Abstract
Many groups of insects are specialists in exploiting sensory cues to locate food resources or conspecifics. To achieve orientation, bees and ants analyze the polarization pattern of the sky, male moths orient along the females' odor plume, and cicadas, grasshoppers, and crickets use acoustic signals to locate singing conspecifics. In comparison with olfactory and visual orientation, where learning is involved, auditory processing underlying orientation in insects appears to be more hardwired and genetically determined. In each of these examples, however, orientation requires a recognition process identifying the crucial sensory pattern to interact with a localization process directing the animal's locomotor activity. Here, we characterize this interaction. Using a sensitive trackball system, we show that, during cricket auditory behavior, the recognition process that is tuned toward the species-specific song pattern controls the amplitude of auditory evoked steering responses. Females perform small reactive steering movements toward any sound patterns. Hearing the male's calling song increases the gain of auditory steering within 2–5 s, and the animals even steer toward nonattractive sound patterns inserted into the speciesspecific pattern. This gain control mechanism in the auditory-to-motor pathway allows crickets to pursue species-specific sound patterns temporarily corrupted by environmental factors and may reflect the organization of recognition and localization networks in insects.
Keywords: localization, phonotaxis
Orientation in a complex environment is a challenging computational task, especially for insects with small nervous systems (1, 2). Different groups have evolved to become successful specialists in foraging and mate attraction (3) by adapting their sensory structures (4), specializing and tuning central neural processing (5), and/or modifying their behavioral strategies (6). The use of acoustic signals between mates is widespread among cicadas and Orthoptera (7, 8). The mates must both recognize and localize the species-specific sound pattern as they home in on the sound source (9). One model system to study this auditory behavior, which is called phonotaxis, is the female cricket. The animals walk or fly toward singing males, attracted by the temporal structure of their calling song (10). During phonotaxis, the females make rapid (latency, 55–60 ms), reflexlike steering responses toward individual sound pulses (11–13). Proposed models of pattern recognition (7, 14, 15) are too slow to be directly involved in the steering process. To understand how the recognition process interacts with localization, we analyzed the dynamics of female phonotactic walking by using a highly sensitive trackball system. We demonstrate that recognition of the species-specific song increases the gain of nonselective auditory steering and thus transiently modulates the auditory responsiveness of the animal.
Materials and Methods
Methods were described in detail in refs. 12 and 13 and are only briefly summarized.
Animals. Female crickets were isolated as last instars from our colony in the Department of Zoology at the University of Cambridge and raised individually. The tethered animals walked on a trackball under open-loop conditions while their body position and orientation remained the same during phonotaxis. Experiments were performed in the dark at 24–28°C. The trackball movements were continuously monitored and revealed the animals' walking and steering velocity.
Trackball. The trackball (56.5-mm diameter, 3.0-g weight), made from Rohacell 31 (Röhm, Darmstadt, Germany), was fitted into a transparent half-sphere, drilled with 24 evenly spaced holes, and mounted on a small cylinder. A constant air stream was passed through the cylinder to gently lift the trackball so that it was free to rotate. A 2D optical mouse sensor (ADNS-2051, Agilent Technologies, Palo Alto, CA) was positioned underneath the trackball. The light of the sensor was reflected off the ball onto the sensor that monitored forward/backward and left/right movements. The optical sensor and electronic circuitry generated brief coding pulses for every movement increment of 127 μm. These pulses were sampled online at 10 kHz per channel and transferred directly onto a computer through an A/D board (National Instruments PCI-Mio16-E-4). The frequency of coding pulses that could be resolved was from 0 to 3 kHz, corresponding to velocities of 0–38 cm/s.
Analysis. Data analysis was performed offline by using custommade software. The lateral deviation of the walking cricket, i.e., the deviation from a straight line, was integrated from the left/right lateral steering velocities. The translation velocity of the walking cricket was calculated from forward/backward and left/right movements of the trackball. For the tuning curves, we calculated the mean lateral deviation per chirp, i.e., how much the animal deviated from a straight line for each pattern.
Acoustic Stimulation. Acoustic stimuli were presented from two speakers (SRS A57, Sony, Tokyo) positioned 87 cm from the cricket at 45° from its longitudinal axis. All acoustic stimuli were 4.8 kHz, 75 dB sound pressure level (spl) rms relative to 20 μPa as measured with a half-inch microphone (type 4191, Brüel & Kjaer Instruments, Naerum, Denmark) and measuring amplifier (type 2610). All sound pulses had a rising and falling ramp of 2 ms. Sound was presented for 30s from the left and right speaker, and the responses of the animals were recorded. We tested the animals' response toward a series of sound patterns with 50% duty cycle with increasing syllable periods (SPs); a standard pattern used in previous experiments to analyze phonotactic tuning in crickets (10). Test pulses demonstrating the decay in auditory responsiveness were 100 ms long and repeated at 2 Hz.
Results
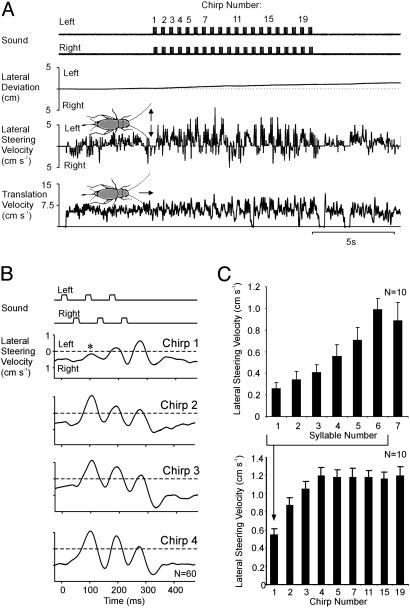
Steering at the Start of Phonotaxis. Male crickets (Gryllus bimaculatus) produce a species-specific calling song by rubbing their front wings together. Their songs consist of sound pulses, also called syllables, with three to five pulses (21 ms long with intervals of 21 ms) grouped into chirps, which are repeated at 2–3 Hz (8, 10). We used the females' rapid steering response to individual sound pulses as a measure of phonotaxis. First, we asked how recognition and steering are activated at the start of a song. Crickets were repeatedly exposed to a 10-s song sequence of 75 dB spl in which consecutive sound pulses of the chirps were presented from opposite sides (split-song paradigm; refs. 10 and 12), and the evoked steering responses toward individual pulses were analyzed (Fig. 1). The females made fast steering movements to the left and then to the right speaker in response to the split-song paradigm (Fig. 1 A and B). The net result was that, overall, they walked straight forward or had a slight bias to the side of the first sound pulse in the chirp (Fig. 1 A Top). Walking velocity varied at ≈5 cm·s–1 (Fig. 1 A Bottom). Averaging the steering velocity to all first, second, etc., chirps of the split-song paradigm demonstrated that small steering movements were made even toward the first syllable of the first chirp (Fig. 1B; asterisk). A recognition process based on the species-specific pattern could not have influenced this response so that at least weak auditory steering is present independent of pattern recognition. During the first chirp, steering became larger from pulse to pulse. Steering then further increased over the next three chirps and reached its maximum at the fourth chirp (Fig. 1B Bottom). We pooled the data from 10 crickets to reveal the dynamics of the steering response to the first seven sound pulses (Fig. 1C Upper), the dynamics for all chirps (Fig. 1C Lower) and the overall distance walked during a chirp (data not shown). From the first pulse to the sixth and last pulse of the first chirp, the steering velocity increased by 281% from 2.6 ± 0.6 mm·s–1 to 9.9 ± 1.1 mm·s–1 (mean ± SEM). The steering velocity remained at an elevated level (8.9 ± 1.7 mm·s–1) for the seventh pulse, i.e., the first pulse of the second chirp occurring after an interval of 250 ms (Fig. 1C Upper). The mean steering velocity to all pulses of a chirp increased by 120% from 5.4 ± 0.7 mm·s–1 to 11.9 ± 0.7 mm·s–1 over the first four chirps and then remained almost constant (Fig. 1C Lower). In the same period, the distance walked increased only by 20% from 17.4 ± 1.0 mm per chirp to 20.8 ± 1.3 mm per chirp from the first to the second chirp and then remained constant. These results demonstrate that the steering response was strongly enhanced within 2 s of the onset of the song, and this enhancement was maintained during the chirp intervals when no sound was presented. We assumed that the enhancement of auditory steering was coupled with pattern recognition.
Fig. 1.
Onset dynamics of pattern recognition and steering analyzed with a split-song paradigm. (A) The velocity of lateral steering increased with the beginning of the split-song and revealed steering movements toward the left and right side. The path of the cricket deviated slightly to the left, the side from which the first pulse of each chirp was presented. (B) The onset dynamics of pattern recognition and steering was revealed by averaging the response (n = 60) to the first four chirps. Crickets steered toward the first pulse of the first chirp (asterisk). (C Upper) The histogram reveals that steering increased during the first seven pulses presented. (Lower) The average lateral steering velocity for selected chirps of the sound pattern. The response increased from the first to the fourth chirp and then remained constant. Error bars indicate the SEM.
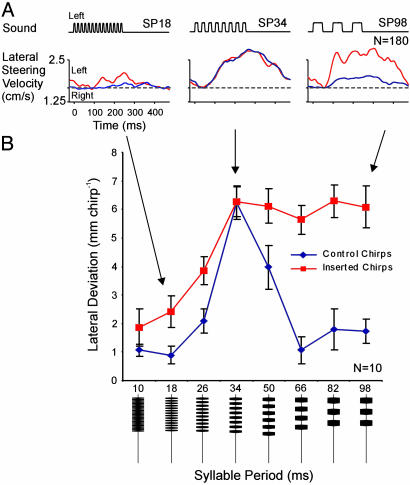
Temporal Selectivity During Phonotaxis. To analyze further the interaction between pattern recognition and steering, we combined species-specific chirps that elicit phonotaxis with nonattractive chirps. We presented sound patterns with different SPs (see the lower part of Fig. 2B) and averaged the steering velocity evoked by chirps with different SPs (Fig. 2 A, blue trace). The strongest response was elicited by sound patterns of SP34 (17-ms pulse and 17-ms interval) closely resembling the natural song. Short (SP18, 9-ms pulse, 9-ms interval) and long SPs (SP98, 49-ms pulse, 49-ms interval) also elicited some small-amplitude steering. Pooling the data for 10 crickets showed that the tuning curve of phonotaxis based on the amplitude of steering was sharply tuned to SP34 (Fig. 2B, blue trace) as described in ref. 10. Steering responses of 15–25% of the peak response also occurred toward SP10 and SP98 but always remained small. We then presented the same crickets with a series of attractive SP34 chirps and replaced every fourth chirp with a nonattractive chirp covering the range of all SP patterns. If chirps of SP50–SP98 were inserted, the steering response toward the inserted chirps increased by 400% to the same level as the response to SP34 chirps (Fig. 2 A and right half of B, red traces). Inserted chirps with SP10–SP26 also caused an increase (80–120%), although it was less dramatic (Fig. 2 A and left half of B, red traces). This result demonstrates that the activation of pattern recognition has a considerable impact on the cricket's auditory responsiveness. The animal's temporal selectivity broadens, and it steers toward inserted SP patterns that it would normally ignore (e.g., SP66).
Fig. 2.
Change in tuning of cricket steering during phonotaxis. Steering responses were analyzed toward series of chirps with different SPs (see bottom of figure). (A, blue lines) Crickets steered toward species-specific SP34 chirps but showed only minor responses toward nonattractive chirps with either short (18-ms) or long (98-ms) SP that did not correspond to the normal song. (A, red lines) Steering behavior toward the same nonattractive chirps when these were inserted into a sequence of cricket song. The animals made increased steering responses toward SP18 and SP98 chirps (steering velocity averaged for 180 chirps for blue and red curves, respectively). (B, blue lines) Tuning of cricket phonotactic steering toward chirps with different SP when these were presented on their own. Phonotaxis is tuned to SP34 and animals do not respond toward chirps with short or long SPs. (B, red lines) Tuning of phonotactic steering toward nonattractive chirps inserted into a sequence of SP34 chirps. Crickets steered toward chirps with SP of 50–98 ms with the same magnitude as to attractive SP34 chirps. Steering increased less intensely toward inserted chirps with SP of 10–26 ms. Data are pooled from 10 animals, error bars indicate the SEM.
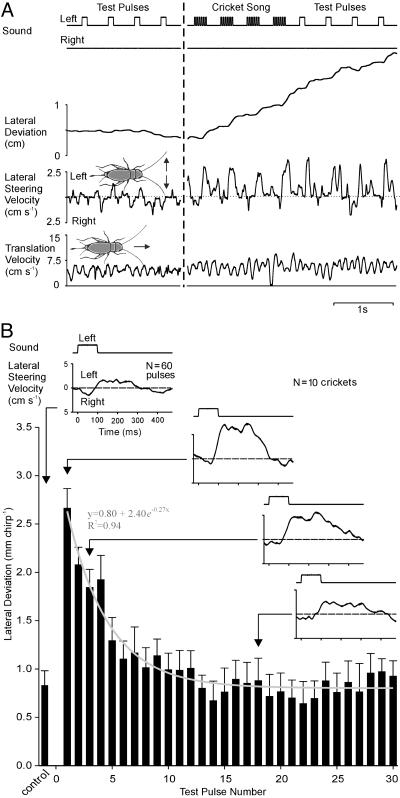
Decay of Auditory Responsiveness. To determine how long this change in auditory responsiveness is maintained, we presented 10 crickets with a series of 100-ms test pulses (75 dB spl) immediately before and after a 10-s song sequence. Although the crickets were walking, they made only small steering movements (0.8 ± 0.2 mm per pulse) toward the pulses before the song (Fig. 3, left of the dashed line) and showed no obvious lateral deviation or phonotaxis. The crickets made large-amplitude steering movements toward the pattern resembling its song (Fig. 3A center pulses) and then, when presented with subsequent test pulses, continued to show large-amplitude steering movements (Fig. 3A right pulses). These responses were 238% higher (2.7 ± 0.2 mm per pulse) than those to the initial test pulses (Fig. 3B). The decay of the steering response to test pulses after a song is described by the exponential function y = 0.8 + 2.4e–0.27x that fitted the raw data with an R2 value of 0.94 (Fig. 3B, gray line). After ≈5 s (i.e., test pulse no. 10), the response reached the same value as to test pulses presented before phonotaxis. Testing the asymptote of the curve (0.8 mm per pulse) against the lateral deviation to the control pulses with a two-tailed one-sample t test revealed no significant difference (P = 0.858, t = 0.185, df = 9). Because the test pulses presented on their own (Fig. 3A) did not initiate phonotaxis, the decay function indicates the gradual decline of the animal's auditory responsiveness toward nonattractive sound pulses. This result may explain transient steering to nonattractive signals, which has been reported after crickets walked toward species-specific songs (10, 16).
Fig. 3.
Phonotactic responses to acoustic test pulses presented before and after exposure to a sequence of species-specific song. (A) During presentation of the 100-ms test pulses (left of dashed line), the animals showed only minor responses, and lateral deviation, steering velocity, and translation velocity remained basically unchanged. Stimulation with 10 s of cricket song (right of the dashed line) elicited phonotaxis, with strong steering toward the sound source. After stimulation with cricket song, steering also occurred toward the test pulses. The translation velocity was not altered. (B) Decay time of steering response to 30 test pulses after stimulation with 10 s of cricket song. Superimposed is the exponential function describing the decay of the steering response (gray line). The average lateral deviation toward the test pulses returned to the control level after 5 s (10 chirps) from the termination of cricket song. Data are pooled from 10 crickets. Error bars indicate the SEM. Insets show the averages (n = 60) of the steering velocity to the control test pulse presented before the song (Upper Left) and to different test pulses at a particular time after the song.
Discussion
Our experiments revealed the following three crucial properties of cricket phonotaxis: (i) an initial gradual increase of auditory steering, (ii) steering toward nonattractive chirps, and (iii) a gradual decay of steering amplitude after a song. From these findings, we conclude that the recognition of the species-specific song causes a change in the animals' auditory responsiveness, leading to an increase in the gain of auditory steering. The modulation of auditory processing occurs only when the speciesspecific sound pattern activates phonotaxis (compare Figs. 2 and 3). Walking alone does not initiate this modulation; only small steering movements are made by a walking cricket to nonattractive sounds when presented alone (Fig. 2 A Left and Right, blue curves). Furthermore, walking, nonmotivated females produce no or only very small steering responses toward the speciesspecific song pattern. This behavior differs from bat avoidance behavior of crickets, where the flight motor pattern gates the response to ultrasound (17).
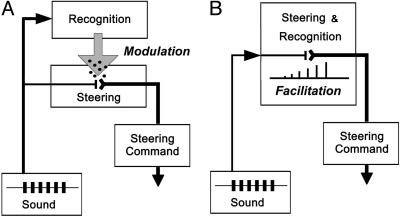
For crickets, a serial organization of pattern recognition and localization has been proposed (18, 19). Suggested recognition mechanisms in the brain (7, 14, 15), however, are far too slow to be directly involved in mediating the animals' short-latency (55–60 ms), reflex-like steering responses to individual sound pulses (11–13) (see Fig. 1). Orientation to nonattractive chirps (compare Figs. 2 and 3) should not occur if the steering response is governed by a series of temporal filters (14) or depends on template matching (7, 15). Furthermore, crickets made small steering movements even to nonattractive sounds, demonstrating that localization is activated independent of any pattern recognition. The possibility that pattern recognition could modulate a separate localization process has been discussed (11, 16, 20). Our data now actually demonstrate that the impact of pattern recognition on localization is a modulatory process on a time scale of seconds, which increases the gain of auditory steering at the start of phonotaxis (see Fig. 1B). How could this behavior be organized? One possibility is that recognition and localization take place in parallel networks, with the recognition network modulating the steering pathway (Fig. 4A), which appears to be similar to grasshoppers (19). Intracellular stimulation of auditory neurons in walking crickets implicated a control of steering through the brain (21). The target of the modulation could be descending brain interneurons (22) and/or thoracic sensory-motor pathways involved in the control of walking. In the most parsimonious organization, the recognition process could be an integral part of an auditory steering pathway. The temporal pattern of acoustic stimulation would then slowly facilitate synaptic processing underlying the steering responses (Fig. 4B). In both cases, the pathways allow nonselective steering responses. Recordings in walking and orienting crickets should demonstrate which of these options is implemented in the CNS.
Fig. 4.
Organization of steering and pattern recognition in crickets. (A) Pattern recognition controls the gain of auditory steering in a parallelorganized system. (B) Facilitation of synaptic processing activated by the species-specific song pattern leads to a gradual increase in the gain of the auditory steering-recognition pathway.
The increase in gain of auditory steering by pattern recognition on a time scale of seconds has considerable consequences for cricket behavior. Because the animals use reactive steering responses to sound pulses, they do not calculate an overall walking direction. Instead, the walking path in crickets emerges from sequential steering reactions toward the male's song (12, 13). Therefore, the directionality of walking is lost as soon as the sound signal ceases. This behavior is fundamentally different from complex vector and landmark navigation in desert ants (2, 5) and bees (23–25), which continuously integrate their foraging paths (ants) and may use landmarks to calculate a vector pointing toward their nests (bees). This vector is the animal's internal reference signal for navigation and is stored in its memory. The orienting cricket uses external signal cues, but in a complex natural biotope, acoustic communication signals are distorted in the time and frequency domain as they spread through vegetation (26). In such circumstances, tolerating transient signal distortion allows animals to approach a sound source with greater reliability. Once phonotaxis is activated, the cricket's auditory steering system can tolerate a loss of signal for several hundred milliseconds, with only a minor decrease in auditory responsiveness. The downside of the change in auditory responsiveness is that briefly nonattractive chirps may be approached as well. Because enhanced auditory responsiveness is maintained only by the species-specific sound pattern, selective orientation of the female is guaranteed.
The change in the animals' responsiveness has serious implications for the design of mate-choice experiments in which the attractiveness of a pattern is tested by simultaneous presentation of certain stimuli (27). Tuning curves derived from these tests are often different, compared with tests monitoring the ongoing localization behavior of the animal (28–30). This difference could result from the modulation of nonselective behavioral responses due to the activation of the recognition network by simultaneous presentation of a species-specific stimulus. The impact of pattern recognition on localization also comes with an implication for cricket song structures (31). Once recognition is activated, even sound pulses that are not attractive when presented alone can contribute to auditory steering (16). During evolution, this fact may have shaped the structure of complex songs composed of chirps and intermitting trills.
The impact of pattern recognition on localization has similarities with feeding behavior in flies where sucrose presentation can elicit a central excitatory state that induces proboscis extension even to nonsucrose stimuli (27). Thus, in both behaviors, activation of a recognition process modulates an unselective behavioral response that decays over a period of seconds. This strategy is efficient for the release of motor activity if the recognition mechanism is activated only by specific sensory cues. A control and modulation of reflexlike motor responses by recognition networks may represent a general strategy in the evolution of insect behavior during which existing, preadapted structures or motor networks are incorporated into new contexts (4, 32).
Acknowledgments
We thank M. Burrows, R. Johnson, R. Patterson, G. de Polavieja, R. Reeve, S. Rogers, and B. Webb for valuable discussion and/or comments, and M. Knepper for continuous development of software tools. The Biotechnology and Biological Sciences Research Council and the Royal Society supported this work.
Author contributions: J.F.A.P. and B.H. designed research, performed research, analyzed data, and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SP, syllable period; SPL, sound pressure level.
References
- 1.Menzel, R. & Giurfa, M. (2001) Trends Cognit. Sci. 5, 62–71. [DOI] [PubMed] [Google Scholar]
- 2.Wehner, R. (2003) J. Comp. Physiol. A 189, 579–588. [DOI] [PubMed] [Google Scholar]
- 3.Lehrer, M., ed. (1997) Orientation and Communication in Arthropods, (Birkhäuser, Basel).
- 4.Fullard, H. F. & Yack, J. Y. (1993) Trends Ecol. Evol. 8, 248–252. [DOI] [PubMed] [Google Scholar]
- 5.Wehner, R. (1987) J. Comp. Physiol. A 161, 511–531.3316619 [Google Scholar]
- 6.Mason, A. C., Oshinsky, M. L. & Hoy, R. R. (2001) Nature 410, 686–690. [DOI] [PubMed] [Google Scholar]
- 7.Hoy, R. R. (1978) Fed. Proc. 37, 2316–2323. [PubMed] [Google Scholar]
- 8.Gerhardt, H. C. & Huber, F. (2002) Acoustic Communication in Insects and Anurans (Univ. Chicago Press, Chicago).
- 9.Pollack, G. S. (2000) Curr. Opin. Neurobiol. 10, 763–767. [DOI] [PubMed] [Google Scholar]
- 10.Weber, T. & Thorson, J. (1989) in Cricket Behavior and Neurobiology, eds. Huber, F., Moore, T. E. & Loher, W. (Cornell Univ. Press, Ithaca, NY), pp. 310–339.
- 11.Pollack, G. S. & Hoy, R. R. (1981) J. Insect Physiol. 27, 41–45. [Google Scholar]
- 12.Hedwig, B. & Poulet, J. F. A. (2004) Nature 430, 781–785. [DOI] [PubMed] [Google Scholar]
- 13.Hedwig, B. & Poulet, J. F. A. (2005) J. Exp. Biol. 208, 915–927. [DOI] [PubMed] [Google Scholar]
- 14.Schildberger, K. (1984) J. Comp. Physiol. A 155, 171–185. [Google Scholar]
- 15.Hennig, R. M. (2003) J. Comp. Physiol. A 189, 589–598. [DOI] [PubMed] [Google Scholar]
- 16.Doherty, J. A. (1991) J. Comp. Physiol. A 168, 213–222. [Google Scholar]
- 17.Nolen, T. G. & Hoy, R. R. (1984) Science 226, 992–994. [DOI] [PubMed] [Google Scholar]
- 18.Stabel, J., Wendler, G. & Scharstein, H. (1989) J. Comp. Physiol. A 165, 165–177. [Google Scholar]
- 19.von Helversen, D. & von Helversen, O. (1995) J. Comp. Physiol. A 177, 767–774. [Google Scholar]
- 20.Weber, T. & Thorson, J. (1988) J. Comp. Physiol. A 163, 13–22. [Google Scholar]
- 21.Schildberger, K. & Hörner, M. (1988) J. Comp. Physiol. A 163, 621–631. [Google Scholar]
- 22.Staudacher, E. M. (2001) J. Comp. Physiol. A 187, 1–17. [DOI] [PubMed] [Google Scholar]
- 23.Collet, M. & Collet, T. S. (2000) Biol. Cybern. 83, 245–259. [DOI] [PubMed] [Google Scholar]
- 24.Riley, J. R., Greggers, U., Smith, A. D., Stach, S., Reynolds, D. R., Stollhoff, N., Brandt, R., Schaupp, F. & Menzel, R. (2003) Proc. R. Soc. London Ser. B. 270, 2421–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menzel, R., Greggers, U., Smith, A., Berger, S., Brunke, S., Bundrock, G., Hülse, S., Plümpe, T., Schaupp, F., Schüttler, E., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 3040–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury, J. W. & Vehrenkamp, S. L. (1998) Principles of Animal Communication (Sinauer, Sunderland MA), pp. 113–139.
- 27.Dethier, V. G. (1976) The Hungry Fly (Harvard Univ. Press, Cambridge, MA).
- 28.Wagner, W. E. (1998) Anim. Behav. 55, 1029–1042. [DOI] [PubMed] [Google Scholar]
- 29.Doherty, J. A. (1985) J. Comp. Physiol. A 157, 279–289. [Google Scholar]
- 30.Pollack, G. S. & Hoy, R. R. (1979) Science 204, 429–432. [DOI] [PubMed] [Google Scholar]
- 31.Alexander, R. D. (1962) Evolution (Lawrence, Kans.) 16, 443–467. [Google Scholar]
- 32.Dumont, J. P. C. & Robertson, R. M. (1986) Science 233, 849–853. [DOI] [PubMed] [Google Scholar]