Abstract
A number of insect effector genes activated by the steroid hormone 20-hydroxyecdysone (20E) are dually controlled by the ecdysteroid receptor (EcR/USP) and products of ecdysteroid early responsive genes (E74, E75, and Broad). However, the molecular mechanism of this dual action is poorly understood. Here we examined transcriptional activation of the vitellogenin (Vg) gene in the yellow fever mosquito, Aedes aegypti, by EcR/USP and E74 in response to an elevation of 20E titers. There are two isoforms of the Aedes E74 gene, AaE74A and AaE74B, which have a common C-terminal Ets DNA-binding domain and isoform-specific N termini in the female mosquito. Inhibiting expression of AaE74B but not AaE74A by RNA interference led to substantial reduction in the Vg gene expression. AaE74B and the ecdysteroid receptor synergistically enhanced 20E-induced transcription of the Vg promoter. This action required the E74-binding sites and the ecdysone response elements in the Vg 5′ regulatory region. Two-hybrid assays and coimmunoprecipitation analyses demonstrated direct interaction between AaE74B and AaEcR/AaUSP. Moreover, disruption of this interaction by a dominant negative E74 mutant abolished the enhanced activation of Vg. Therefore, the cooperative interaction between AaE74B and the ecdysteroid receptor is required for high-level expression of the Vg gene in vivo. The synergistic activation is accomplished through their 20E-dependent protein-protein interaction on the gene promoter. This study reveals how the 20E direct-indirect regulation of an effector gene is achieved at the molecular level.
Keywords: Ets transcription factor, mosquito egg maturation, nuclear hormone receptor
The steroid hormone ecdysteroid is a key regulator in the coordination of multiple developmental processes in insects, including embryogenesis, metamorphosis, and reproduction (1-6). The molecular mechanism of 20-hydroxyecdysone (20E) action has been studied extensively during metamorphosis in Drosophila melanogaster (2, 3, 7). Based on the observations of the effects of 20E on puffing patterns in Drosophila polytene chromosomes, Ashburner et al. have proposed a hierarchal model of ecdysteroid action (8). Subsequent molecular studies have confirmed and extended this model (2, 3, 7). Ecdysteroid signaling is mediated by the insect ecdysteroid receptor, which comprises the ecdysone receptor (EcR) and Ultraspiracle (USP) (9-11). The ecdysteroid receptor EcR/USP directly induces several early response genes, including E74, E75, and Board. In turn, products of these early genes activate late effector genes that control stage- and tissue-specific developmental responses to 20E (2, 3).
Although this initial hypothesis has postulated indirect action of the hormone on the effector genes via early gene products, further studies have revealed the existence of a more complex direct-indirect mode of effector gene regulation that involves the EcR/USP and one or several products of early genes (3, 5, 6, 12-15). Mechanistic understanding of how the EcR/USP and an early gene product interact during transcription activation of an effector gene with direct-indirect control is still lacking.
The mosquito vitellogenin (Vg) gene represents an excellent system to address this question. This gene, which encodes the major yolk protein precursor for developing eggs, is highly expressed exclusively in the mosquito female fat body, the metabolic tissue analogous to the vertebrate liver. The expression of this gene is initiated only after the mosquito female feeds on vertebrate blood. Blood feeding triggers the production of 20E, which in turn activates the Vg gene. 20E is required for both the Vg activation and the sustained high level of its expression (5, 6). Because of the intricate link between egg development, blood feeding, and pathogen transmission, detailed understanding of molecular mechanisms governing egg development is essential for future strategies of mosquito control.
Two EcR isoforms (AaEcRa and AaEcRb) and two USP isoforms (AaUSPa and AaUSPb) have been characterized in the mosquito Aedes aegypti (16-18). The mosquito AaEcR/AaUSP binds to the ecdysone response elements (EcRE) located in the Vg promoter and activates low-level expression of this gene (15). The EcR/USP binding activity occurs in fat body nuclei in a pattern correlating well with Vg gene expression (15, 19). Chromatin immunoprecipitation (ChIP) experiments have shown that the AaEcR/AaUSP complex occupies the Vg promoter in vivo shortly after blood feeding (20).
Genetic studies using Drosophila and Aedes transformation have identified a region in the 5′ regulatory region of the Vg gene that contains putative binding sites for E74 and is essential for stage-specific hormonal enhancement of Vg gene expression (14). The protein product of the E74 gene belongs to the Ets domain transcription factor family, which binds to the core DNA motif 5′-GGAA/T-3′ (21). The Aedes E74 gene encodes two isoforms, AaE74A and AaE74B, which have a common C-terminal Ets DNA-binding domain and isoform-specific N termini (22). AaE74B activates the Vg gene in vitro, strongly suggesting that it is a positive regulator of this gene in vivo (23). Furthermore, the DNA binding activities of AaE74B and AaEcR/AaUSP tested with their respective binding sites are temporally similar in the nuclear extract of vitellogenic fat bodies (23).
We report here that a high level of Vg expression requires synergistic action of the EcR/USP and AaE74B through their direct protein-protein interaction on the Vg promoter. This study reveals how the 20E direct-indirect regulation of an effector gene is achieved at the molecular level.
Materials and Methods
Animals. Mosquitoes of the A. aegypti Rockefeller/UGAL strain were used in this study. They were reared as described in ref. 24. Vitellogenesis was initiated by feeding 3- to 5-day-old (postemergence) females on white laboratory rats.
RNA Interference (RNAi) Assay. Synthesis of dsRNAs and microinjection were performed as described in ref. 25. The dsRNA corresponding to the AaE74B isoform-specific region, where no other significant homology was found by blast search (National Center for Biotechnology Information, Bethesda), was prepared by using the HiScribe RNAi Transcription Kit (New England Biolabs) following the manufacturer's instructions. The integrity of the dsRNA was assessed by gel electrophoresis, and concentration was determined by spectrophotometry. Two days after emergence, mosquito females were each microinjected intrathoracically with ≈1 μg of dsRNA. The injected mosquitoes were allowed a period of 2 days for recovery and then were fed on blood. The transcription of each gene was analyzed by real-time PCR as described in ref. 25.
Plasmid Constructs. Three reporter constructs, Vg1071ΔCluster-luciferase (Luc) with mutation of E74 cluster, Vg1071ΔC10-Luc with mutation of C10, and double-mutation construct Vg1071ΔDB-Luc, were generated by using the QuikChange Site-Directed Mutagenesis System (Stratagene) to change the E74 core recognition sequence GGAA to TTAA. These mutations in the E74 binding sites abolished their binding to E74B (data not shown). The EcRE-deletion plasmid, Vg1071Δ EcRE-Luc, was created by insertion of a fragment from the Vg promoter (-1,071 to -618 bp) into a vector VgΔRE1, 2-Luc, described in ref. 15. The serial deletions of Vg 5′ region luciferase reporter constructs Vg2100-Luc, Vg1071-Luc, Vg618-Luc, Vg348-Luc, pAcAaE74B, pAcAaEcR, and pAcAaUSP have been described elsewhere (15, 26). The reporter phRL-CMV (Promega) was used to normalize transfection efficiency.
DNA fragments encoding the isoform-specific region or common region of AaE74B were subcloned into the pM vector (Clontech) to create GAL4-AaE74B fusion proteins. All plasmids were confirmed by restriction digestion and partial sequencing.
Cell Culture and Transient Transfection Assay. Transfection of the Drosophila S2 cell line (Invitrogen) was conducted as described in ref. 15. EcR-deficient D. melanogaster L57-3-11 cell line, generously provided by Lucy Cherbas (Indiana University, Bloomington), was transfected according to instructions of Hu et al. (27). The green African monkey kidney CV-1 cell line (American Type Culture Collection) was used for the mammalian two-hybrid assay and transfected as described elsewhere (25).
Antibodies. Generation of rabbit polyclonal antibodies against AaUSP and AaEcR, as well as against AaE74A and AaE74B, was reported in refs. 20 and 23. The monoclonal antibody against DmUSP (anti-DmUSP) was a gift from Fotis C. Kafatos (European Molecular Biological Laboratories, Heidelberg, Germany).
EMSA. EMSAs for E74 were performed as described in ref. 22. For competition reactions, unlabeled oligonucleotides were added in excess of 50-fold.
Coimmunoprecipitation Assay. In vitro expressed and 35S-labeled proteins or Drosophila S2 cell nuclear extracts were used as protein sources. Expression vectors of AaE74B, AaEcR, and AaUSP were cotransfected into Drosophila S2 cells in the presence or absence of 20E. The nuclear extracts were prepared from the transfected S2 cells as described in ref. 19 and incubated with AaEcR, AaUSP, AaE74A, or AaE74B proteins that were synthesized in vitro and labeled with [35S]methionine. Antibodies against AaEcR, AaE74B, and Drosophila USP were used for this analysis. Assays were performed by using the Immunoprecipitation Kit (Roche Applied Science, Indianapolis) following the manufacturer's instructions. The resulting immune complexes were precipitated by addition of protein A-agarose beads. After extensive washing, the complexes were fractionated by SDS/PAGE and subjected to autoradiography. When the experiment was conducted with 20E, 20E was added into the binding and washing buffers.
Results
Functional Analysis of AaE74 during Vitellogenesis. The two AaE74 isoforms show distinct expression profiles after blood ingestion in mosquitoes, implying that AaE74B is a potential activator of Vg gene expression whereas AaE74A is a negative regulator (22, 23). To elucidate the physiological functions of the two AaE74 isoforms in vivo, we used RNAi to attenuate expression of the AaE74 isoforms. dsRNA, corresponding to the isoform-specific region of either AaE74A or AaE74B, was injected into the thorax of female mosquitoes 2 days postemergence. In parallel, dsRNA complementary to the bacterial malE gene was used as a negative control. After a 2-day recovery, these mosquitoes were given a blood meal, and the fat bodies were subsequently collected at various time points and subjected to the analysis of gene expression.
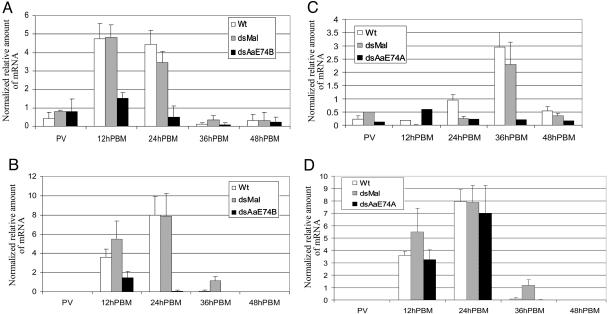
In the AaE74B RNAi mosquitoes, the mRNA levels of AaE74B declined dramatically at 12 h and 24 h postblood meal (PBM) compared with the uninjected (WT) and malE (dsMal) dsRNA-injected female adults (Fig. 1A). Significantly, a substantial reduction in the expression of the Vg gene was observed in AaE74B RNAi mosquitoes at 12 h and 24 h PBM (Fig. 1B). In control mosquitoes injected with malE dsRNA, expression profiles of AaE74B and Vg were similar to those in the untreated mosquitoes. These experiments indicated that AaE74B expression was effectively suppressed by RNAi, which resulted in virtual shutdown of Vg gene expression. We concluded that AaE74B is required for activation and sustained expression of this gene in vivo.
Fig. 1.
AaE74 RNAi assay. dsRNAs of AaE74B or AaE74A were injected into thoraces of female mosquitoes as described in Materials and Methods. mRNA expression was measured by real-time PCR at the indicated time points after blood feeding. Each sample was analyzed in triplicate and normalized to the internal control, β-actin mRNA (25). (A) AaE74B mRNA levels in mosquitoes injected with AaE74B dsRNA. (B) Vg mRNA levels in AaE74B RNAi mosquitoes. (C) AaE74A mRNA levels in AaE74A RNAi mosquitoes. (D) Vg mRNA levels in mosquitoes injected with AaE74A dsRNA. Wt, uninjected A. aegypti Rockefeller/UGAL strain; dsMal, injected with dsRNA complementary to bacterial malE; dsAaE74B, injected with AaE74B dsRNA; dsAaE74A, injected with AaE74A dsRNA; PV, previtellogenic.
In mosquitoes injected with the AaE74A-specific dsRNA, the levels of AaE74A mRNA showed dramatic reduction at 36 h PBM when the AaE74A mRNA level was normally high in the untreated mosquitoes. Such a reduction in the AaE74A mRNA level was not observed in malE dsRNA-injected females (Fig. 1C). However, RNAi-dependent silencing of AaE74A expression exhibited no effect on Vg gene expression at 12 h and 24 h PBM (Fig. 1D). From these reverse-genetic analyses, it appeared that AaE74A was not required for the expression of the Vg gene. Therefore, we focused on investigating the role of AaE74B in Vg gene expression.
Characterization of E74-Binding Sites in the Vg 5′ Regulatory Region. To determine whether the Vg gene is a direct target of E74, we studied transcriptional activation of the Vg promoter by E74B by using a transient transfection assay. Four luciferase reporter constructs, Vg2100-Luc, Vg1071-Luc, Vg618-Luc, and Vg348-Luc, were analyzed in Drosophila Schneider 2 cells (S2). Each construct contains progressive deletions of the 5′ regulatory region of Vg gene (Fig. 6 A and B, which is published as supporting information on the PNAS web site). The results indicated that the region from -618 bp to -1,071 bp is critical for the regulation of Vg by AaE74B.
Sequence analysis of the 2.1-kb Vg 5′ regulatory region revealed 10 potential E74 binding sites, named C1-C10. The EMSA competition assay showed that the order of binding ability to AaE74B is C10, C5 > C2, C3, C4, C9 > C1, C6, C7, and C8 (Fig. 6C). The binding sites C3, C4, and C5 form a cluster ≈50 bp long located at -963 bp to -912 bp and were designated the E74 cluster. It is worth noting that both the E74 cluster and C10 are located in the median region of the Vg promoter (-1,071 bp to -618 bp), which is essential for E74B-dependent transcriptional activation in cell transfection experiments.
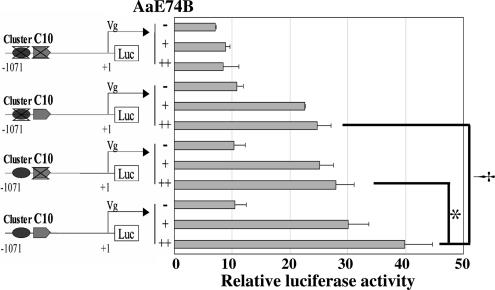
To further investigate the function of the putative E74-binding sites in the median region of the Vg promoter, we made three derivative reporter constructs: Vg1071Δcluster-Luc with mutation of the E74 cluster, Vg1071ΔC10-Luc with mutation of the C10 site, and Vg1071ΔDB-Luc with mutations of both the E74 cluster and the C10 site. In S2 cells cotransfected with the AaE74B expression vector, both Vg1071Δcluster-Luc and Vg1071ΔC10-Luc displayed significantly reduced transcription in comparison with the intact construct Vg1071-Luc (Fig. 2). Transactivation by E74 was abolished in the assay with the double-mutation construct Vg1071ΔDB-Luc. Collectively, these experiments indicated that transactivation of the Vg gene by E74B required both the E74 cluster and the C10 binding site in the Vg 5′ regulatory region.
Fig. 2.
The E74 cluster and C10 are required for transcriptional activation of the Vg promoter by AaE74B. A schematic diagram of the deletion reporter constructs is depicted in Left. Drosophila S2 cells were transfected with 100 ng of phRL-CMV (Renilla luciferase control vector), 100 ng of different reporter plasmids, and expression vector pAcAaE74B. Transfections without the pAcAaE74B are indicated as “-,” and those with increasing amounts of AaE74B are indicated as “+” (50 ng) and “++” (100 ng). Data in Right represent the ratios of firefly luciferase to Renilla luciferase activity (relative luciferase activity). Significant differences (t test) between single deletion and intact reporter constructs are indicated (* and †, P < 0.05). Values are means of triplicate independent transfection experiments, with error bars representing the SD of the mean.
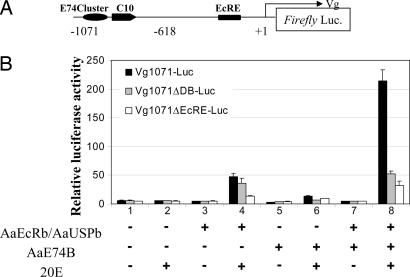
Synergistic Activation of Vg Expression by AaE74B and AaEcR/AaUSP. A functional ecdysteroid response element (VgEcRE) in the Vg 5′ regulatory region was identified as necessary to elicit transcriptional induction by 20E (15). Moreover, the VgEcRE was shown to be occupied by the AaEcR/AaUSP complex in fat body nuclei in the early vitellogenic stage (3-12 h PBM) (20). Because the functional AaE74B protein was also observed at this stage (23), AaE74B might act in concert with AaEcR/AaUSP on the Vg promoter. This possibility was explored with a transient transfection assay. EcR-deficient D. melanogaster L57-3-11 cells transfected with the Vg1071-Luc reporter (Fig. 3A) construct alone did not respond to 10-6 M 20E (Fig. 3B, lanes 1 and 2). AaEcR/AaUSP up-regulated the reporter activity by ≈9-fold in response to 20E treatment (Fig. 3B, lanes 3 and 4). Similarly, transcriptional activity of the Vg promoter was induced 4-fold by cotransfection with AaE74B (Fig. 3B, lanes 5 and 6). Importantly, cotransfection of AaE74B together with AaEcR/AaUSP resulted in 42-fold activation of the Vg promoter after addition of 20E (Fig. 3B, lanes 7 and 8).
Fig. 3.
AaE74B acts synergistically with AaEcR/AaUSP in activating the Vg promoter. (A) Schematic illustration of the luciferase reporter construct Vg1071-Luc. (B) Activation of the Vg promoter by AaEcR/AaUSP and AaE74B. Drosophila L57-3-11 cells were transfected by expression vectors as indicated, together with different reporter constructs: Vg1071-Luc, Vg1071ΔDB-Luc, and Vg1071ΔEcRE-Luc. Vg1071ΔDB-Luc denotes a Vg1071-Luc derivative with mutations in the E74 cluster and C10, and Vg1071ΔEcRE-Luc represents a Vg1071-Luc derivative that lacks the functional EcRE as identified in ref. 15. After transfection, cells were cultured in medium with or without 1 × 10-6 M 20E. Data represent ratios of firefly luciferase to Renilla luciferase activity (relative luciferase activity). Shown are mean values ± SD from three independent transfection experiments.
To evaluate whether the E74 binding sites (E74 cluster and C10) and VgEcRE in the Vg promoter are indispensable for this synergistic activation, we used two reporter constructs: Vg1071ΔDB-Luc and Vg1071ΔEcRE-Luc with mutations of the E74 binding sites (precise base pair changes in E74 cluster and C10) or VgEcRE, respectively. The cotransfection of AaE74B and AaEcR/AaUSP led to no synergistic induction with either of these reporter constructs (Fig. 3B, lane 8). Thus, both the E74 binding sites and VgEcRE were required for robust activation of the Vg gene by AaE74B and AaEcR/AaUSP.
Protein-Protein Interaction of AaE74B with AaEcR/AaUSP. To examine whether AaE74B directly interacts with AaEcR/AaUSP during transactivation of the Vg gene, we used coimmunoprecipitation and two-hybrid analyses.
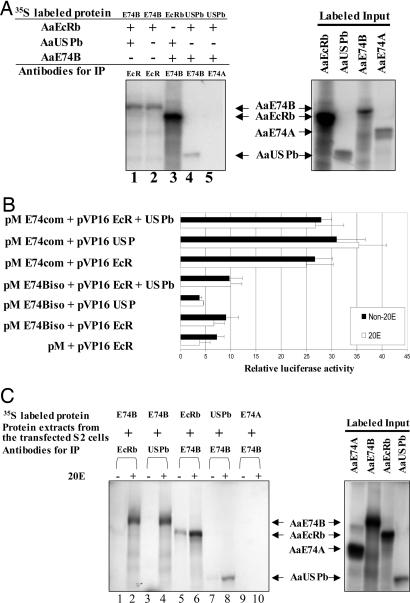
In the first series of coimmunoprecipitation experiments, we used in vitro synthesized and labeled AaE74B, AaE74A, AaEcR, and AaUSP proteins. The proteins were mixed in pair-wise combinations, incubated in the presence or absence of 20E, and precipitated with the antibodies specific to AaE74B, AaEcR, or AaUSP. The results indicated that AaE74B bound either AaEcR or AaUSP in the absence of 20E (Fig. 4A).
Fig. 4.
Association of AaE74B with AaEcR and AaUSP. (A) Proteins were synthesized and were labeled by using the TnT Coupled Transcription/Translation System. These proteins were then incubated for 4 h in the presence or absence of 1 × 10-6 M 20E, followed by immunoprecipitation with the indicated antibodies. The resulting immune complexes were precipitated by addition of protein A-agarose beads. After extensive washing, the complexes were analyzed by SDS/PAGE and autoradiography. (B) Interaction of AaE74B with AaEcR and AaUSP in mammalian two-hybrid assay. CV-1 cells were cotransfected with control reporter pCMV-Luc and reporter construct pFR-Luc together with indicated plasmids. Luciferase activity was normalized with Renilla luciferase activity. The results represent the mean values obtained from three independent experiments. (C) Drosophila S2 cells were cotransfected collectively by vectors for AaE74B, AaEcRb, and AaUSPb and cultured in the presence or absence of 20E. Nuclear extracts prepared from the transfected cells were incubated with [35S]methionine-labeled AaEcRb, AaUSPb, or AaE74B, followed by immunoprecipitation.
To further investigate the interaction of AaE74B with AaEcR and AaUSP, we performed two-hybrid analysis in CV1 cells, which have low levels of RXR (the USP homolog) but no endogenous E74, EcR, or USP protein. The N-terminal AaE74B isoform-specific region (E74Biso) or the C-terminal region common for both E74 isoforms (E74com) was fused to the GAL4 DNA binding domain (DBD). Conversely, AaEcR and AaUSP truncated forms (pV16EcR and pV16USP) containing the DNA-binding and ligand-binding domains (domains C-F) were expressed as fusions to the acidic activation domain of the herpes simplex virus transcriptional activator VP16. No interaction was observed between the AaE74B isoform-specific region and EcR or USP (Fig. 4B). However, the reporter activity increased significantly when pM-E74com was expressed together with either pV16EcR or pV16USP, suggesting that the C-terminal region of E74B was required for protein-protein contact with EcR and USP. Most importantly, the interaction between E74B and AaEcR was not affected by either the addition of full-length AaUSP or the presence of 20E (Fig. 4B).
We also studied the interactions of AaE74B, AaEcRb, and AaUSPb with proteins expressed in Drosophila S2 cells. AaE74B, AaE74A, AaEcRb, and AaUSPb proteins were in vitro-synthesized and labeled with [35S]methionine. The labeled proteins were incubated individually with nuclear extract from S2 cells cotransfected concurrently with AaE74B, AaEcRb, and AaUSPb expression plasmids, followed by immunoprecipitation with the antibodies specific to AaEcR, AaUSP, or AaE74B (Fig. 4C). The AaEcR and AaUSP antibodies precipitated radioactively labeled AaE74B in the presence of 20E but not without 20E (Fig. 4C, lanes 1-4). Similarly, AaE74B was associated with AaUSPb only in the presence of 20E (Fig. 4C, lanes 7 and 8). A low level of AaEcRb was precipitated by AaE74B antibodies in the absence of 20E (Fig. 4C, lane 5). However, the intensity of precipitated AaEcRb was enhanced dramatically after the addition of 20E (Fig. 4C, lane 6). AaE74A could not be recognized by the AaE74B antibody, indicating that the interactions were specific (Fig. 4C, lanes 9 and 10). We concluded that in S2 cells AaE74B specifically interacted with AaEcR/AaUSP in a 20E-dependent manner. This difference in hormonal dependence suggested that the interaction between E74B and EcR/USP in vivo requires participation of accessory factors and/or posttranslational modifications of the E74B, EcR, and USP proteins.
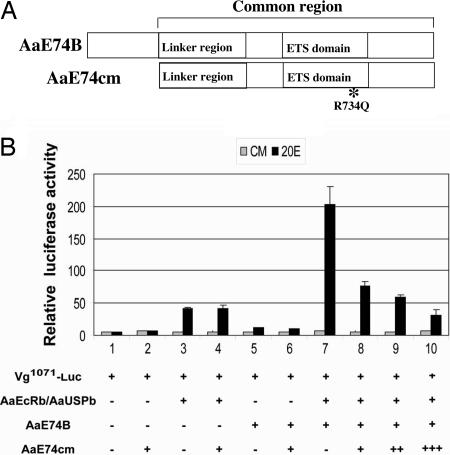
To test whether the protein-protein interaction between E74 and EcR/USP is essential for the synergistic activation of the Vg promoter in the response to 20E, we constructed a mutant of E74B (E74cm) that covered the common region of E74B and contained a single amino acid residue substitution (R734Q) (Fig. 5A). This arginine residue in the ETS domain has been shown to be critical for DNA binding (28). As shown in Figs. 7 and 8, which are published as supporting information on the PNAS web site, the E74B protein with the R734Q mutation failed to recognize its cognate DNA-binding sequence, whereas E74cm retained most of its binding to either EcR or USP. Coexpression of E74cm showed no significant effects on activation of the Vg promoter by either E74B or EcR/USP (Fig. 5B). However, the activation carried out in synergy by EcR/USP and E74B was indeed inhibited by E74cm in a dose-dependent manner, presumably through disrupting the association of EcR/USP and E74B.
Fig. 5.
Protein interaction between E74 and EcR/USP is essential for their synergistic activation of the Vg promoter. (A) Schematic illustration of the E74B mutant. (B) Drosophila L57-3-11 cells were transfected with indicated expression plasmids. E74cm represented expression vector for dominant negative E74B mutant. After transfection, cells were cultured in medium with or without 1 × 10-6 M 20E. Data represent ratios of firefly luciferase to Renilla luciferase activity (relative luciferase activity), and values shown are the mean of three independent experiments, with error bars representing the SD of the mean.
Discussion
The regulation of many effector genes is under the direct-indirect control of 20E that involves the EcR/USP and one or several products of ecdysone early responsive genes (3, 5, 6, 12-14). These types of controls not only amplify the magnitude of hormonal signals but also provide flexibility to modulate timing, duration, and scale-of-target gene expression in response to a 20E signal. In this work, we used the Vg gene from the mosquito A. aegypti to elucidate how the 20E direct-indirect regulation of an effector gene was achieved. We showed here that high-level expression of the Vg gene requires synergistic action of the EcR/USP and an early gene product AaE74B through their direct protein-protein interaction on the Vg promoter.
Posttranscriptional gene silencing by dsRNA has been used successfully in mosquitoes (25, 29, 30). Microinjection of AaE74B dsRNA into previtellogenic female mosquitoes resulted in attenuation of the Vg gene expression after blood ingestion, indicating that AaE74B is essential for transcription of the Vg gene in vivo. Using a cell transfection assay, we demonstrated that activation of the Vg gene by AaE74B required the median region (-1,071 bp to -618 bp) of the Vg promoter. The importance of this region for the 20E-dependent enhancement of Vg gene expression is substantiated by a previous study employing Drosophila and Aedes transformation (14). Furthermore, cell transfection experiments clearly showed that AaE74B and AaEcR/AaUSP cooperatively activate transcription of the Vg promoter and that their synergistic activity requires their respective binding sites in the 5′ regulatory region of the gene.
In mosquito A. aegypti, formation of EcR/USP heterodimer appears shortly after blood feeding. The E74B gene, in turn, is activated in fat body by the rising titer of 20E. E74B protein peaks at ≈6 h PBM, when the rate of Vg expression increases dramatically. Moreover, synergistic action of AaE74B and AaEcR/AaUSP was also observed when ectopic expression of these transcription factors activated, albeit weakly, the endogenous Vg gene in response to 20E in a mosquito larval cell line, in which this female and stage-specific gene was normally repressed (G.S. and A.S.R., unpublished observation). Together, these results lend support to our previous hypothesis that the EcR/USP heterodimer is the developmental timer controlling the switch-on of the tissue- and stage-specific 20E-response genes, whereas their full expression requires the participation of additional transcriptional regulators, including products of the ecdysone early genes (20).
Combinatorial control is a characteristic property of Ets family transcription factors to which E74 belongs. Ets proteins interact with other key transcription factors, such as GATA and nuclear receptors, and coordinate many cellular events by either synergistic transactivation or reciprocal repression of the target genes (31, 32). For example, the transcription factor Ets-1 can cooperate with several nuclear receptors, such as the vitamin D receptor, the estrogen receptor, and the peroxisome proliferator-activated receptor α, and it can induce a conformational change in the nuclear receptor, creating an active interaction surface with coactivators (33). In our study, the AaE74cm protein abolishes the cooperative activation of the Vg promoter by E74B and EcR/USP, but it does not show clear effects on activation by E74B or EcR/USP alone. It is most likely that, in addition to cofactors interacting with E74 or EcR/USP alone, E74B and EcR/USP interaction leads to recruitment of supplementary coactivators required for high-level expression of an effector gene.
Despite the fact that E74A and E74B share the common protein region required for both DNA binding and interaction with EcR/USP, E74A does not affect transcriptional activation of the Vg promoter either in vivo or in vitro. This finding implies that the isoform-specific N-terminal regions are major determinants of the transactivation activity of E74 and that they are targets for protein interactions and posttranslational modifications.
The coimmunoprecipitation analysis presented in this study has established that AaE74B physically interacts with the AaEcR/AaUSP complex in vitro in a 20E-independent manner. This kind of interaction is not without precedent. Tolon et al. (33) have reported that, through physical interaction with Ets-1, the vitamin D receptor changes from a normal ligand-dependent transcriptional activator to a ligand-independent one and that this direct interaction per se is also ligand-independent. Likewise, Ets-1 confers the ligand-independent feature to other nuclear receptors such as estrogen receptor and peroxisome proliferator-activated receptor α (33). In contrast, AaEcRb and AaUSPb expressed in Drosophila S2 cells bind AaE74B weakly in the absence of 20E, whereas this association is enhanced by the presence of 20E. This discrepancy in hormonal dependence suggests that additional signaling transduction and unknown auxiliary factors may be implicated in mediating the interaction between E74B and EcR/USP in vivo. It is of great importance to elucidate how 20E modulates this specific protein-protein interaction in the future.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant AI-36959 (to A.S.R.).
Author contributions: G.S., J.Z., and A.S.R. designed research; G.S., J.Z., and L.C. performed research; G.S., J.Z., and L.C. analyzed data; and G.S., J.Z., and A.S.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 20E, 20-hydroxyecdysone; dsMal, malE dsRNA-injected female adult A. aegypti; EcR, ecdysone receptor; EcRE, ecdysone response elements; PBM, postblood meal; RNAi, RNA interference; USP, Ultraspiracle.
References
- 1.Riddiford, L. M. (1993) in The Development of Drosophila melanogaster, eds. Bate, M. & Martinez-Arias, A. (Cold Spring Harbor Lab. Press, Cold Spring Harbor, NY), pp. 899-939.
- 2.Thummel, C. S. (1996) Trends Genet. 12, 306-310. [DOI] [PubMed] [Google Scholar]
- 3.Thummel, C. S. (2002) Insect Biochem. Mol. Biol. 32, 113-120. [DOI] [PubMed] [Google Scholar]
- 4.Kozlova T. & Thummel, C. S. (2003) Science 301, 1911-1914. [DOI] [PubMed] [Google Scholar]
- 5.Raikhel, A. S., Kokoza, V. A., Zhu, J. S., Martin, D., Wang, S. F., Li, C., Sun, G. Q., Ahmed, A., Dittmer, N. & Attardo, G. (2002) Insect Biochem. Mol. Biol. 32, 1275-1286. [DOI] [PubMed] [Google Scholar]
- 6.Raikhel, A. S., McGurk, L. & Bownes, M. (2003) in Encyclopedia of Hormones, eds. Henry, H. L. & Norman, A. W. (Academic, San Diego), Vol. 1, pp. 451-459. [Google Scholar]
- 7.Bender, M. (2003) in Encyclopedia of Hormones, eds. Henry, H. L. & Norman, A. W. (Academic, San Diego), Vol. 1, pp. 438-446. [Google Scholar]
- 8.Ashburner, M., Chihara, C., Meltzer, P. & Richards, G. (1974) Cold Spring Harbor Symp. Quant. Biol. 38, 655-662. [DOI] [PubMed] [Google Scholar]
- 9.Yao, T. P., Segraves, W. A., Oro, A. E., McKeown, M. & Evans, R. M. (1992) Cell 71, 63-72. [DOI] [PubMed] [Google Scholar]
- 10.Yao, T. P., Forman, B. M., Jiang, Z., Cherbas, L., Chen, J. D., McKeown, M., Cherbas, P. & Evans, R. M. (1993) Nature 366, 476-479. [DOI] [PubMed] [Google Scholar]
- 11.Thomas, H. E., Stunnenberg, H. G. & Stewart, A. F. (1993) Nature 362, 471-475. [DOI] [PubMed] [Google Scholar]
- 12.Antoniewski, C., Laval, M., Dahan, A. & Lepesant, J. A. (1994) Mol. Cell. Biol. 14, 4465-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann, M. & Korge, G. (1995) EMBO J. 14, 716-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokoza, V. A., Martin, D., Mienaltowski, M. J., Ahmed, A., Morton, C. M. & Raikhel, A. S. (2001) Gene 274, 47-65. [DOI] [PubMed] [Google Scholar]
- 15.Martin, D., Wang, S. F. & Raikhel, A. S. (2001) Mol. Cell. Endocrinol. 173, 75-86. [DOI] [PubMed] [Google Scholar]
- 16.Kapitskaya, M., Wang, S., Cress, D. E., Dhadialla, T. S. & Raikhel, A. S. (1996) Mol. Cell. Endocrinol. 121, 119-132. [DOI] [PubMed] [Google Scholar]
- 17.Wang, S. F., Li, C., Zhu, J., Miura, K., Miksicek, R. J. & Raikhel, A. S. (2000) Dev. Biol. 218, 99-113. [DOI] [PubMed] [Google Scholar]
- 18.Wang, S. F., Li, C., Sun, G., Zhu, J. & Raikhel, A. S. (2002) Mol. Cell. Endocrinol. 196, 29-42. [DOI] [PubMed] [Google Scholar]
- 19.Miura, K., Wang, S. F. & Raikhel, A. S. (1999) Mol. Cell. Endocrinol. 156, 111-120. [DOI] [PubMed] [Google Scholar]
- 20.Zhu, J., Miura, K., Chen, L. & Raikhel, A. S. (2003) Proc. Natl. Acad. Sci. USA 100, 544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urness, L. D. & Thummel, C. S. (1995) EMBO J. 14, 6239-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, G. Q., Zhu, J. S., Li, C., Tu, Z. J. & Raikhel, A. S. (2002) Mol. Cell. Endocrinol. 190, 147-157. [DOI] [PubMed] [Google Scholar]
- 23.Sun, G. Q., Zhu, J. S., Li, C. & Raikhel, A. S. (2004) Mol. Cell. Endocrinol. 218, 95-105. [DOI] [PubMed] [Google Scholar]
- 24.Hays, A. R. & Raikhel, A. S. (1990) Roux's Arch. Dev. Biol. 199, 114-121. [DOI] [PubMed] [Google Scholar]
- 25.Zhu, J., Chen, L. & Raikhel, A. S. (2003) Proc. Natl. Acad. Sci. USA 100, 13338-13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, S. F., Miura, K., Miksicek, R. J., Segraves, W.A. & Raikhel, A. S. (1998) J. Biol. Chem. 273, 27531-27540. [DOI] [PubMed] [Google Scholar]
- 27.Hu, X., Cherbas, L. & Cherbas, P. (2003) Mol. Endocrinol. 17, 716-731. [DOI] [PubMed] [Google Scholar]
- 28.Miley, G. R., Fantz, D., Glossip, D., Lu, X., Saito, R. M., Palmer, R. E., Inoue, T., Van Den Heuvel, S., Sternberg, P. W. & Kornfeld, K. (2004) Genetics 167, 1697-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blandin, S., Moita, L. F., Kocher, T., Wilm, M., Kafatos, F. C. & Levashina, E. A. (2002) EMBO Rep. 3, 852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen, I. A., Attardo, G. M., Park, J.-H., Peng, Q. & Raikhel, A. S. (2004) Proc. Natl. Acad. Sci. USA 101, 10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, R., Pei, H. & Watson, D. K. (2000) Oncogene 19, 6514-6523. [DOI] [PubMed] [Google Scholar]
- 32.Darby, T. G., Meissner, J. D., Ruhlmann, A., Mueller, W. H. & Scheibe, R. J. (1997) Oncogene 15, 3067-3082. [DOI] [PubMed] [Google Scholar]
- 33.Tolon, R. M., Castillo, A. I., Jimenez-Lara, A. M. & Aranda, A. (2000) Mol. Cell. Biol. 20, 8793-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.