Abstract
Friend spleen focus-forming virus (SFFV) causes rapid erythroleukemia in mice due to expression of its unique envelope glycoprotein, gp55. Erythroid cells expressing SFFV gp55 proliferate in the absence of their normal regulator erythropoietin (Epo) because of constitutive activation of Epo signal transduction pathways. Although SFFV infects many cell types, deregulation of cell growth occurs only when SFFV infects erythroid cells, suggesting that these cells express unique proteins that the virus requires to mediate its biological effects. Not only do erythroid cells express the Epo receptor (EpoR), but those from mice susceptible to SFFV-induced erythroleukemia also express a short form of the receptor tyrosine kinase Stk (sf-Stk). In erythroid cells, SFFV gp55 interacts with the EpoR complex and sf-Stk, leading to activation of the kinase and constitutive activation of signal transducing molecules. In this study, we demonstrate that SFFV gp55 can also deregulate the growth of nonerythroid cells when it is coexpressed with sf-Stk. Expression of SFFV gp55 in rodent fibroblasts engineered to express sf-Stk induced their transformation, as demonstrated by focus formation and anchorage-independent growth in vitro. This transformation by SFFV gp55 requires the kinase activity of sf-Stk and the presence of its extracellular domain but not expression of the EpoR or the tyrosine kinase Jak2, which is required for activation of signal transduction pathways through the EpoR. Thus, expression of SFFV gp55 in nonerythroid cells coexpressing sf-Stk results in their uncontrolled growth, demonstrating a previously unrecognized mechanism for retrovirus transformation of rodent fibroblasts and providing insight into SFFV-induced disease.
Keywords: fibroblast transformation, retroviral envelope protein
The Friend spleen focus-forming virus (SFFV) is a highly pathogenic retrovirus that exclusively induces erythroleukemia in susceptible strains of mice within weeks of inoculation (for review, see ref. 1). The primary pathogenic determinant for this virus is localized to the env gene, which encodes a 55-kDa protein (gp55) that interacts with the erythropoietin receptor (EpoR) complex at the surface of erythroid cells and other hematopoietic cells engineered to express murine EpoR, abrogating Epo dependence (1). Susceptibility to SFFV-induced erythroleukemia is strain-specific and depends on several different host genes (2). These genes include those that interfere with viral entry and integration as well as the Fv-2 gene, which functions at the level of the erythroid target cell. The Fv-2 gene encodes the receptor tyrosine kinase Stk/RON, a member of the Met family of receptor tyrosine kinases (3). Susceptibility to SFFV-induced disease is associated with expression of a short form of Stk, termed sf-Stk, that is transcribed from an internal promoter within the Stk gene of susceptible mice (Fv-2ss), but not resistant mice (Fv-2rr) (3), and is abundantly expressed in erythroid cells (4). Infection of Fv-2ss erythroid cells with the polycythemia-inducing strain of SFFV (SFFV-P) induces Epo-independent proliferation and differentiation, whereas erythroid cells infected with the anemia-inducing strain of SFFV (SFFV-A) proliferate in the absence of Epo but still require Epo for differentiation (5). We recently demonstrated that sf-Stk interacts with SFFV-P gp55 in hematopoietic cells that express the EpoR and that this interaction induces sf-Stk activation (6). Furthermore, exogenous expression of sf-Stk, but not a kinase-inactive mutant of sf-Stk, in bone marrow cells from Fv-2rr mice can restore Epo-independent erythroid colony formation in response to SFFV infection (7, 8). Thus, SFFV appears to exert its biological effects on erythroid cells by activating sf-Stk.
The erythroid specificity of SFFV-induced disease is not due to the failure of the virus to infect or replicate in nonerythroid cells but may be due to the lack of expression in other cell types of cooperating cellular proteins, such as the EpoR or sf-Stk, that the virus needs to exert its biological effects. In this study, we demonstrate that SFFV can transform fibroblasts when it is coexpressed with sf-Stk. Both strains of SFFV have this property, and neither the EpoR nor Jak2, the tyrosine kinase responsible for phosphorylation of the EpoR, are required. These results demonstrate a unique mechanism for retrovirus-induced fibroblast transformation and suggest that SFFV may be capable of altering the growth of cells from multiple lineages if they express sf-Stk.
Materials and Methods
Plasmids. pMXsf-Stk-IRES-EGFP, pMXgp55-IRES-sf-Stk, and pMXgp55-IRES-K190M are described in refs. 6 and 8. A bicistronic vector with the tyrosines within the sf-Stk multifunctional protein docking site (Y429 and Y436) mutated to phenylalanine was generated by using the QuikChange site-directed mutagenesis kit (Stratagene) with the primer (mutated codons are italicized) 5′-TGGGGACCACTTTGTGCAGCTGACAGCAGCTTTTGTGAACGTAGG-3′ (sense Y429F and Y436F). Sf-Stk (6) and sf-Stk K190M (8) were PCR-amplified and cloned into the pEF6 vector (Invitrogen), which contains the elongation factor-1α promoter and the blasticidin resistance gene, generating sf-Stk WT and sf-Stk KD, respectively. The following deletion constructs of sf-Stk were generated by PCR using the following forward primers: sf-Stk TM/C (extracellular sequences of sf-Stk are deleted), 5′-ACGGATCCAACATGGCCTCACAGAGGATACTCCTTATTGC-3′; sf-Stk C (extracellular and transmembrane sequences of sf-Stk are deleted), 5′-ACGGATCCAACATGAACTCCCGAAGACGGAAAAAGCAGCTAGG-3′. The reverse primer 5′-TGAATTCCAAGTGGGCAGGGGTGGCTCTGAGAGAGGC-3′ was used. Each BamHI and EcoRI fragment was cloned into the pEF6 vector.
Cell Lines. NIH 3T3 cells, NIH 3T3/SFFV-P, and NIH 3T3/SFFV-A were maintained in DMEM supplemented with 10% FBS. To establish NIH 3T3/sf-Stk cells, NIH 3T3 cells were transfected with pMX sf-Stk-IRES-EGFP (6) and sorted with a FACS to select those cells expressing EGFP. NIH 3T3 cells stably expressing sf-Stk WT, sf-Stk TM/C, sf-Stk C, and sf-Stk KD were established after transfection with each plasmid as described above and selection with blasticidin.
Viruses. SFFV-P- and SFFV-A-containing virus preparations were obtained from SFFVAP–L and SFFVA–L transfected NIH 3T3 cells, respectively, that had been superinfected with Friend murine leukemia virus (F-MuLV) (9). A SFFV BB6-containing virus preparation was obtained by infecting SFFV BB6 clone 13 NIH 3T3 cells (10) with F-MuLV. F-MuLV- and Friend mink cell focus-inducing MuLV-containing virus preparations were obtained from productively infected NIH 3T3 cells. Infectious stocks of the pMXgp55-sf-Stk bicistronic vector were obtained as described in ref. 8.
Protein Analysis. Cell lysates were prepared by resuspending cells in lysis buffer (20 mM Tris·HCl, pH 7.5/150 mM NaCl/10% glycerol/1% Triton X-100/2 mM EDTA/0.5 mM phenylmethylsulfonyl fluoride/1 mM Na3VO4/1 μg each of aprotinin and leupeptin per ml) followed by incubation on ice for 20 min. Insoluble components were removed by centrifugation, and protein concentrations were determined with a protein assay kit (Bio-Rad). Immunoprecipitations were performed as described in ref. 11 by using anti-Stk polyclonal rabbit antibody. Immunoprecipitated proteins or whole-cell lysates were separated by electrophoresis on Tris-glycine or Bis-Tris minigels (Invitrogen) and then transferred electrophoretically to nitrocellulose filters for Western blotting with rat anti-SFFV gp55 (12), rabbit anti-Stk, or goat anti-R-MuLV gp70 antiserum, followed by visualization using enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech).
Jak2-Deficient Fibroblasts. Jak2+/- mice (13) were obtained from Klaus Pfeffer (Technical University of Munich, Munich) and maintained at the National Cancer Institute–Frederick by breeding with C57BL/6 mice. To obtain Jak2-deficient embryo fibroblasts, we mated Jak2 heterozygotes and harvested embryos after 12.5 days of gestation. The genotype of the embryos was determined by PCR as described in ref. 14 by using the primers 5′-CCACAAGCTTTAGAAGCAGC-3′, 5′-CTTTGTACGCTGTCCACCCAG-3′, and 5′-TCGCCTTCTATCGCCTTCTTG-3′ and Jak2-/- and Jak2+/- fibroblasts prepared from the appropriate embryos. The cells were maintained in DMEM with 20% heat-inactivated FBS at 37°C in a humidified atmosphere with 5% CO2. Fibroblasts at second passage were used in transformation assays.
Transformation Assay. Cells plated in 60-mm dishes were transfected with 4 μg of plasmid DNA with or without 0.6 μg of pSV2neo plasmid DNA using lipofectamine 2000 reagent (Invitrogen). Approximately 48 h after transfection, cells were replated in 60-mm dishes and maintained in the presence of G418 or blasticidin for 4 wk, with the media replaced every 3–4 days, before transformed foci were counted.
Colony-Formation Assays. Cells were seeded in agar suspension in 60-mm dishes as described in ref. 15. Dishes were incubated for 3 wk before colonies were counted.
Results
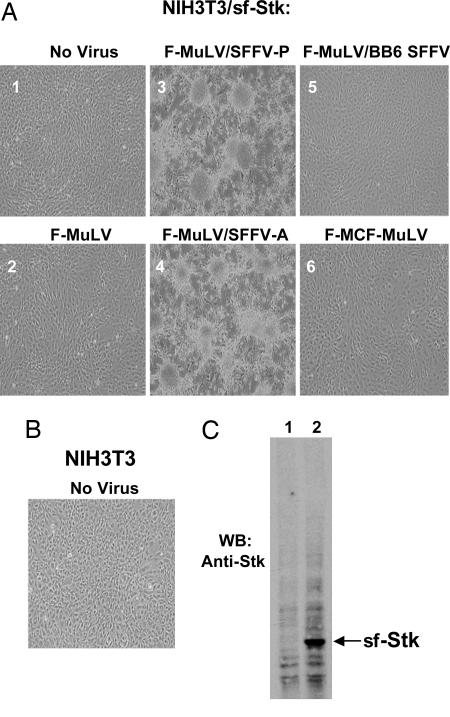
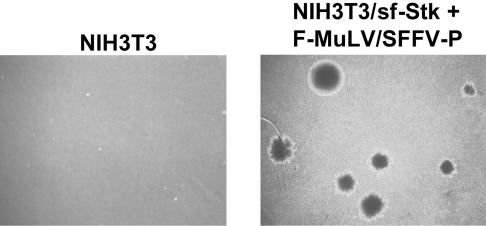
Coexpression of SFFV and sf-Stk Results in Transformation of Rodent Fibroblasts. It has previously been shown that infection of rodent fibroblasts with SFFV does not result in their transformation (16). However, unlike erythroid cells, fibroblasts do not express sf-Stk, which is essential for induction of erythroleukemia by SFFV. To determine whether SFFV could transform fibroblasts in cooperation with sf-Stk, we first established NIH 3T3 cells that stably express sf-Stk (NIH 3T3/sf-Stk). These cells express high levels of sf-Stk (Fig. 1C, lane 2) and do not show any obvious morphological changes in comparison with NIH 3T3 cells (compare Fig. 1 A1 with Fig. 1B). We did not detect endogenous Stk or sf-Stk in NIH 3T3 cells by Western blot analysis (Fig. 1C, lane 1) or by RT-PCR (data not shown). When NIH 3T3/sf-Stk cells were infected with F-MuLV pseudotypes of SFFV-P and SFFV-A and observed 2–3 wk later, they became morphologically transformed, as recognized by their fusiform shape, the loss of contact inhibition, and focus formation (Fig. 1 A 3 and 4). The transformed cells showed anchorage-independent properties, as witnessed by their capacity to grow in soft agar (Fig. 2 and Table 1). Infection of NIH 3T3/sf-Stk cells with F-MuLV alone did not result in transformation (Fig. 1 A2 and Table 1). Transfection of expression plasmids containing only the gp55 coding sequences from SFFV-P or SFFV-A also resulted in transformation of NIH 3T3/sf-Stk cells (data not shown), indicating that the envelope proteins of these viruses were responsible for fibroblast transformation of sf-Stk-expressing NIH 3T3 cells. In contrast, infection of NIH 3T3/sf-Stk cells with BB6 SFFV, a variant of SFFV-P that activates the EpoR but not sf-Stk (6), failed to induce their transformation (Fig. 1 A5 and Table 1), suggesting that activation of sf-Stk by SFFV is necessary for fibroblast transformation. Friend mink cell focus-inducing MuLV, which expresses an envelope protein highly related to SFFV gp55 (1), did not induce any morphological changes in NIH 3T3/sf-Stk cells (Fig. 1 A6 and Table 1). Also, infection of NIH 3T3/sf-Stk cells with other MuLV strains, including amphotrophic MuLV, PVC-211 MuLV, CasBrM MuLV, FB29 MuLV, or 10A1 MuLV, failed to transform these cells (data not shown). These results indicate that both SFFV-P and SFFV-A, unlike other MuLVs, can transform rodent fibroblasts in cooperation with sf-Stk.
Fig. 1.
Coexpression of SFFV and sf-Stk causes transformation of NIH 3T3 cells. (A) NIH 3T3 cells were stably transfected with sf-Stk (NIH 3T3/sf-Stk) and then left uninfected (1) or infected with F-MuLV (2), F-MuLV/SFFV-P (3), F-MuLV/SFFV-A (4), F-MuLV/SFFV mutant BB6 (5), or Friend mink cell focus-inducing MuLV (6). The cells were observed over 3 wk for morphological changes and are shown at ×100 magnification. (B) NIH 3T3 cells shown for comparison. (C) Western blot analysis of extracts from NIH 3T3 (lane 1) or NIH 3T3/sf-Stk (lane 2) cells with anti-Stk antiserum.
Fig. 2.
Anchorage-independent growth of sf-Stk-expressing NIH 3T3 cells after infection with SFFV. NIH 3T3 cells and NIH 3T3/sf-Stk cells infected with F-MuLV/SFFV-P were grown in soft agar and photographed after 3 wk at ×10 magnification.
Table 1. Anchorage-independent growth of sf-Stk-expressing NIH 3T3 cells after infection with SFFV.
| Colonies per dish, 3 wk
|
||
|---|---|---|
| Cells | 250 cells per dish | 500 cells per dish |
| NIH 3T3 | 0 | 0 |
| NIH 3T3/sf-Stk | 0 | 0 |
| NIH 3T3/sf-Stk plus F-MuLV | 0 | 0 |
| NIH 3T3/sf-Stk plus F-MuLV/SFFV-P | 27 ± 3 | 63 ± 5 |
| NIH 3T3/sf-Stk plus F-MuLV/SFFV-A | 28 ± 2 | 48 ± 8 |
| NIH 3T3/sf-Stk plus F-MuLV/BB6 SFFV | 0 | 0 |
| NIH 3T3/sf-Stk plus F-MCF MuLV | 0 | 0 |
NIH 3T3 cells or NIH 3T3/sf-Stk cells infected with the various MuLVs were grown in soft agar at 250 cells or 500 cells per 60-mm dish, and after 3 wk, the number of colonies was counted. F-MCF, Friend mink cell focus-inducing.
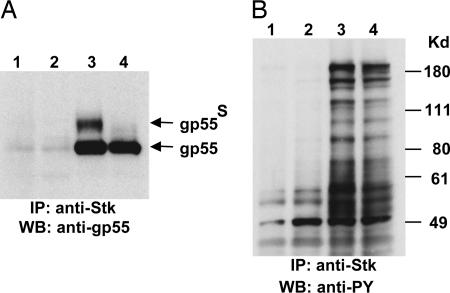
Our previous studies showed that SFFV gp55 interacts with sf-Stk in BaF3 cells expressing the EpoR (BaF3-EpoR) and that the EpoR, sf-Stk, and SFFV gp55 form a complex at the cell surface, resulting in activation of sf-Stk signal transduction pathways (6). To determine whether SFFV gp55 was interacting with sf-Stk in NIH 3T3 cells, cell lysates from NIH 3T3/sf-Stk cells infected with SFFV-P or SFFV-A were immunoprecipitated with anti-Stk antiserum and then immunoblotted with anti-SFFV gp55 (7C10) or anti-phosphotyrosine antibody (4G10). As shown in Fig. 3A, when anti-Stk immunoprecipitates were immunoblotted with anti-gp55 antiserum, bands corresponding to SFFV gp55-P (lane 3) and SFFV gp55-A (lane 4) as well as the surface forms of these proteins (gp55s) were detected. When anti-Stk immunoprecipitates were immunoblotted with anti-phosphotyrosine antibody, a large number of phosphotyrosine-containing proteins were detected only in lysates from NIH 3T3 cells coexpressing sf-Stk and gp55 (Fig. 3B, lanes 3 and 4). NIH 3T3 cells expressing sf-Stk in the absence of SFFV gp55 did not result in the detection of tyrosine-phosphorylated proteins associated with sf-Stk (Fig. 3B, lane 2).
Fig. 3.
SFFV gp55 and sf-Stk interact in NIH 3T3 cells, resulting in tyrosine phosphorylation of multiple proteins. (A and B) Lysates from NIH 3T3 (lanes 1), NIH 3T3/sf-Stk-F-MuLV (lanes 2), NIH 3T3/sf-Stk-F-MuLV/SFFV-P (lanes 3), and NIH 3T3/sf-Stk-F-MuLV/SFFV-A (lanes 4) were immunoprecipitated (IP) with anti-Stk antibody, and then Western blot (WB) analysis was performed with anti-SFFV gp55 (A) or anti-phosphotyrosine (PY) (B) antibody.
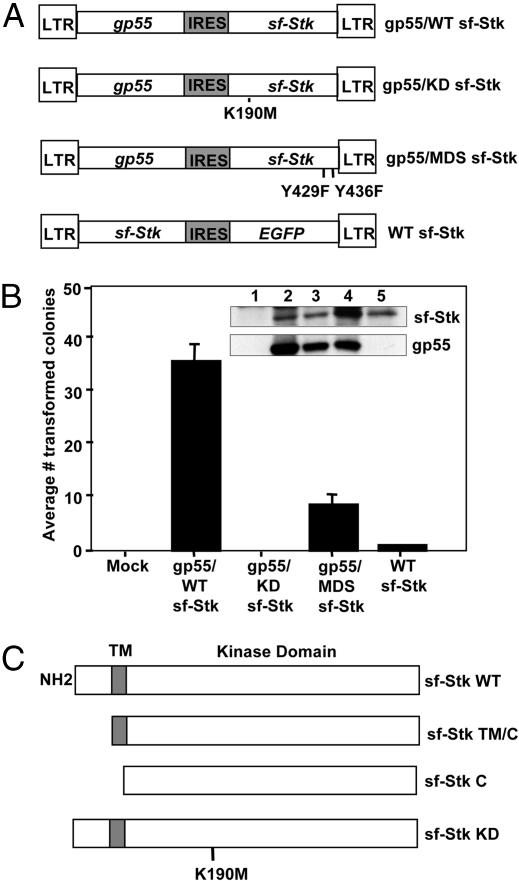
The Kinase Domain of sf-Stk Is Required for Fibroblast Transformation by SFFV. To test whether the kinase activity of sf-Stk is required for fibroblast transformation by SFFV, we carried out transformation assays on NIH 3T3 cells transfected with a bicistronic plasmid that coexpresses SFFV gp55 and sf-Stk (pMXgp55-sfStk) (8) as well as bicistronic plasmids expressing SFFV gp55 and various mutants of sf-Stk (Fig. 4A). As shown in Fig. 4B, transfection of NIH 3T3 cells with the bicistronic vector expressing gp55 and WT sf-Stk (gp55/WT sf-Stk) resulted in their transformation, with an average of 35.5 foci per 60-mm plate (SE = 3.3) at 4 wk posttransfection. In contrast, a vector expressing SFFV gp55 and a kinase-inactive mutant of sf-Stk (gp55-KD sf-Stk) was unable to induce foci formation in NIH 3T3 cells, although the cells expressed gp55 and sf-Stk protein (Fig. 4B Inset, lane 3). Transfection of NIH 3T3 cells with a plasmid expressing WT sf-Stk alone did not induce foci formation. Identical results were obtained when the rat embryonic fibroblast cell line 208F was used (data not shown). Furthermore, as shown in Fig. 4B, a bicistronic plasmid expressing gp55 and a sf-Stk mutant that has both Y429 and Y436 in the multifunctional docking site changed to phenylalanine (gp55-MDS sf-Stk) caused significantly fewer foci in NIH 3T3 cells (an average of 8.5 foci; SE = 1.9), although the cells express high levels of sf-Stk and gp55 (Fig. 4B Inset, lane 4). Also, foci induced by gp55-MDS sf-Stk were much smaller in size than those induced by gp55-WT sf-Stk (data not shown). The same results were obtained from transformation assays using the rat fibroblast line 208F (data not shown). The results obtained indicate that kinase activity of sf-Stk and the subsequent activation of sf-Stk signal transduction pathways are required for transformation of fibroblasts in conjunction with SFFV.
Fig. 4.
The kinase and extracellular domains of sf-Stk are required for transformation of fibroblasts in conjunction with SFFV. (A) Diagrams of the retroviral vectors used in B. (B) NIH 3T3 cells were transfected with the indicated plasmids along with pSV2neo as described in Materials and Methods. After 4 wk, transformed foci were counted. Four independent experiments were performed, and the average number of colonies ± SE is shown. (Inset) Sf-Stk and gp55 expression was detected by Western blot analysis at 3 days posttransfection. Lane 1, mock; lane 2, gp55/WT sf-Stk; lane 3, gp55/KD sf-Stk; lane 4, gp55/MDS sf-Stk; lane 5, WT sf-Stk. (C) Diagrams of the plasmids used in Table 2.
Extracellular Sequences of sf-Stk Are Required for Transformation of Fibroblasts in Conjunction with SFFV. We constructed deletion mutants of sf-Stk in which the extracellular domain (55 aa) (sf-Stk TM/C) or both the extracellular and transmembrane domains (81 aa) (sf-Stk C) were deleted (see Fig. 4C) to determine whether these sequences are crucial for induction of fibroblast transformation in conjunction with SFFV. Compared with WT sf-Stk, sf-Stk TM/C, and sf-Stk C were unable to induce foci when transfected into NIH 3T3 cells infected with F-MuLV pseudotypes of SFFV-P or SFFV-A (Table 2). WT sf-Stk was able to transform SFFV-P- and SFFV-A-infected NIH 3T3 cells equally well. These results indicate that the extracellular sequences of sf-Stk are crucial for efficient transformation of fibroblasts by SFFV.
Table 2. The kinase and extracellular domains of sf-Stk are required for transformation of fibroblasts in conjunction with SFFV.
| Colonies per dish
|
||||
|---|---|---|---|---|
| Transfected cells | sf-Stk WT | sf-Stk TM/C | sf-Stk C | sf-Stk KD |
| SFFV-P-NIH 3T3 | ||||
| Experiment 1 | 62, 61 | 0,0 | 0,0 | 0,0 |
| Experiment 2 | 53, 48 | 0,0 | 0,0 | 0,0 |
| Experiment 3 | 57, 55 | 0,0 | 0,0 | 0,0 |
| SFFV-A-NIH 3T3 | ||||
| Experiment 1 | 38, 46 | 0,0 | 0,0 | 0,0 |
| Experiment 2 | 16, 10 | 0,0 | 0,0 | 0,0 |
| Experiment 3 | 78, 44 | 0,0 | 0,0 | 0,0 |
NIH 3T3 cells infected with SFFV-P or SFFV-A were transfected with the indicated plasmids (Fig. 4C), and after 4 wk, transformed foci were counted. Three independent experiments were performed.
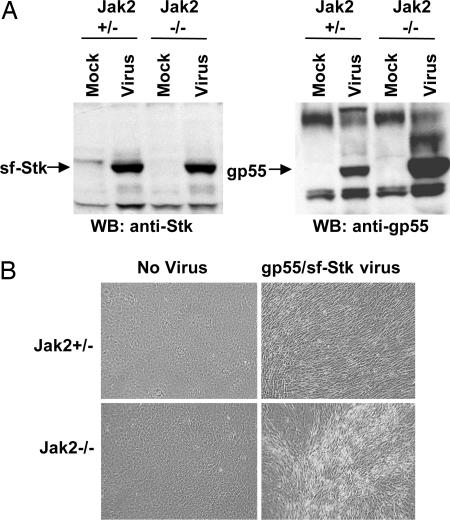
Jak2 Is Not Required for Transformation of Fibroblasts by SFFV gp55-Activated sf-Stk. Jak2 is thought to be a key kinase in EpoR signaling (17). We investigated whether fibroblast transformation by SFFV gp55-activated sf-Stk is Jak2-dependent. For this study, we used primary cultures of Jak2-/- fibroblasts and Jak2+/- fibroblasts and infected them with a virus expressing bicistronic gp55-sf-Stk (8). Cells were passaged several times to allow for virus spread and were shown to express high levels of both sf-Stk and gp55 (Fig. 5A). Over the next few weeks, the cultures were observed for transformation. As shown in Fig. 5B, fibroblasts from both Jak2+/- and Jak2-/- mice showed foci of transformation after infection with the gp55-sf-Stk virus. In contrast, neither Jak2+/- nor Jak2-/- cells infected with SFFV alone or with a virus encoding only sf-Stk showed any signs of transformation (data not shown). These results indicate that Jak2 is dispensable for fibroblast transformation by SFFV gp55-activated sf-Stk.
Fig. 5.
Jak2 is not required for transformation of fibroblasts by SFFV-activated sf-Stk. Primary embryo fibroblasts prepared from Jak2+/- and Jak2-/- mice were infected with pMXgp55-sf-Stk virus and observed after 4 wk. (A) Expression of SFFV envelope protein and sf-Stk by Western blot analysis in the transformed cells. (B) Photomicrographs of fibroblasts infected with pMXgp55-sf-Stk virus or left uninfected. Magnification is ×100.
Discussion
Unlike retroviruses carrying classical oncogenes, the erythroleukemia-inducing Friend SFFV cannot transform rodent fibroblasts (16). However, as demonstrated in this study, SFFV can transform NIH 3T3 cells when they are engineered to express a short form of the receptor tyrosine kinase Stk, a kinase previously shown to be essential for mediating the biological effects of the virus in vivo (3). Infection of NIH 3T3 cells stably expressing sf-Stk with either the polycythemia-inducing variant (SFFV-P) or the anemia-inducing variant (SFFV-A) of the virus induced transformed foci and caused their anchorage-independent growth in soft agar. In studies not shown, fibroblasts expressing both SFFV gp55 and sf-Stk were also able to grow as s.c. tumors in nude mice. Infection of sf-Stk-expressing NIH 3T3 cells with viruses expressing only the envelope proteins of SFFV-P or SFFV-A or infection of NIH 3T3 cells with a bicistronic vector expressing SFFV-P gp55 and sf-Stk also resulted in their transformation, indicating that gp55, the unique envelope protein encoded by SFFV, is responsible. Furthermore, primary embryonic mouse fibroblasts and the rat embryonic cell line 208F also became transformed when SFFV was coexpressed with sf-Stk. In contrast, infection of NIH 3T3/sf-Stk cells with BB6 SFFV, a variant of SFFV that does not interact with sf-Stk (6), or with various ecotropic, amphotropic, and polytropic MuLVs did not alter their cellular morphology. We did not detect transformation of NIH 3T3 cells, 208F cells, or primary embryonic fibroblasts by sf-Stk alone, whether we used expression vectors driven by a retroviral LTR, the elongation factor-1α promoter, or the CMV immediate early promoter (data not shown). We also failed to see foci when a constitutively activated point mutant of sf-Stk (M330T) (8) was expressed in NIH 3T3 cells. Unlike sf-Stk, full-length Stk, which does not interact with SFFV gp55 (6), was unable to cooperate with SFFV to induce transformation of rodent fibroblasts (data not shown). These results indicate that SFFV is a fibroblast-transforming virus when it is coexpressed with sf-Stk and demonstrate a previously unrecognized mechanism by which a retrovirus lacking an oncogene can transform rodent fibroblasts. We recently reported that mice infected with a bicistronic virus coexpressing SFFV gp55 and sf-Stk developed clinical signs not previously associated with SFFV-induced disease (8). That observation, combined with those presented here, suggests that SFFV may be capable of transforming other cell types that it infects, provided that the cell expresses sf-Stk.
We previously demonstrated that SFFV gp55-P physically interacts with sf-Stk in hematopoietic cells expressing the EpoR, SFFV gp55, and sf-Stk (6). In this study, we show that in NIH 3T3 cells, both the intracellular and cell surface forms of SFFV gp55 interact with sf-Stk and that this interaction induces sf-Stk activation, resulting in tyrosine phosphorylation of multiple proteins and transformation. Sf-Stk kinase activity is essential for SFFV transformation of fibroblasts, and maximal transformation occurs when the multifunctional docking site within the kinase is tyrosine-phosphorylated. By using deletion mutants of sf-Stk, we were able to conclude that the extracellular sequences of sf-Stk are critical for induction of transformation in conjunction with SFFV gp55, suggesting that the extracellular domain of the kinase interacts with SFFV gp55. Consistent with this idea are data obtained with the SFFV variant BB6, which does not cause transformation of sf-Stk-expressing NIH 3T3 cells. The envelope protein of BB6 SFFV has a transmembrane domain identical to SFFV gp55 but contains a 53-aa deletion in its extracellular domain (10). Our previous studies indicated that the BB6 envelope protein fails to activate sf-Stk (6), suggesting that the deleted extracellular sequences in BB6 SFFV are those that interact with sf-Stk.
It is interesting that both SFFV-P and SFFV-A transform fibroblasts expressing sf-Stk with similar efficiency, because the two viruses differ in their biological effects on erythroid cells. Erythroid cells infected with SFFV-P either in vitro or in vivo can proliferate and differentiate in the absence of Epo, whereas those infected with SFFV-A can only proliferate, suggesting that the two viruses differ in their ability to activate differentiation signals in erythroid cells. Furthermore, SFFV-P, but not SFFV-A, can render BaF3-EpoR cells, which do not express sf-Stk, factor-independent, a difference that is thought to be due to disparities in the quality of the interaction between the envelope proteins and the EpoR (18). Our present study supports this idea. Because both SFFV-P and SFFV-A gp55 can interact with sf-Stk to activate proliferation signals in the absence of the EpoR, the failure of SFFV-A to induce Epo-independent erythroid cell differentiation is more likely due to its inefficient interaction with the EpoR than with its ability to interact with sf-Stk.
SFFV gp55-activated sf-Stk transformed rodent fibroblasts, but a constitutively activated mutant of sf-Stk did not, even though a constitutively activated mutant of full-length Stk/RON has been shown to transform NIH 3T3 cells (19). However, unlike full-length Stk/RON, sf-Stk lacks signal sequences to direct it to the cell surface, where it could function to activate signal transduction pathways (4). Interaction of the cell surface form of SFFV gp55 with sf-Stk, as shown in Fig. 3A, could efficiently carry sf-Stk to the cell surface, where it can interact with other signaling proteins to cause transformation. Our previous data demonstrated that interaction between SFFV gp55 and sf-Stk increases the stability of sf-Stk, (8) and this stabilizing interaction may also be important for assuring sufficient levels of sf-Stk in the cell to cause transformation.
The tyrosine kinase Jak2 is a key molecule for signaling through the EpoR (17), and a recent study suggested that full-length Stk is downstream of the EpoR and Jak2 (20). In this study, we demonstrated that Jak2 is not required for transformation of rodent fibroblasts by SFFV-activated sf-Stk. Thus, SFFV infection of primary murine erythroid cells that abundantly express sf-Stk could induce a proliferative signal, leading to Epo-independent erythroid burst formation, without activation of EpoR/Jak2. It will be interesting to determine which signal transduction pathways are activated in sf-Stk-expressing NIH 3T3 cells infected with SFFV and which of these are required for transformation.
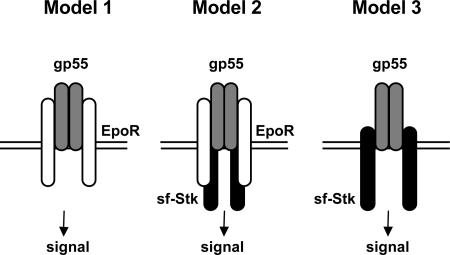
Three putative models for how SFFV gp55 may activate proliferative signals in cells are shown in Fig. 6. Model 1 is represented by the induction of Epo independence by SFFV-P or BB6 SFFV in BaF3-EpoR cells, which express the EpoR but not sf-Stk. In this model, dimers of SFFV gp55 or BB6 gp42 interact with the EpoR, which may cause homodimerization of the receptor and activation of Jak2 and various Epo signal transducing molecules. SFFV-A does not induce factor independence in BaF3-ER cells, suggesting that SFFV-A gp55 cannot sufficiently activate the EpoR (18). Model 2 is represented by erythroid cells infected with SFFV-P or SFFV-A. In this model, dimers of SFFV gp55 interact with the EpoR and/or sf-Stk, causing the activation of the EpoR/Jak2 and/or sf-Stk and their associated signal transduction pathways. SFFV-P, which can interact with both the EpoR and sf-Stk, would be expected to activate both EpoR signaling pathways and pathways downstream of sf-Stk, whereas SFFV-A, which is not efficient in activating the EpoR, would be expected only to activate sf-Stk signaling pathways. Model 3 is represented by nonerythroid cells, such as rodent fibroblasts expressing both sf-Stk and SFFV gp55. In this model, SFFV-P or SFFV-A gp55 interacts with sf-Stk to activate sf-Stk signal transduction pathways, an event that should also occur in erythroid cells from mice infected with SFFV-P or SFFV-A.
Fig. 6.
Putative models for the induction of Epo independence and fibroblast transformation by SFFV. Model 1 shows induction of Epo-independent growth of BaF3-EpoR cells by SFFV-P or BB6 SFFV. Model 2 shows induction of Epo-independent growth of erythroid cells by SFFV-P or SFFV-A. Model 3 shows transformation of rodent fibroblasts by sf-Stk and SFFV-P or SFFV-A or Epo-independent growth of erythroid cells infected with SFFV-P or SFFV-A. White, EpoR; gray, SFFV gp55; black, sf-Stk.
It is well established that retroviruses carrying oncogenes can transform rodent fibroblasts. However, data are accumulating to indicate that envelope proteins encoded by retroviruses may have transforming capacities. Expression of the envelope protein of JSRV (Jaagsiekte sheep retrovirus) in rodent fibroblasts can result in their transformation (21, 22), as can expression of the envelope protein of the avian hemangioma retrovirus (23), and a recent study demonstrated that the envelope protein of the mouse mammary tumor virus can transform mammary epithelial cells in culture (24). We demonstrate that another retroviral envelope protein, SFFV gp55, can also transform fibroblasts, provided that the cells express a particular protein, sf-Stk, which can be activated by the viral envelope protein. A short form of RON, the human counterpart of Stk, has recently been detected in human cancer cells (25). It is possible that this short form of RON may interact with a partner like SFFV gp55 in human cancer cells and contribute to their transformation.
In conclusion, our study demonstrates a unique mechanism for retrovirus-induced transformation and provides insight pertaining to the biological effects of Friend SFFV.
Acknowledgments
We thank Roy Geib (Indiana University School of Medicine, Terra Haute) for SFFV BB6 clone13 NIH 3T3 cells, Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle) for 208F cells, and Klaus Pfeffer for Jak2-deficient mice. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author contributions: K.N. and S.R. designed research; K.N., C.H., T.J., and D.T. performed research; K.N. and S.R. analyzed data; and K.N. and S.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: SFFV, spleen focus-forming virus; SFFV-P, polycythemia-inducing strain of SFFV; SFFV-A, anemia-inducing strain of SFFV; Epo, erythropoietin; EpoR, Epo receptor; sf-Stk, short form of Stk; F-MuLV, Friend murine leukemia virus.
References
- 1.Ruscetti, S. K. (1999) Int. J. Biochem. Cell Biol. 31, 10-35. [DOI] [PubMed] [Google Scholar]
- 2.Ney, P. A. & D'Andrea, A. D. (2000) Blood 96, 3675-3680. [PubMed] [Google Scholar]
- 3.Persons, D. A., Paulson, R. F., Loyd, M. R., Herley, M. T., Bodner, S. M., Bernstein, A., Correll, P. H. & Ney, P. A. (1999) Nat. Genet. 23, 159-165. [DOI] [PubMed] [Google Scholar]
- 4.Iwama, A., Okano, K., Sudo, T., Matsuda, Y. & Suda, T. (1994) Blood 83, 3160-3169. [PubMed] [Google Scholar]
- 5.Hankins, W. D. & Troxler, D. (1980) Cell 22, 693-699. [DOI] [PubMed] [Google Scholar]
- 6.Nishigaki, K., Thompson, D., Hanson, C., Yugawa, T. & Ruscetti, S. (2001) J. Virol. 75, 7893-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein, L. D., Ney, P. A., Liu, Q.-P., Paulson, R. F. & Correll, P. H. (2002) Oncogene 21, 3562-3570. [DOI] [PubMed] [Google Scholar]
- 8.Rulli, K., Yugawa, T., Hanson, C., Thompson, D., Ruscetti, S. & Nishigaki, K. (2004) J. Virol. 78, 4573-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff, L. & Ruscetti, S. (1985) Science 228, 1549-1552. [DOI] [PubMed] [Google Scholar]
- 10.Majumdar, M. K., Cho, C.-L., Fox, M. T., Eckner, K. L., Kozak, S., Kabat, D. & Geib, R. W. (1992) J. Virol. 66, 3652-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishigaki, K., Hanson, C., Ohashi, T., Thompson, D., Muszynski, K. & Ruscetti, S. (2000) J. Virol. 74, 3037-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff, L., Koller, R. & Ruscetti, S. (1982) J. Virol. 43, 472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer, H., Cumano, A., Muller, M., Wu, H., Huffstadt, U. & Pfeffer, K. (1998) Cell 93, 397-409. [DOI] [PubMed] [Google Scholar]
- 14.Radosevic, N., Winterstein, D., Keller, J. R., Neubauer, H., Pfeffer, K. & Linnekin, D. (2004) Exp. Hematol. 32, 149-156. [DOI] [PubMed] [Google Scholar]
- 15.Ruscetti, S. K., Janesch, N. J., Chakraborti, A., Sawyer, S. T. & Hankins, W. D. (1990) J. Virol. 64, 1057-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troxler, D. H., Parks, W. P., Vass, W. C. & Scolnick, E. M. (1977) Virology 76, 602-615. [DOI] [PubMed] [Google Scholar]
- 17.Willhuhn, B. A., Quelle, F. W., Silvennoinen, O., Yi, T., Tang, B., Miura, O. & Ihle, J. N. (1993) Cell 74, 227-236. [DOI] [PubMed] [Google Scholar]
- 18.Constantinescu, S. N., Keren, T., Russ, W. P., Ubarretxena-Belandia, I., Malka, Y., Kubatzky, K. F., Engelman, D. M., Lodish, H. F. & Henis, Y. I. (2003) J. Biol. Chem. 278, 43755-43763. [DOI] [PubMed] [Google Scholar]
- 19.Santoro, M. M., Penengo, L., Minetto, M., Orecchia, S., Cilli, M. & Gaudino, G. (1998) Oncogene 17, 741-749. [DOI] [PubMed] [Google Scholar]
- 20.Van den Akker, E., van Dijk, T., Parren-van Amelsvoort, M., Grossmann, K. S., Schaeper, U., Toney-Earley, K., Waltz, S. E., Lowenberg, B. & von Lindern, M. (2004) Blood 103, 4457-4465. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, N., Palmarini, M., Murgia, C. & Fan, H. (2001) Proc. Natl. Acad. Sci. USA 98, 4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai, S. K., Juh, F.-M., Vigdorovich, V., Danilkovitch-Miagkova, A., Lerman, M. I. & Miller, A. D. (2001) Proc. Natl. Acad. Sci. USA 98, 4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alain, A., Sela-Donenfeld, D., Panet, A. & Eldor, A. (2000) Virology 276, 161-168. [DOI] [PubMed] [Google Scholar]
- 24.Katz, E., Lareef, M. H., Rassa, J. C., Grande, S. M., King, L. B., Russo, J., Ross, S. R. & Monroe, J. G. (2005) J. Exp. Med. 201, 431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardella, C., Costa, B., Maggiora, P., Patane, S., Olivero, M., Ranzani, G. N., De Bortoli, M., Comoglio, P. M. & DiRenzo, M. F. (2004) Cancer Res. 64, 5154-5161. [DOI] [PubMed] [Google Scholar]