Abstract
Matrix metalloproteinases (MMPs) are thought to be involved in the growth, destabilization, and eventual rupture of atherosclerotic lesions. Using the mouse brachiocephalic artery model of plaque instability, we compared apolipoprotein E (apoE)/MMP-3, apoE/MMP-7, apoE/MMP-9, and apoE/MMP-12 double knockouts with their age-, strain-, and sex-matched apoE single knockout controls. Brachiocephalic artery plaques were significantly larger in apoE/MMP-3 and apoE/MMP-9 double knockouts than in controls. The number of buried fibrous layers was also significantly higher in the double knockouts, and both knockouts exhibited cellular compositional changes indicative of an unstable plaque phenotype. Conversely, lesion size and buried fibrous layers were reduced in apoE/MMP-12 double knockouts compared with controls, and double knockouts had increased smooth muscle cell and reduced macrophage content in the plaque, indicative of a stable plaque phenotype. ApoE/MMP-7 double knockout plaques contained significantly more smooth muscle cells than controls, but neither lesion size nor features of stability were altered in these animals. Hence, MMP-3 and MMP-9 appear normally to play protective roles, limiting plaque growth and promoting a stable plaque phenotype. MMP-12 supports lesion expansion and destabilization. MMP-7 has no effect on plaque growth or stability, although it is associated with reduced smooth muscle cell content in plaques. These data demonstrate that MMPs are directly involved in atherosclerotic plaque destabilization and clearly show that members of the MMP family have widely differing effects on atherogenesis.
Keywords: animal models, atherosclerosis
Matrix metalloproteinases (MMPs) are a group of >20 zinc-containing endopeptidases that are either secreted or expressed at the cell surface of all of the main vascular cell types. MMPs have overlapping specificities, but each can process at least one extracellular matrix component, and many nonmatrix substrates have also been described. Given this complexity it is not surprising that multiple roles for MMPs have been proposed, including regulation of cell migration, proliferation, and death. As a result, roles for MMPs in atherosclerotic plaque growth and fibrous cap formation have been suggested (1). On the other hand, the presence of elevated mRNA, protein, and activity levels of MMPs within atherosclerotic lesions, particularly at the shoulder regions of the fibrous cap (2–5), has led to the suggestion that they degrade strength-giving extracellular matrix components, including fibrillar collagens. By this mechanism MMPs could promote atherosclerotic plaque destabilization, the main cause of myocardial infarction in humans.
Intervention studies in animal models have been used to investigate the potential roles of MMPs in cardiovascular disease. For example, inhibition of MMP activity by adenovirus-mediated delivery of the gene for human tissue inhibitor of metalloproteinases (TIMP)-1 reduced lesion size in the aortic root of apolipoprotein E (apoE) knockout mice (6). However, atherosclerotic lesion size and stability were unaffected in the aortas of mice deficient for TIMP-1 (7). Additionally, administration of a broad-spectrum MMP inhibitor drug failed to exert any beneficial effect on atherosclerosis measured in the aortas of low-density lipoprotein receptor knockout mice (8). Contradictory results have also been obtained in studies of MMP transgenic and knockout mice (7, 9–11). However, none of these studies directly addressed the issue of plaque instability.
We have recently described a model of plaque instability in which spontaneous plaque ruptures and rupture-related events are observed at an early time point in the proximal part of the brachiocephalic arteries of fat-fed apoE knockout mice (12). Acute plaque disruption and fibrin deposition correlate closely with the presence of buried fibrous layers (akin to human healed plaque ruptures) in lesions from mice at this time point. Therefore, buried fibrous layers can be used as a surrogate marker of previous plaque disruption/instability, and we are able to compare the effects of several MMP gene deletions in the same defined anatomical location. We investigated the contributions of four members of the MMP family that have been suggested to play a role in atherosclerotic plaque instability: MMP-3 (stromelysin-1), MMP-7 (matrilysin-1), MMP-9 (gelatinase B), and MMP-12 (macrophage metalloelastase). We determined their effects on progression and atherosclerotic lesion phenotype in the brachiocephalic arteries of apoE knockout mice.
Methods
Animals. MMP-3–/– (background strain B10.RIII), MMP-7–/– (background strain C57BL/6), MMP-9–/– (background strain CD1), and MMP-12–/– (background strain 129) mice were kindly provided by J. Mudgett (Merck Research Laboratories, Rahway, NJ) and S. Shapiro (Harvard Medical School, Boston). ApoE–/– mice were kindly provided by J. Breslow (The Rockefeller University, New York). The strain background of the apoE–/– animals was 71% C57BL/6, 29% 129, as determined by fingerprinting of tail-tip DNA. ApoE–/– mice were crossed with MMP knockouts to generate apoE–/–/MMP-3–/–, apoE–/–/MMP-7–/–, apoE–/–/MMP-9–/–, and apoE–/–/MMP-12–/– double knockout mice as well as their relevant age-, strain-, and sex-matched apoE–/– single knockout littermate controls. Genomic DNA was extracted from tail tips for genotyping by PCR. The housing and care of the animals and all of the procedures used in these studies were performed in accordance with the guidelines and regulations of the University of Bristol and the United Kingdom Home Office.
Experimental Design. As demonstrated (12), atherosclerotic lesions develop more rapidly in the brachiocephalic arteries of male mice compared with females; therefore only male animals were used in these studies. To evaluate any effect of MMP gene knockout on normal development of the brachiocephalic artery, subsets of animals were fed standard chow diet from weaning for 6 weeks and then terminated. To evaluate atherosclerosis, animals of 8 weeks of age were fed high-fat diet containing 21% (wt/wt) pork lard and supplemented with 0.15% (wt/wt) cholesterol (Special Diet Services, Witham, U.K.) for 8 weeks. To ensure that the studies were adequately powered, group sizes in excess of 20 animals were used.
Plasma Lipid Profile. Heparinized plasma samples were collected at termination, and plasma lipid profiles were determined as described (12).
Termination. Animals were anaesthetized by i.p. injection of sodium pentobarbitone before exsanguination by arterial perfusion via the abdominal aorta with PBS at a constant pressure of 100 mmHg (1 mmHg = 133 Pa), with outflow through the incised jugular veins. This process was followed by constant pressure perfusion with zinc-HCl fixative [0.05% (wt/vol) calcium acetate, 0.5% (wt/vol) zinc acetate, 0.5% (wt/vol) zinc chloride, 0.1 mol/liter Tris·HCl, pH 7.4]. The brachiocephalic artery was removed from each animal.
Histology. Brachiocephalic arteries were embedded in paraffin or optimum cutting temperature compound (OCT, BDH Laboratory Supplies, Poole, Dorset, U.K.). Sections were cut at 3 μm for paraffin-embedded sections and 7 μm for OCT-embedded sections. Sections were stained with hematoxylin and eosin or Miller's elastin/van Gieson stain.
Immunohistochemistry. Serial 3-μm paraffin sections were dewaxed and rehydrated. Endogenous peroxidase activity was inhibited by incubation with 3% hydrogen peroxide. After blocking sections with 20% (vol/vol) goat serum in PBS, sections were incubated overnight at 4°C with either a purified rat mAb against mouse macrophages (Mac2) (BD Biosciences, Oxford, U.K.) at 3.12 μg/ml or mouse mAb against α-smooth muscle actin (Sigma) at 82 μg/ml in 1% (wt/vol) BSA in PBS. Sections were then incubated with the appropriate biotinylated secondary antibodies (DAKO) diluted 1:200 in 1% (wt/vol) BSA in PBS, and then horseradish peroxidase-labeled Extravidin [diluted 1:400 in 1% (wt/vol) BSA in PBS]. Color was developed with 0.05% (wt/vol) 3,3′-diaminobenzidine, and then the nuclei were counterstained with Mayer's hematoxylin. Positive cells were counted and expressed as a percentage of the total number of nucleated cells. A negative control, where the primary antibody was replaced with either mouse or rat IgG at the same dilution, was always included.
Morphological Analysis. Sections were inspected for the presence of buried fibrous layers, smooth muscle cell-rich layers, invested with elastin, and usually overlain with foam cells, within the body of the plaque. These represent previous healed plaque ruptures (12) and were also counted.
Statistical Analysis. Values are expressed as mean ± SEM. For the comparison of group means, a check was first made for similar variances: if the check was passed, then an unpaired two-sample two-tailed Student's t test was carried out. If the variances were significantly different, then an unpaired two-sample two-tailed t test with Welch's correction was used. Discontinuous data (incidence of buried fibrous layers) were analyzed with the Mann–Whitney test. In all cases, statistical significance was concluded where the two-tailed probability was <0.05.
Results
Morphometry of the Brachiocephalic Artery in Chow-Fed Animals. There were no statistically significant differences in lumen size, medial area, or length of the internal elastic lamina in brachiocephalic arteries of any of the apoE/MMP double knockout mice compared with their respective strain-matched apoE single knockout controls, after 6 weeks of chow diet (Table 1). This finding suggests that there is no significant difference in development of the brachiocephalic artery as a result of the deficiency of individual MMPs.
Table 1. Brachiocephalic artery morphometry in chow-fed apoE/MMP double knockout mice.
| Study group | Lumen area, ×103 μm2 | Media area, ×103 μm2 | Total vessel area, ×103 μm2 | Internal elastic lumina length, μm |
|---|---|---|---|---|
| ApoE-/-/MMP-3+/+ (n = 6) | 149 ± 15 | 70 ± 3 | 218 ± 17 | 1,364 ± 69 |
| ApoE-/-/MMP-3-/- (n = 6) | 147 ± 13 | 67 ± 7 | 214 ± 20 | 1,358 ± 59 |
| ApoE-/-/MMP-7+/+ (n = 6) | 185 ± 12 | 60 ± 4 | 245 ± 14 | 1,521 ± 51 |
| ApoE-/-/MMP-7-/- (n = 6) | 196 ± 13 | 57 ± 8 | 254 ± 19 | 1,568 ± 52 |
| ApoE-/-/MMP-9+/+ (n = 6) | 156 ± 11 | 59 ± 5 | 239 ± 25 | 1,481 ± 85 |
| ApoE-/-/MMP-9-/- (n = 6) | 179 ± 21 | 59 ± 7 | 215 ± 18 | 1,396 ± 85 |
| ApoE-/-/MMP-12+/+ (n = 6) | 168 ± 9 | 48 ± 4 | 202 ± 13 | 1,385 ± 52 |
| ApoE-/-/MMP-12-/- (n = 6) | 153 ± 12 | 57 ± 3 | 225 ± 10 | 1,449 ± 39 |
Double knockout mice and single knockout controls were fed chow diet and terminated at 6 weeks of age, and the brachiocephalic artery was removed. Vessel compartment areas were determined by computerized morphometry. There were no significant differences between double knockout and single knockout control mice. Values represent mean ± SEM.
Plasma Lipid Profile. There were no statistically significant differences in plasma total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein cholesterol, or triglycerides between MMP-3, MMP-7, and MMP-9 double knockout mice and their respective apoE single knockout controls, after 8 weeks of high-fat feeding (Table 2). MMP-12 double knockout mice had significantly higher plasma concentrations of HDL cholesterol (+30%; P < 0.01) and triglycerides (+205%; P < 0.001) than their strain-matched apoE single knockout controls. These data are summarized in Table 2.
Table 2. Plasma lipids in fat-fed apoE/MMP double knockout mice.
| Study group | Total cholesterol, mmol/liter | High-density lipoprotein cholesterol, mmol/liter | Low-density lipoprotein cholesterol, mmol/liter | Triglycerides, mmol/liter |
|---|---|---|---|---|
| ApoE-/-/MMP-3+/+ (n = 26) | 32.9 ± 2.2 | 2.5 ± 0.1 | 29.3 ± 2.2 | 2.2 ± 0.5 |
| ApoE-/-/MMP-3-/- (n = 26) | 31.7 ± 3.0 | 2.7 ± 0.1 | 28.1 ± 2.7 | 1.9 ± 0.4 |
| ApoE-/-/MMP-7+/+ (n = 24) | 29.0 ± 3.1 | 2.1 ± 0.2 | 26.2 ± 2.8 | 2.9 ± 0.7 |
| ApoE-/-/MMP-7-/- (n = 20) | 28.6 ± 2.5 | 2.3 ± 0.2 | 25.5 ± 2.2 | 2.0 ± 0.6 |
| ApoE-/-/MMP-9+/+ (n = 24) | 32.4 ± 1.4 | 2.9 ± 0.1 | 28.6 ± 1.3 | 1.8 ± 0.2 |
| ApoE-/-/MMP-9-/- (n = 25) | 27.6 ± 3.1 | 2.6 ± 0.3 | 23.8 ± 2.6 | 2.6 ± 0.7 |
| ApoE-/-/MMP-12+/+ (n = 21) | 36.7 ± 2.9 | 2.9 ± 0.2 | 33.2 ± 2.4 | 1.4 ± 0.3 |
| ApoE-/-/MMP-12-/- (n = 20) | 43.4 ± 1.1 | 3.5 ± 0.1* | 37.9 ± 1.0 | 4.2 ± 0.4* |
Double knockout mice and single knockout controls were fed a high-fat diet for 8 weeks then plasma lipid analyses were performed on terminal bloods drawn from the abdominal aorta. Values represent mean ± SEM. *, P < 0.01 versus single knockout control.
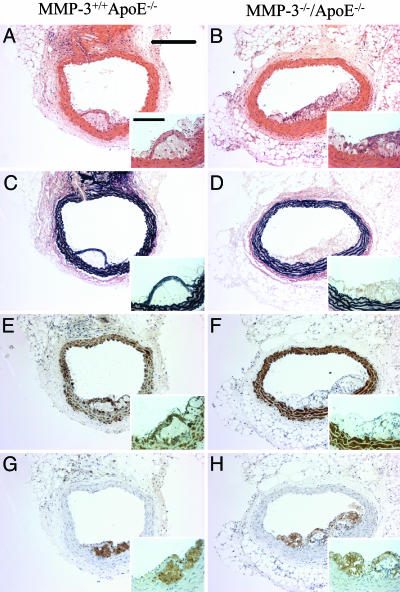
Effects of MMP Gene Knockout on Atherosclerosis Development and Plaque Rupture. MMP-3. Atherosclerotic plaque area was 4-fold greater (P < 0.01) in the brachiocephalic arteries of apoE/MMP-3 double knockout mice (31 ± 7 × 103 μm2) than in strain-matched apoE single knockout controls (7 ± 3 × 103 μm2). The number of buried fibrous layers was increased 8-fold in the double knockouts (0.31 ± 0.11 versus 0.04 ± 0.04 buried fibrous layers per plaque; P < 0.05). ApoE/MMP-3 double knockouts had a significantly lower plaque smooth muscle cell content (24 ± 3%) than strain-matched apoE single knockout controls (45 ± 7%; P < 0.01), but there was no difference in macrophage content. These data are summarized in Table 3, and representative vessel sections are shown in Fig. 1.
Table 3. Brachiocephalic artery plaque morphometry and stability in fat-fed apoE/MMP double knockout mice.
| Study group | Plaque area, × 103 μm2 | Buried fibrous layers, per plaque | Smooth muscle content, % | Macrophage content, % |
|---|---|---|---|---|
| ApoE-/-/MMP-3+/+ (n = 26) | 7 ± 3 | 0.04 ± 0.04 | 45 ± 7 | 41 ± 6 |
| ApoE-/-/MMP-3-/- (n = 26) | 31 ± 7* | 0.31 ± 0.11* | 24 ± 3* | 43 ± 6 |
| ApoE-/-/MMP-7+/+ (n = 24) | 69 ± 11 | 0.64 ± 0.10 | 9 ± 1 | 24 ± 4 |
| ApoE-/-/MMP-7-/- (n = 20) | 64 ± 9 | 0.44 ± 0.13 | 16 ± 3* | 25 ± 3 |
| ApoE-/-/MMP-9+/+ (n = 24) | 41 ± 8 | 0.42 ± 0.12 | 55 ± 4 | 31 ± 3 |
| ApoE-/-/MMP-9-/- (n = 25) | 91 ± 13* | 0.84 ± 0.12* | 39 ± 2* | 43 ± 2* |
| ApoE-/-/MMP-12+/+ (n = 21) | 116 ± 12 | 1.33 ± 0.21 | 9 ± 1 | 32 ± 4 |
| ApoE-/-/MMP-12-/- (n = 20) | 56 ± 7* | 0.55 ± 0.14* | 23 ± 3* | 15 ± 4* |
Double knockout mice and single knockout controls were fed a high-fat diet for 8 weeks, then terminated, and the brachiocephalic artery was removed. Lesion characteristics were assessed by computerized morphometry. Values represent mean ± SEM. *, P < 0.05 versus single knockout control.
Fig. 1.
Effect of knocking out MMP-3 on apoE knockout mouse atherosclerosis. Histological appearance of representative sections of brachiocephalic arteries from apoE single knockout control mice (A, C, E, and G) and MMP-3/apoE double knockout mice (B, D, F, and H). Sections are stained with hematoxylin and eosin (A and B) or elastin van Gieson (C and D) or immunostained for α-smooth muscle actin (smooth muscle cells; E and F) or Mac-2 (macrophages; G and H). (Scale bars: 200 μm; Inset, 50 μm.)
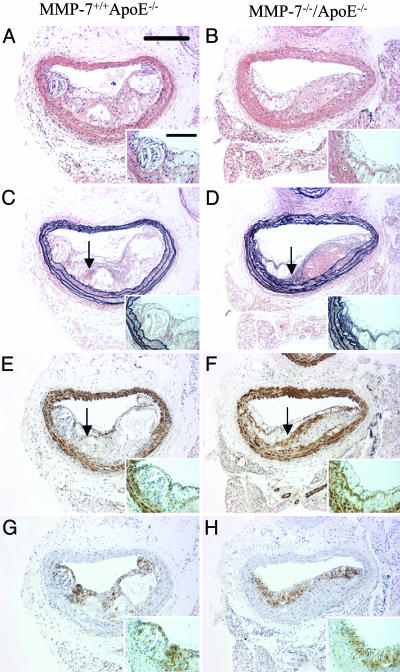
MMP-7. No statistically significant differences in brachiocephalic artery lesion area, media area, or the number of buried fibrous caps were detected between apoE/MMP-7 double knockout mice and their strain-matched apoE single knockout controls. However, a 78% increase in plaque smooth muscle cell content was observed (16 ± 3% versus 9 ± 1%; P < 0.01). The macrophage content of lesions was comparable between both groups. These data are summarized in Table 3, and representative vessel sections are shown in Fig. 2.
Fig. 2.
Effect of knocking out MMP-7 on apoE knockout mouse atherosclerosis. Histological appearance of representative sections of brachiocephalic arteries from apoE single knockout control mice (A, C, E, and G) and MMP-7/apoE double knockout mice (B, D, F and H). Sections are stained with hematoxylin and eosin (A and B) or elastin van Gieson (C and D) or immunostained for α-smooth muscle actin (smooth muscle cells; E and F) or Mac-2 (macrophages; G and H). Arrows indicate buried fibrous layers rich in smooth muscle cells and invested with elastin. (Scale bars: 200 μm; Inset, 50 μm.)
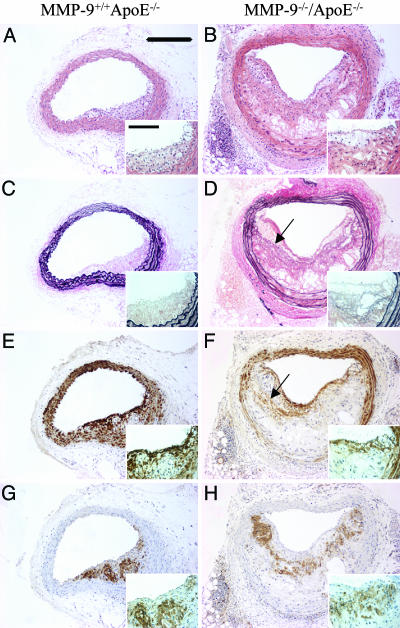
MMP-9. Lesion area was significantly greater in brachiocephalic arteries from apoE/MMP-9 double knockout mice (91 ± 13 × 103 μm2) than strain-matched apoE single knockout controls (41 ± 8 × 103 μm2; P < 0.001). A 2-fold increase in the number of buried fibrous caps was also observed (0.84 ± 0.12 versus 0.42 ± 0.12 buried fibrous layers per plaque; P < 0.05).
A 29% reduction in lesion smooth muscle cell content was observed in apoE/MMP-9 double knockout mice (39 ± 2% versus 55 ± 4%; P < 0.001), together with a 39% increase in the macrophage content (43 ± 2% versus 31 ± 3%; P < 0.01). These data are summarized in Table 3, and representative vessel sections are shown in Fig. 3.
Fig. 3.
Effect of knocking out MMP-9 on apoE knockout mouse atherosclerosis. Histological appearance of representative sections of brachiocephalic arteries from apoE single knockout control mice (A, C, E, and G) and MMP-9/apoE double knockout mice (B, D, F, and H). Sections are stained with hematoxylin and eosin (A and B) or elastin van Gieson (C and D) or immunostained for α-smooth muscle actin (smooth muscle cells; E and F) or Mac-2 (macrophages; G and H). Arrows indicate buried fibrous layers rich in smooth muscle cells and invested with elastin. (Scale bars: 200 μm; Inset, 50 μm.)
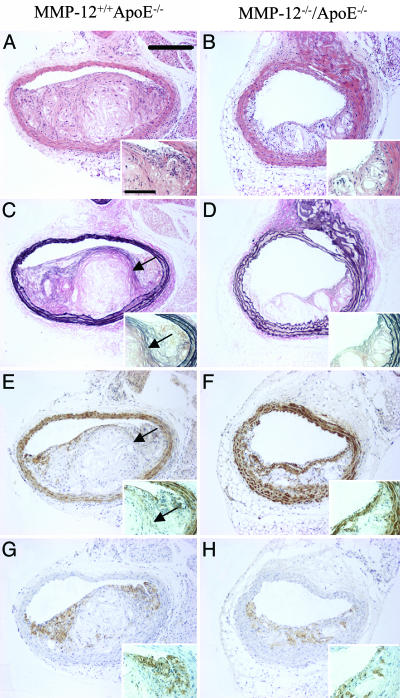
MMP-12. Atherosclerotic plaque area was decreased by 52% in apoE/MMP-12 double knockout mice (56 ± 7 × 103 μm2) compared with strain-matched apoE single knockout controls (116 ± 12 × 103 μm2; P < 0.001). In addition, the number of buried fibrous caps was reduced by 59% in apoE/MMP-12 double knockout animals (0.55 ± 0.14 versus 1.33 ± 0.21 buried fibrous layers per plaque; P < 0.01).
With regard to the cellular composition of the lesions, the smooth muscle cell content was 2.5-fold greater in the double knockouts (23 ± 3% versus 9 ± 1%; P < 0.05). The abundance of macrophages was 53% lower (15 ± 4% versus 32 ± 4%; P < 0.05). These data are summarized in Table 3, and representative vessel sections are shown in Fig. 4.
Fig. 4.
Effect of knocking out MMP-12 on apoE knockout mouse atherosclerosis. Histological appearance of representative sections of brachiocephalic arteries from apoE single knockout control mice (A, C, E, and G) and MMP-12/apoE double knockout mice (B, D, F, and H). Sections are stained with hematoxylin and eosin (A and B) or elastin van Gieson (C and D) or immunostained for α-smooth muscle actin (smooth muscle cells; E and F) or Mac-2 (macrophages; G and H). Arrows indicate buried fibrous layers rich in smooth muscle cells and invested with elastin. (Scale bars: 200 μm; Inset, 50 μm.)
Discussion
Certain characteristics of human atherosclerotic plaques, such as a greater lesion size, decreased smooth muscle content, increased macrophage number, and evidence of healed silent plaque rupture, are associated with instability and rupture (13–15). Similar phenotypic features are observed in unstable brachiocephalic atherosclerotic lesions in the apoE knockout mouse (12), suggesting that mouse lesions mimic at least some of the processes that culminate in plaque rupture in humans. We have therefore used these phenotypic features to quantify the effects of knocking out specific MMPs on plaque stability.
This study shows that knocking out specific MMP genes has diverse effects on the development and phenotype of atherosclerotic plaques. It shows that the hypothesis that MMPs induce atherosclerotic plaque instability by degrading the extracellular components of the fibrous cap is inaccurate: MMP-3 and MMP-9 appear actually to be protective, in that animals with these genes knocked out have accelerated atherosclerotic lesion development and the lesions are significantly more complex with evidence of previous disruption. This study also suggests that MMP-7 has no effect on atherosclerosis. MMP-12 seems to play a detrimental role, supporting atherosclerotic lesion growth and increasing the susceptibility to rupture.
MMP-3. A previous study in fat-fed apoE/MMP-3 double knockout mice, examining atherosclerotic lesions in the thoracic aortas, also demonstrated larger lesions with fewer smooth muscle cells, implying a less stable plaque phenotype (10). Interestingly, it has been reported that a genetic variant of the human MMP-3 promoter that results in reduced gene expression is associated with accelerated atherosclerosis and increased incidence of cardiovascular events (16, 17), consistent with larger, less stable lesions. Hence in both human and murine atherosclerotic lesions there may be a requirement for a certain minimum level of MMP-3 expression to support their stability.
MMP-3 has a broad substrate specificity for extracellular matrix components and also activates many of the other MMPs (18). Therefore many mechanisms could account for the apparent beneficial effect of MMP-3 within atherosclerotic lesions. Galis and colleagues (3) showed that all of the smooth muscle cells in human atherosclerotic lesions, and the majority of those in the media, express MMP-3 protein. It has been suggested MMP-3 facilitates the degradation of cell-to-matrix and cell-to-cell contacts during proliferation and migration of smooth muscle cells (19–22), and in the current study there were indeed fewer smooth muscle cells in atherosclerotic lesions from apoE/MMP-3 double knockout mice than controls (Table 3).
The plaques in strain-matched apoE single knockout controls in the MMP-3 study were small and relatively immature at the time point used (Fig. 1), and consequently had few buried fibrous layers. This finding suggests that the precise genetic background of apoE knockout mice is an important determinant of plaque development, possibly through changes in proteinase expression (23). Knocking out MMP-3 appeared to accelerate plaque growth so that the lesions reached a level of complexity where an unstable plaque phenotype was more prevalent (Table 3).
MMP-7. The data in the current study are in line with previous reports that MMP-7 may not make a major contribution to atherosclerotic lesion development or stability (3, 4). However, compared with apoE single knockout controls, the smooth muscle content of lesions from apoE/MMP-7 double knockout mice was almost 2-fold higher (Table 3). It has been shown that MMP-7 has apoptotic properties (24) and therefore may have the potential to promote smooth muscle cell apoptosis, thus accounting for the increased number of smooth muscle cells in the double knockout animals.
It is possible that knocking out the MMP-7 gene has other effects on arterial physiology that counteract the beneficial changes in smooth muscle cell content, because MMP-7/apoE double knockout mice had the same number of buried fibrous layers as their apoE single knockout controls. In this context it is interesting to note that MMP-7 has been implicated in the maintenance of arterial tone, and that therefore MMP-7 knockout mice may suffer increased tensile force across the fibrous cap as a result of reduced medial tone (25). Hence the greater stability conferred on the lesion by increased smooth muscle cell number may be negated by increased strain on the fibrous cap.
MMP-9. In our study, atherosclerotic lesions from apoE/MMP-9 double knockout mice exhibited characteristics suggesting a heightened transition to an unstable plaque phenotype compared with apoE single knockout controls (Table 3). Interestingly, previous studies performed in MMP-9 knockout mice examining the effects of arterial injury (26), flow cessation (27), and atherosclerosis formation (11) on smooth muscle cell behavior have suggested that MMP-9 may directly modulate smooth muscle migration. On the other hand, evidence from Lessner and colleagues (28) suggests that MMP-9 does not play a major role in macrophage movement. Hence deletion of MMP-9 would be expected to reduce smooth muscle cell but not macrophage content, consistent with the changes in cellular composition observed in the present study. These data could account for the augmented unstable plaque phenotype observed in apoE/MMP-9 double knockout mice.
Two recent studies have suggested another intriguing possibility: MMP-9 might directly affect thrombus maturation and dissolution. MMP-9 is able to inhibit platelet aggregation in response to thrombin or collagen (29) and has the capability to degrade fibrin (30). Furthermore, thrombus formation can induce MMP-9 release from surrounding cells (31), and MMP-9 knockout mice have an increased propensity to hemorrhage (32). Therefore, MMP-9 could reduce thrombus size, and its increased expression may promote smooth muscle cell-directed healing after atherosclerotic plaque rupture, resulting in retardation of plaque expansion and increased stability of the newly forming cap.
Several studies have shown that patients with acute coronary syndromes have elevated plasma levels of MMP-9 compared with patients suffering from stable effort angina (33, 34). These findings and those of others (35) have been interpreted as evidence that MMP-9 is causally involved in the process of acute plaque rupture. Our studies suggest an alternative explanation: when an acute plaque rupture takes place, MMP-9-dependent processes are activated that initiate a healing response that involves the recruitment of smooth muscle cells and the elaboration of matrix. Hence, after an acute coronary event, the increase in plasma MMP-9 concentration may be the consequence of the healing response rather than the initial plaque rupture.
MMP-12. Brachiocephalic arteries from apoE/MMP-12 double knockout mice harbor smaller atherosclerotic lesions that exhibit characteristics of a stable plaque phenotype (Table 3), supporting the hypothesis that MMP-12 may act as a destructive protease promoting atherosclerotic plaque instability. Additionally, the decreased lesional content of macrophages in apoE/MMP-12 double knockout plaques suggests that MMP-12 is involved in macrophage behavior (Table 3). These data are contrary to those of Luttun and colleagues (11), who have recently showed that atherosclerotic lesion size is unaffected in the descending aorta and aortic sinus of apoE/MMP-12 double knockout mice and that there is no difference in lesion macrophage content. However, Luttun and colleagues evaluated atherosclerosis at arterial sites harboring aneurysms, which may not be comparable to a nonaneurysmal site (36) such as the brachiocephalic artery.
Two recent studies have used macrophage-specific overexpression of MMP-12 in animal models of inflammation to evaluate the role this protease plays in inflammatory cell infiltration (37, 38). Both reported increased macrophage recruitment to sites of pre-induced inflammation in animals overexpressing MMP-12 compared with controls, together with increased extracellular matrix degradation and augmented inflammation. Moreover, in mice without a functional MMP-12 gene, allergen-induced lung inflammation was ameliorated relative to controls, principally because of inhibition of inflammatory cell recruitment and infiltration (39). A study exploring the function of macrophages from MMP-12 knockout mice has demonstrated that these cells have a markedly diminished capacity to degrade extracellular matrix components (40). Furthermore, it was shown that MMP-12 knockout macrophages fail to digest soluble elastin and are unable to penetrate reconstituted basement membranes in vitro and in vivo. This result suggests that MMP-12 is necessary for macrophage-mediated extracellular matrix proteolysis and tissue invasion at sites of inflammation and may explain the reductions in plaque size and macrophage content in apoE/MMP-12 double knockout mice in our study.
MMP-12 may affect atherosclerosis indirectly, through an effect on plasma lipid composition (Table 2). However, both proatherogenic and antiatherogenic plasma lipids were affected, and the greatest effect was on triglycerides, which are proatherogenic (41). Therefore these changes, as yet unexplained, are unlikely to be responsible for the reduction in plaque size and stability observed in MMP-12/apoE double knockout mice.
In summary, these studies in double knockout mice highlight divergent roles for different members of the MMP family in atherosclerotic plaque development and destabilization. The widely differing effects of knocking out various MMPs suggest that their functions cannot be completely compensated for by other proteases. The fact that some MMPs (i.e., MMP-3 and MMP-9) appear protective whereas others (i.e., MMP-12) appear harmful may indicate why treatment with nonspecific MMP inhibitors does not have the expected beneficial effect on atherosclerosis in mice (8, 36) and humans (42). This study emphasizes the need for inhibitors that are selective for individual MMPs, and MMP-12 would appear to be a suitable enzyme to target.
Acknowledgments
We thank Drs. John Mudgett, Steven Shapiro, and Jan Breslow for the supply of knockout mice; and Dr. Ray Bush for expert assistance with the mouse breeding programs. This work was supported by the British Heart Foundation.
Author contributions: J.L.J. and C.L.J. designed research; J.L.J. performed research; J.L.J., S.J.G., A.C.N., and C.L.J. analyzed data; and J.L.J., S.J.G., A.C.N., and C.L.J. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MMP, matrix metalloproteinase; apoE, apolipoprotein E.
References
- 1.Newby, A. C. (2005) Physiol. Rev. 85, 1–31. [DOI] [PubMed] [Google Scholar]
- 2.Henney, A. M., Wakeley, P. R., Davies, M. J., Foster, K., Hembry, R., Murphy, G. & Humphries, S. (1991) Proc. Natl. Acad. Sci. USA 88, 8154–8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. (1994) J. Clin. Invest. 94, 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpert, I., Sires, U. I., Roby, J. D., PotterPerigo, S., Wight, T. N., Shapiro, S. D., Welgus, H. G., Wickline, S. A. & Parks, W. C. (1996) Proc. Natl. Acad. Sci. USA 93, 9748–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, J. L., Jackson, C. L., Angelini, G. D. & George, S. J. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1707–1715. [DOI] [PubMed] [Google Scholar]
- 6.Rouis, M., Adamy, C., Duverger, N., Lesnik, P., Horellou, P., Moreau, M., Emmanuel, F., Caillaud, J. M., Laplaud, P. M., Dachet, C. & Chapman, M. J. (1999) Circulation 100, 533–540. [DOI] [PubMed] [Google Scholar]
- 7.Lemaître, V., Soloway, P. D. & D'Armiento, J. (2003) Circulation 107, 333–338. [DOI] [PubMed] [Google Scholar]
- 8.Prescott, M. F., Sawyer, W. K., Von Linden-Reed, J., Jeune, M., Chou, M., Caplan, S. L. & Jeng, A. Y. (1999) Ann. N.Y. Acad. Sci. 878, 179–190. [DOI] [PubMed] [Google Scholar]
- 9.Lemaître, V., O'Byrne, T. K., Borczuk, A. C., Okada, Y., Tall, A. R. & D'Armiento, J. (2001) J. Clin. Invest. 107, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silence, J., Lupu, F., Collen, D. & Lijnen, H. R. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1440–1445. [DOI] [PubMed] [Google Scholar]
- 11.Luttun, A., Lutgens, E., Manderveld, A., Maris, K., Collen, D., Carmeliet, P. & Moons, L. (2004) Circulation 109, 1408–1414. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J., Carson, K., Williams, H., Karanam, S., Newby, A., Angelini, G., George, S. & Jackson, C. (2005) Circulation 111, 1422–1430. [DOI] [PubMed] [Google Scholar]
- 13.Davies, M. J. (1996) Circulation 94, 2013–2020. [DOI] [PubMed] [Google Scholar]
- 14.Mann, J. & Davies, M. J. (1999) Heart 82, 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke, A. P., Kolodgie, F. D., Farb, A., Weber, D. K., Malcom, G. T., Smialek, J. & Virmani, R. (2001) Circulation 103, 934–940. [DOI] [PubMed] [Google Scholar]
- 16.Beyzade, S., Zhang, S., Wong, Y., Day, I. N. M. & Eriksson, P. (2003) J. Am. Coll. Cardiol. 41, 2130–2137. [DOI] [PubMed] [Google Scholar]
- 17.Ye, S., Eriksson, P., Hamsten, A., Kurkinen, M., Humphries, S. E. & Henney, A. M. (1996) J. Biol. Chem. 271, 13055–13060. [DOI] [PubMed] [Google Scholar]
- 18.Sternlicht, M. D. & Werb, Z. (2001) Annu. Rev. Cell Dev. Biol. 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blindt, R., Vogt, F., Lamby, D., Zeiffer, U., Krott, N., Hilger-Eversheim, K., Hanrath, P., vom Dahl, J. & Bosserhoff, A.-K. (2002) J. Vasc. Res. 39, 340–352. [DOI] [PubMed] [Google Scholar]
- 20.Webb, K. E., Henney, A. M., Anglin, S., Humphries, S. E. & McEwan, J. R. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 1837–1844. [DOI] [PubMed] [Google Scholar]
- 21.Ugwu, F., Lemmens, G., Collen, D. & Lijnen, H. R. (2001) Thromb. Res. 102, 61–69. [DOI] [PubMed] [Google Scholar]
- 22.Dwivedi, A. & George, S. J. (2004) Trends Cardiovasc. Med. 14, 100–105. [DOI] [PubMed] [Google Scholar]
- 23.Shi, W., Brown, M. D., Wang, X., Wong, J., Kallmes, D. F., Matsumoto, A. H., Helm, G. A., Drake, T. A. & Lusis, A. J. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1901–1906. [DOI] [PubMed] [Google Scholar]
- 24.Powell, W. C., Fingleton, B., Wilson, C. L., Boothby, M. & Matrisian, L. M. (1999) Curr. Biol. 9, 1441–1447. [DOI] [PubMed] [Google Scholar]
- 25.Hao, L., Du, M., Lopez-Campistrous, A. & Fernandez-Patron, C. (2004) Circ. Res. 94, 68–76. [DOI] [PubMed] [Google Scholar]
- 26.Cho, A. & Reidy, M. A. (2002) Circ. Res. 91, 845–851. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, C. & Galis, Z. S. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 54–60. [DOI] [PubMed] [Google Scholar]
- 28.Lessner, S. M., Martinson, D. E. & Galis, Z. S. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 2123–2129. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Patron, C., Martinez-Cuesta, M. A., Salas, E., Sawicki, G., Wozniak, M., Radomski, M. W. & Davidge, S. T. (1999) Thromb. Haemostasis 82, 1730–1735. [PubMed] [Google Scholar]
- 30.LeLongt, B., Bengatta, S., Delauche, M., Lund, L. R., Werb, Z. & Ronco, P. M. (2001) J. Exp. Med. 193, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontaine, V., Jacob, M.-P., Houard, X., Rossignol, P., Plissionnier, D., Angles-Cano, E. & Michel, J.-B. (2002) Am. J. Pathol. 161, 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, J., Liu, J., Zhou, C., Alexander, J. S., Nanda, A., Granger, D. N. & Zhang, J. H. (2004) J. Cereb. Blood Flow Metab. 24, 1133–1145. [DOI] [PubMed] [Google Scholar]
- 33.Inokubo, Y., Hanada, H., Ishizaka, H., Fukushi, T., Kamada, T. & Okumura, K. (2001) Am. Heart J. 141, 211–217. [DOI] [PubMed] [Google Scholar]
- 34.Blankenberg, S., Rupprecht, H. J., Poirier, O., Bickel, C., Smieja, M., Hafner, G., Meyer, J., Cambien, F. & Tiret, L. (2003) Circulation 107, 1579–1585. [DOI] [PubMed] [Google Scholar]
- 35.Libby, P., Geng, Y. J., Aikawa, M., Schoenbeck, U., Mach, F., Clinton, S. K., Sukhova, G. K. & Lee, R. T. (1996) Curr. Opin. Lipidol. 7, 330–335. [DOI] [PubMed] [Google Scholar]
- 36.Manning, M. W., Cassis, L. A. & Daugherty, A. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 483–488. [DOI] [PubMed] [Google Scholar]
- 37.Wang, X., Liang, J., Koike, T., Sun, H., Ichikawa, T., Kitajima, S., Morimoto, M., Shikama, H., Watanabe, T., Sasaguri, Y. & Fan, J. (2004) Am. J. Pathol. 165, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan, J., Wang, X., Wu, L., Matsumoto, S.-I., Liang, J., Koike, T., Ichikawa, T., Sun, H., Shikama, H., Sasaguri, Y. & Watanabe, T. (2004) Transgenic Res. 13, 261–269. [DOI] [PubMed] [Google Scholar]
- 39.Warner, R. L., Lukacs, N. W., Shapiro, S. D., Bhagarvathula, N., Nerusu, K. C., Varani, J. & Johnson, K. J. (2004) Am. J. Pathol. 165, 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shipley, J. M., Wesselschmidt, R. L., Kobayashi, D. K., Ley, T. J. & Shapiro, S. D. (1996) Proc. Natl. Acad. Sci. USA 93, 3942–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeichi, S., Yukawa, N., Nakajima, Y., Osawa, M., Saito, T., Seto, Y., Nakano, T., Saniabadi, A. R., Adachi, M., Wang, T. & Nakajima, K. (1999) Atherosclerosis 142, 309–315. [DOI] [PubMed] [Google Scholar]
- 42.Brown, D. L., Desai, K. K., Vakili, B. A., Nouneh, C., Lee, H.-M. & Golub, L. M. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 733–738. [DOI] [PubMed] [Google Scholar]