and saccade conditions. The inclusion of a constant fixation condition controlled for any individual differences and systematic biases in temporal judgements. Even under optimal conditions, duration estimates derived from comparison tasks like that used here are often biased. This bias, known as the time order error, is notoriously difficult to predict (Allan & Gibbon, 1994; Hellstroem, 1985). It may explain the non-significant trend evident in both experiments for subjective duration estimates to exceed the veridical reference stimulus duration under constant fixation conditions. The time order error should be identical in our constant fixation and saccade conditions. We therefore estimated the magnitude of the chronostasis illusion as the difference between these conditions.
Although chronostasis was clearly present for all types of saccade tested here, there was a trend towards larger effect sizes in the second experiment. While a number of procedural changes between the two experiments may have been responsible, differences as large as these are often seen for different groups of subjects in separate chronostasis experiments, even when the procedure is essentially unchanged (cf. Yarrow et al., 2001). For this reason, our statistical inferences were based only on differences between conditions within a single group of subjects. Chronostasis is constant across different reference stimulus durations (Yarrow et al., submitted) and when the timing of the mid-saccadic stimulus change is altered (Yarrow et al., 2001) so these changes are unlikely to have influenced our results across experiments. The illusion has been shown to increase approximately linearly with the duration of a saccade (Yarrow et al., 2001) and saccades were slightly slower in Experiment 2, but the size of this difference (around 15 ms) suggests that it can only offer a partial explanation of the increase in illusion size. It is possible that the subtlety of the mid-saccadic stimulus change in Experiment 2 (a change from red to green for a stimulus initially perceived only in the periphery) may have enhanced the illusion, given that noticeable changes in the position of the target stimulus at this time are known to substantially reduce chronostasis (Yarrow et al, 2001). Changes in form, like those used in Experiment 1, may have been registered as a greater disruption to the continuity of the visual scene.
The main results of the two experiments are, however, very clear. In the first experiment the hypothesis that chronostasis would increase in the anti-saccade condition was not supported. Reaction times between these two conditions differed by 61 ms; if chronostasis involved antedating the post-saccadic percept to a pre-motor event occurring prior to the (re)specification of saccade parameters required in the anti-saccade condition, an increase in effect size of similar magnitude would be expected. This did not emerge. These data therefore suggest that volition is not a critical factor in producing chronostasis. The results of the second experiment reinforce this conclusion. The illusion appears to be a relatively low-level phenomenon since it even arises for highly reflexive eye movements. Thus the time-marking signal employed to trigger chronostasis probably arises from some basic motor process downstream of any volitional intervention.
Turning to the issue of anatomical localisation, the express saccade condition provides the most revealing result. Because this kind of saccade is the most basic studied thus far (Hopp et al., 2002) it effectively limits the regions from which a relevant movement-related command might arise. More complex types of saccades make use of higher cortical areas, but all saccades ultimately rely on areas projecting directly to the brainstem burst generator (Scudder et al., 2002). Since chronostasis appears ubiquitous and of comparable magnitude for all saccades, those cortical areas involved in more “cognitive” saccades, but which are not typically involved in low-level saccade generation, cannot be the source of the signal that triggers the illusion. Single pulse transcranial magnetic stimulation of the posterior parietal cortex, for example, has been shown to affect the onset time and metrics of memory guided saccades (Muri et al., 2000). This area is almost certainly not involved in express saccades however (Hopp et al., 2002). Since chronostasis was found for express saccades, posterior parietal cortex is unlikely to give rise to signals important in triggering the effect, though it may contribute to generating the subjective content of the illusion.
Expanding on this point, the chronostasis effect recalls pre-saccadic receptive field shifts originally demonstrated for neurones in the lateral intraparietal area (Duhamel et al., 1992). However, such remapping is evident in other regions including the superior colliculus, frontal eye fields and early visual cortical areas (Walker et al., 1995; Umeno et al., 1997; Nakamura & Colby, 2002) so a remapping explanation of chronostasis remains viable. More importantly, remapping in regions like the lateral intraparietal area is presumably triggered by efference-copy signals arising from other saccade-related areas (Sommer & Wurtz, 2002). When excluding cortical areas based on their failure to play a role in the generation of express saccades, we consider only the source of any signal initiating the processes that underlie saccadic chronostasis. The location from which this signal arises need not be the same as the location(s) which construct the experiences of subjective time and visual awareness.
Aside from parietal cortex, a number of other cortical sites are undermined by our results from express saccades. The prefrontal cortex can be excluded by similar arguments to those used for parietal areas. Transcranial magnetic stimulation over prefrontal cortex increases the likelihood of express saccades, suggesting that it typically inhibits these responses and does not play a role when they occur spontaneously (Muri et al., 1999). The frontal eye fields are better candidates. They project to the superior colliculus and have weak but direct projections to the brainstem burst generator (although the extent and functional significance of these links is debatable; see Scudder et al., 2002, for an overview). They also show retinotopic mapping like that in the superior colliculus and consistent with an important role in determining the spatial metrics of saccades (Thompson & Bichot, 1999) and have recently been shown to facilitate responses in early visual areas in a manner consistent with premotor views of attention (Moore & Armstrong, 2003). These areas are candidate loci for a signal giving rise to chronostasis. However, some authors have suggested that they are relevant for voluntary but not reflexive saccades (Schneider & Deubel, 2002). Crucially, their causal role in the generation of express saccades is questionable (Hopp et al., 2002). Schiller and colleagues (Schiller, Sandell, & Maunsell, 1987) showed that lesions of the frontal eye fields had no long-term effects on the production of express saccades, in contrast to lesions of the superior colliculus. It has recently been suggested that neurons in the frontal eye fields discharge in a manner consistent with a role in express saccade generation (Everling & Munoz, 2000). However, this conclusion remains contentious (Hopp et al., 2002); the observed patterns of cell discharges may reflect efference copy information arriving from the superior colliculus rather than motor commands. There is therefore good reason to doubt that the signal triggering chronostasis arises in the frontal eye fields.
A number of other anatomical regions are uncontroversially involved in all the saccade types employed here. In particular, afferent information from the oculomotor plant remains a viable source. The delayed nature of sensory feedback places this interpretation at odds with the remapping account of chronostasis, which suggests that the system responsible for timing the post-saccadic stimulus is effectively being switched on early during saccade generation. However, feedback from ocular muscles and related sources might reasonably result in the transient increases in arousal suggested by Hodinott-Hill et al. (2002). Such feedback could also play a role if temporal experience is modified “after the event”, perhaps reflecting the ongoing revision of conscious awareness in response to updated information (Dennett & Kinsbourne, 1992). Future experiments could investigate the role of afferent information by assessing chronostasis following externally induced perturbations of eye position.
Returning to an outflow explanation, the brainstem burst generator is clearly a good candidate for the signal triggering chronostasis, since it is the final source of efferent information for saccade generation. This region is presumably active in all saccades. However, we suggest a region active slightly earlier than the brainstem burst generator in movement generation: the superior colliculus. This suggestion is based on this region’s known anatomical connections, and in particular on recent data demonstrating the transmission of efference-copy information upstream from the superior colliculus to cortical areas (Sommer et al., 2002). The superior colliculus is widely regarded to be the primary interface with the burst generator (e.g. Scudder et al., 2002). Lesions here impair saccades, but do not completely prevent saccades occurring via other routes (Sparks, 1986). Electrical stimulation in the superior colliculus can produce saccades with a delay of only 20–30 ms (Moschovakis et al., 1996) but movement-related activation is often seen in collicular cells well in advance of saccade initiation (e.g. Walker et al., 1995). Although the deep layers of the superior colliculus are most widely known for their descending projections involved in the direct control of movement, they also have ascending axons that terminate in the dorsal thalamus and in turn project to the frontal eye fields and the inferior parietal lobule (Sparks, 1986). This pathway has recently been shown to send corollary discharge signals upstream to the frontal eye fields. Monkeys performing a double saccade task failed to fully correct for the displacement caused by the first saccade when this pathway was blocked (Sommer et al., 2002). These authors suggested that signals transmitted via this pathway might trigger receptive field shifts in frontal and parietal regions. Might such a pathway also convey information responsible for chronostasis to areas involved in timekeeping and conscious perception? We speculate that a corollary discharge signal from the superior colliculus could trigger remapping processes in the frontal eye fields or lateral intraparietal area that underlie the extended conscious visual percept experienced in chronostasis experiments. The same collicular signal, or subsequent signals from affected cortical regions, might trigger timing processes, probably involving a network of brain areas including the basal ganglia, lateral cerebellum, supplementary motor area, dorsolateral prefrontal cortex, anterior cingulate cortex and right parietal cortex (Hazeltine, Helmuth, & Ivry, 1997; Macar et al., 2002; Matell & Meck, 2000; Meck, 1996; Meck & Benson, 2002; Rao, Mayer, & Harrington, 2001).
It should be noted that the logic applied here assumes a single neural locus as the origin of the chronostasis trigger signal. Of course this information could be transmitted from different sites and via multiple routes, depending on the nature of the movement. For example, receptive field shifts are known to occur in several brain areas (Duhamel et al., 1992; Walker et al., 1995; Umeno et al., 1997; Nakamura et al., 2002) perhaps with different temporal dynamics. An interaction between various signal sources (and regions receiving and acting upon such signals) might help to explain the trend observed in the express saccade experiment for a greater effect in the cued condition relative to express and self-timed saccades, should future research show it to be reliable.
Saccadic chronostasis appears to be a prime example of a motor act interacting with sensory systems to alter perceptual experience, reflecting the indivisibility of the two systems (Hommel, Musseler, Aschersleben, & Prinz, 2001). The present data show that the signal used to achieve this modification of perception can arise rather late in the chain linking decisions to motor actions, perhaps at the level of the final oculomotor command itself. These experiments point to the generality of the phenomenon of saccadic chronostasis. The process appears to be operating regardless of the duration of the post-saccadic stimulus or the kind of saccade that is being made, suggesting that the illusion is ubiquitous in our everyday experience and may well be filling in the experiential gaps left by saccadic suppression. A detailed neural model of exactly how chronostasis arises will be the topic of future research.
Methods
Statistics
Standard parametric tests were used (repeated-measures ANOVAs, t-tests, logistic regression) with α = 0.05 and Bonferroni corrections applied for multiple comparisons. For repeated measures ANOVAs, sphericity violations were controlled using the Greenhouse-Geisser correction when ɛ was below 0.7 and the Huynh-Feldt correction when Greenhouse-Geisser ɛ was above 0.7 (Howell, 1997).
Pro/anti saccade experiment
26 subjects (15 male, mean age 30.5, SD 7.8) participated. They sat before a 22” CRT colour monitor refreshing at 60 Hz. Eye to screen distance was maintained at 41 cm using an adjustable chin rest. Horizontal eye movements were recorded from the left eye using an infra-red eye tracker (Microguide 1000 spectacles, low-pass filtered at 40 Hz) and sampled at 200 Hz. Stimuli were black or red on a white background, subtending 1.1º (crosses and open circles) or 3.3° (open squares). The experiments were controlled by a PC interfaced with a 12 bit A/D card (National Instruments DAQ 1200).
A repeated-measures design was employed with three conditions: pro-saccade, anti-saccade and control (see below). Trials from each condition were presented in separate blocks, with six blocks per condition. A single block was completed from each condition in turn, with order counterbalanced across subjects (first 24 subjects) or completed in a random order (final two subjects, both investigators, who completed the 18 blocks and a further 12 blocks from two extra conditions not reported here).
For saccade blocks of both kinds, black crosses were initially displayed 20º to the left and right of a central red cross. In pro-saccade blocks, subjects fixated the red cross and initiated the trial with a mouse key press. 500 ms later a black outline box appeared around one or other peripheral cross, directing the subject to make a speeded saccade in that direction. The box appeared randomly to the left or right on each trial. In anti-saccade blocks, the same sequence of stimuli was used, but subjects were required to saccade to the cross around which the box had failed to appear (i.e. in a direction opposite to it). Eye movement triggered the black cross to be replaced with a circle when the saccade had travelled one fifth of the distance to target. The circle remained on screen for 400–1600 ms. It then disappeared, to be replaced by a red circle (the comparison stimulus) after 500 ms. Subjects indicated whether the time they saw the first circle was longer or shorter than that for which the comparison circle was displayed (exactly 1000 ms). The duration of the first circle was controlled by a modified binary search (MOBS) procedure (low boundary 400 ms, high boundary 1600 ms, initial presentation random 600–1400 ms, five reversals to terminate) that used subjects’ previous responses to home in on a value judged equivalent to the duration of the comparison stimulus (Tyrrell & Owens, 1988). Blocks finished when the MOBS had terminated.
Saccade start/end points were calculated automatically using a velocity criterion. Trials where the first saccade recorded did not exceed 90% of the total distance recorded (summed across all detected saccades) were excluded on line and repeated immediately. This led to the rejection of trials in which subjects initially moved their eyes in the wrong direction. In control (constant fixation) trials, subjects initially fixated a peripheral cross at equivalent eccentricity (±20°). It was blanked 400ms after the subject’s mouse key press, then replaced after a further 100 ms by the to-be-judged circle, with subsequent stimulus presentation and subject responses as per saccade trials. Position of the fixation cross alternated every trial.
Blocks were of variable length, typically 6–20 trials (excluding those rejected). Subjective duration estimates were obtained by taking the average of the six MOBS termination values in each condition. In the saccade conditions, each estimate was corrected post hoc to match the time the first circle was on screen following target foveation by subtracting the average time the eye was in motion following the triggered change to a circle. The experiment took around one hour to complete.
Express saccade experiment
Data from 12 subjects (8 male, mean age 32.3, SD 7.0) was used, a further 7 subjects having been initially assessed and found to produce too low a proportion of acceptable express saccades (<10%). Testing was carried out in total darkness. Subjects sat before an adjustable metal frame upon which three two-colour (red/green) LEDs were mounted in horizontal alignment. The LEDs were 22 cm apart, and eye to screen distance was maintained at 60 cm. Saccades from the central to peripheral LEDs were therefore 20° in extent. Other materials were unchanged from Experiment 1.
A repeated-measures design was employed with four conditions: self-timed, cued, express (“gap” paradigm) and control. Trials from each condition were presented in separate blocks, with three blocks per condition. Two subjects completed additional blocks to ensure that logistic regression provided a significant fit in all conditions (see below). Testing was conducted in two sessions (approx. 50 mins each) to prevent fatigue; block order was selected randomly for each subject, with the constraint that at least one block from each condition was presented in each testing session.
For the express saccade blocks, a central red LED provided an initial fixation point. The experimenter sat in an adjacent testing area and viewed a display showing eye position and details of the current trial and block. They initiated the trial with a mouse key press. 300 ms later the LED was extinguished, and after a further 200 ms one of the two peripheral LEDs (randomly selected) was switched on, initially in red, directing the subject to make a speeded saccade in that direction. For cued blocks, the 200 ms blank period was removed. In self-timed blocks, two LEDs were initially switched on (the central one and one randomly selected peripheral one, both red). The experimenter initiated the trial then verbally indicated that the subject could make a saccade whenever they were ready. In all cases, eye movement triggered the red peripheral LED to change colour to green when the saccade had travelled one half of the distance to target. The green LED remained on for 100–900 ms. It then disappeared, appearing again after a break of 500 ms and remaining on for 500 ms (comparison stimulus).
Because many trials were rejected, and this decision could only be made after the trial had been completed (see below), MOBS were not used to control the duration of the first green stimulus and provide subjective duration estimates. Instead, the duration of the first green LED was selected randomly on each trial from a distribution containing values between 100 and 900 ms in 25 ms increments. The distribution was initially uniform, in the region 300–700 ms, but was updated after each accepted trial according to the generalized P’olya urn (GPU) model proposed by Rosenberger and Grill (1997) with initial urn composition type IV and k = 8. This procedure adjusts the distribution from which values are selected based on previous responses. Hence selection is efficient, producing on average more values close to the subject’s subjective duration estimate, but each trial is selected at random, preventing sequential dependency biases that might compromise estimates subsequently produced by logistic regression.
The automatic analysis of saccades was as per the pro/anti saccade experiment, except that the first saccade only had to exceed 80% of the total distance recorded to be acceptable, and the experimenter had on-line access to derived saccade stats and could make corrections. In addition, express saccade trials were only accepted when saccadic RT was between 70 and 130 ms (measured from go stimulus to saccade onset) and cued saccade trials were only accepted when RT was above 130 ms. In control (constant fixation) trials, subjects initially fixated a peripheral red LED (random left/right presentation). It changed to become green 500 ms after the experimenter began the trial, with subsequent stimulus presentation and subject responses as per saccade trials.
Blocks ended after 20 accepted trials. Subjective second estimates were obtained using logistic regression. In the saccade conditions, each trial presentation value was corrected post hoc to match the time the first green LED was displayed following target foveation by subtracting the time the eye was in motion following the triggered change.
Footnotes
Reprint Requests should be sent to: Kielan Yarrow, Sobell Dept., Institute of Neurology, 8-11 Queen Square, London WC1N 3BG., UK. Email k.yarrow@ion.ucl.ac.uk.
References
- Allan LG, Gibbon J. A new temporal illusion or the TOE once again? Perception and Psychophysics. 1994;55:227–229. doi: 10.3758/bf03211669. [DOI] [PubMed] [Google Scholar]
- Allan LG. The influence of the scalar timing model on human timing research. Behavioural Processes. 1998;44:101–117. doi: 10.1016/s0376-6357(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Dennett DC, Kinsbourne M. Time and the observer: The where and when of consciousness in the brain. Behavioral and Brain Sciences. 1992;15:183–247. [Google Scholar]
- Deubel, H. (1996). Visual processing and cognitive factors in the generation of saccadic eye movements. In W.Prinz & B. Bridgeman (Eds.), Handbook of perception and action. Volume 1: Perception (pp. 143–189). London: Academic press.
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Experimental Brain Research. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nature Neuroscience. 2002;5:382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Helmuth LL, Ivry RB. Neural mechanisms of timing. Trends in Cognitive Sciences. 1997;1:163–169. doi: 10.1016/S1364-6613(97)01058-9. [DOI] [PubMed] [Google Scholar]
- Hellstroem A. The time-order error and its relatives: Mirrors of cognitive processes in comparing. Psychological Bulletin. 1985;97:35–61. [Google Scholar]
- Hodinott-Hill I, Thilo KV, Cowey A, Walsh V. Auditory chronostasis: Hanging on the telephone. Current Biology. 2002;12:1779–1781. doi: 10.1016/s0960-9822(02)01219-8. [DOI] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The Theory of Event Coding (TEC): a framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. Investigating the site of human saccadic adaptation with express and targeting saccades. Experimental Brain Research. 2002;144:538–548. doi: 10.1007/s00221-002-1077-x. [DOI] [PubMed] [Google Scholar]
- Howell, D. C. (1997). Statistical Methods for Psychology (4 ed.) Belmont, CA: Wadsworth.
- Kusunoki M, Goldberg ME. The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. Journal of Neurophysiology. 2003;89:1519–1527. doi: 10.1152/jn.00519.2002. [DOI] [PubMed] [Google Scholar]
- Libet B, Wright EWJ, Feinstein B, Pearl DK. Subjective referral of the timing for a conscious sensory experience: a functional role for the somatosensory specific projection system in man. Brain. 1979;102:193–224. doi: 10.1093/brain/102.1.193. [DOI] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Experimental Brain Research. 2002;142:475–485. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AME. Dissecting the brain's internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Progress in Neurobiology. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Muri RM, Gaymard B, Rivaud S, Vermersch A, Hess CW, Pierrot-Deseilligny C. Hemispheric asymmetry in cortical control of memory-guided saccades. A transcranial magnetic stimulation study. Neuropsychologia. 2000;38:1105–1111. doi: 10.1016/s0028-3932(00)00030-0. [DOI] [PubMed] [Google Scholar]
- Muri RM, Rivaud S, Gaymard B, Ploner CJ, Vermersch AI, Hess CW, et al. Role of the prefrontal cortex in the control of express saccades. A transcranial magnetic stimulation study. Neuropsychologia. 1999;37:199–206. doi: 10.1016/s0028-3932(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proceedings of the National Academy of Sciences, USA. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockett S. On subjective back-referral and how long it takes to become conscious of a stimulus: a reinterpretation of Libet's data. Consciousness and Cognition. 2002;11:144–161. doi: 10.1006/ccog.2002.0549. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature Neuroscience. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rosenberger WF, Grill SE. A sequential design for psychophysical experiments: an application to estimating timing of sensory events. Statistics in Medicine. 1997;16:2245–2260. doi: 10.1002/(sici)1097-0258(19971015)16:19<2245::aid-sim656>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends in Neurosciences. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. Journal of Neurophysiology. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Schneider, W. X. & Deubel, H. (2002). Selection-for-perception and selection-for-spatial-motor-action are coupled by visual attention: A review of recent findings and new evidence from stimulus-driven saccade control. In B.Hommel & W. Prinz (Eds.), Attention and Performance XIX: Common mechanisms in perception and action (pp. 609–627). New York: Oxford University Press.
- Scudder CA, Kaneko CRS, Fuchs AF. The brainstem burst generator for saccadic eye movements: A modern synthesis. Experimental Brain Research. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiological Reviews. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. Frontal eye field: A cortical Salience map. Behavioral and Brain Sciences. 1999;22:699–700. [Google Scholar]
- Treisman, M. (1963). Temporal discrimination and the indifference interval: Implications for a model of the “internal clock.” Psychological Monographs, 77 [DOI] [PubMed]
- Treisman M, Faulkner A, Naish PL, Brogan D. The internal clock: Evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception. 1990;19:705–743. doi: 10.1068/p190705. [DOI] [PubMed] [Google Scholar]
- Tyrrell RA, Owens DA. A rapid technique to assess the resting states of the eyes and other threshold phenomena: The Modified Binary Search (MOBS) Behavior Research Methods, Instruments, & Computers. 1988;20:137–141. [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I Predictive visual responses. Journal of Neurophysiology. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Walker MF, Fitzgibbon EJ, Goldberg ME. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. Journal of Neurophysiology. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Edwards H, Fakhri M, Percival A. Why “sounds are judged longer than lights”: Application of a model of the internal clock in humans. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 1998;51B:97–120. doi: 10.1080/713932672. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Haggard P, Heal R, Brown P, Rothwell JCE. Illusory perceptions of space and time preserve cross-saccadic perceptual continuity. Nature. 2001;414:302–305. doi: 10.1038/35104551. [DOI] [PubMed] [Google Scholar]
- Yarrow, K., Haggard, P., & Rothwell, J. C. E. Action, arousal, and subjective time. Submitted for publication. [DOI] [PubMed]
- Yarrow K, Rothwell JCE. Manual chronostasis: Tactile perception precedes physical contact. Current Biology. 2003;13:1334–1339. doi: 10.1016/s0960-9822(03)00413-5. [DOI] [PubMed] [Google Scholar]


