Abstract
The dioxin/aryl hydrocarbon receptor (AhR) functions as a ligand-activated transcription factor regulating transcription of a battery of genes encoding xenobiotic metabolizing enzymes. Known receptor ligands are environmental pollutants including polycyclic aromatic hydrocarbons and polychlorinated dioxins. Loss-of-function (gene-disruption) studies in mice have demonstrated that the AhR is involved in toxic effects of dioxins but have not yielded unequivocal results concerning the physiological function of the receptor. Gain-of-function studies therefore were performed to unravel the biological functions of the AhR. A constitutively active AhR expressed in transgenic mice reduced the life span of the mice and induced tumors in the glandular part of the stomach, demonstrating the oncogenic potential of the AhR and implicating the receptor in regulation of cell proliferation.
The dioxin/aryl hydrocarbon receptor (AhR) belongs to a specific class of transcription factors, the basic helix–loop–helix/Per-Arnt-Sim domain (bHLH/PAS) proteins, which is emerging as a battery of regulatory factors seemingly designed to respond to environmental cues (1). The ligand-activated AhR mediates transcriptional activation of a network of genes encoding enzymes such as CYP1A1, CYP1A2, and glutathione S-transferase Ya that function in the oxidative metabolism of xenobiotics (2). Environmental pollutants, notably polychlorinated dioxins and biphenyls, represent well characterized AhR ligands (1).
AhR-mediated signaling pathways provide a first line of defense against potentially toxic environmental contaminants (1). On the other hand, induction of oxidative metabolic processes by the AhR also can cause the production of highly carcinogenic metabolites, creating a link between AhR activation and chemical carcinogenesis (3). In addition, the receptor seems to mediate by as-yet-unresolved mechanisms a wide range of toxic effects by dioxins including birth defects, impaired reproductive capacity, and immune suppression (1). Although several independent loss-of-function studies performed by gene disruption in mice have indicated a role of the AhR in the development of the liver and vasculature, there is no consensus concerning the physiological function of the receptor (4–8). Moreover, a physiological ligand of the AhR has not yet been identified. Against this background we have performed a gain-of-function study to examine possible biological functions of the AhR. We therefore have created a constitutively active AhR (CA-AhR) mutant and expressed it in transgenic mice to study possible AhR-mediated biological effects that are generated independently of any exposure to AhR ligands.
Materials and Methods
Cell Culture, Reporter Gene, and Immunoblot Assays.
Chinese hamster ovary cells were transiently transfected with an AhR-responsive reporter gene, wild-type mouse AhR, or CA-AhR, treated with 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or vehicle alone (1% DMSO), and analyzed as described (9). To monitor expression of the CA-AhR, whole-cell extracts (30 μg of protein) were separated by 7.5% SDS/PAGE, transferred to nitrocellulose membrane, and detected with anti-AhR antibody (Biomol, Plymouth Meeting, PA).
Mice.
CA-AhR was subcloned into pEμSR (10) containing the mouse Ig heavy chain intron enhancer, a modified simian virus 40 promoter, and the simian virus 40 polyadenylation site. Transgenic mice were created by pronuclear injection of a 5.5-kb KpnI fragment encompassing the EμSR–CA-AhR construct into fertilized C57BL/6 × CBA eggs, resulting in five founder animals carrying the CA-AhR construct in the genome. Three lines were chosen for further studies and subsequently crossed into the C57BL/6 strain for two additional generations. Transgenic CA-AhR and wild-type control animals were of the same mixed genetic background. Homozygosity was verified by Southern blot analysis of genomic DNA from tail biopsies. Animals were held in ventilated filter-top cages, received conventional rodent feed (RM3, Special Diet Services, Essex, U.K.) and tap water ad libitum, and were exposed to a 12-h light/dark cycle. Age-matched wild-type and CA-AhR mice were treated orally with corn oil or various doses of TCDD dissolved in corn oil as indicated in the figure legends. Animals were killed by CO2 asphyxiation followed by cervical dislocation. All animal procedures were approved by the local ethical committee on animal experiments.
RNA Analysis.
Total RNA was prepared by tissue homogenization in a guanidinium thiocyanate buffer followed by CsCl2-gradient centrifugation (11). Poly(A) RNA was isolated from total RNA by using oligo(dT)-coupled magnetic beads (Dynal, Oslo). RNA blot analysis was carried out according to standard methods (11). The filters were hybridized with specific 32P-labeled cDNA fragments (12) and exposed to autoradiographic film, and band intensity was quantified by phosphorimager analysis (Fuji). In indicated cases, RNA was analyzed by reverse transcription–PCR as described.
Histopathological Analysis and Immunohistochemistry.
Tissues were fixed in 4% buffered formaldehyde, embedded in paraffin, and cut into 4-μm-thick sections that were stained with hematoxylin/eosin, Alcian blue (pH 2.5), or van Gieson stain according to standard procedures. Antibodies against proliferating cell nuclear antigen (PCNA, FL-261), CYP1A1 (AB1247), and AhR (SA-210) were purchased from Santa Cruz Biotechnology, Chemicon, and Biomol, respectively. Serial sections were de-paraffined, rehydrated, and heat-treated for 15 min in Tris-EDTA (pH 9.0) in a microwave oven. Endogenous peroxidase was blocked in 3% H2O2 in methanol for 15 min. Incubation with diluted antibodies, secondary antibody (Envision+, anti-rabbit horseradish peroxidase, Dako), and washing was done according to manufacturer's protocol. Diaminobenzidine was used as chromogen substrate.
Results
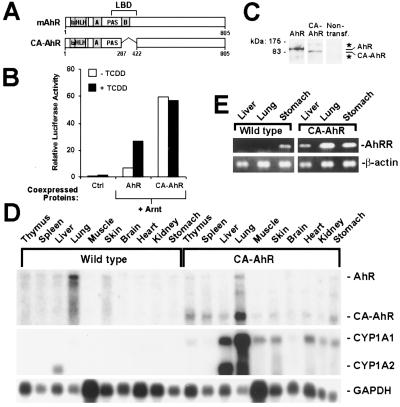
In analogy to nuclear hormone receptors (13), the ligand-binding domain of the AhR mediates both activation of receptor function in the presence of ligand and repression of receptor function in the absence of ligand (14). Deletion of a portion of the minimal ligand-binding domain (amino acids 288–421) of the AhR results in a protein, CA-AhR (Fig. 1A), that fails to bind ligand (9). This truncated receptor was constitutively active with regard to reporter gene activation (Fig. 1B) in transient-transfection experiments. The CA-AhR expression levels matched those of the ligand-dependent wild-type AhR (Fig. 1C).
Figure 1.
Constitutive activity of CA-AhR. (A) Schematic representation of the wild-type mouse AhR (mAhR) and of CA-AhR. bHLH, basic helix–loop–helix; PAS, Per-Arnt-Sim. (B) Functional activity of CA-AhR in Chinese hamster ovary cells. Cells were transiently transfected with an AhR-dependent luciferase reporter gene and expression vectors encoding AhR nuclear translocator (Arnt), wild-type AhR, or CA-AhR. The control lanes (Ctrl) represent activity from the reporter gene alone and empty expression vector. Data are from one experiment performed in duplicate and are representative of at least three independent experiments. (C) Detection of the AhR and CA-AhR proteins expressed after transient transfection of Chinese hamster ovary cells. Whole-cell extracts were analyzed by immunoblotting using anti-AhR antibodies. The star indicates nonspecific immunoreactivity. (D) Expression and functional activity of CA-AhR in 8-month-old female mice: RNA blot analysis showing expression of the endogenous AhR, CA-AhR, and the target genes CYP1A1 and CYP1A2. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown as RNA-loading control of corresponding tissues. (E) AhR repressor (AhRR) and β-actin mRNA expression was assessed by reverse transcription–PCR.
In the transgenic mouse experiments we expressed CA-AhR by using a tissue-restricted expression vector to avoid overt toxic effects in multiple organs, possibly resulting from the use of a potent global expression vector. Therefore CA-AhR was expressed under the control of a modified simian virus 40 promoter and the IgH intron enhancer (10). Mating heterozygous CA-AhR animals yielded wild-type, heterozygous, and homozygous mice at a normal Mendelian 1:2:1 frequency, indicating no prenatal lethality of homozygous mutants. Both heterozygous and homozygous CA-AhR mice were fertile and showed a normal sex ratio. In agreement with other studies using IgH intron enhancer-driven expression constructs (10, 15), CA-AhR mRNA expression was detected in thymus and spleen (Fig. 1D) as well as (except in the lung) at low levels in a number of nonlymphoid tissues (Fig. 1D). With the exception of the lung, CYP1A1 mRNA was not detected in untreated wild-type mice (Fig. 1D). In contrast, all tissues that showed CA-AhR expression also demonstrated at various levels induced expression of CYP1A1 mRNA (Fig. 1D). However, the variation in induced expression of this target gene did not correlate with the expression levels of CA-AhR, indicating that additional tissue-specific factors are important for the regulation of the expression of this target gene. In addition, the transgene also induced mRNA expression of CYP1A2 (Fig. 1D) and AhRR (Fig. 1E), representing other distinct target genes of the AhR (1, 16). Interestingly, expression of AhRR mRNA was detected in the stomach of untreated wild-type mice (Fig. 1E). Taken together, these data demonstrate that CA-AhR is transcriptionally active and mimics the action of the ligand-activated AhR.
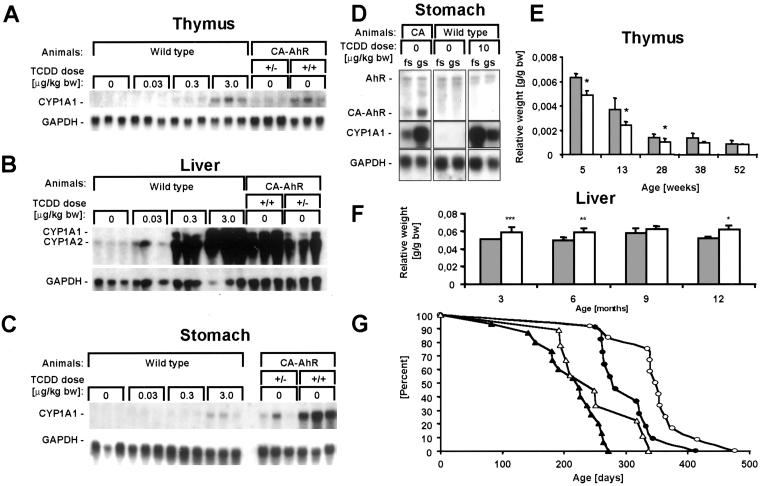
To assess the level of functional activity of CA-AhR, induction of CYP1A1 mRNA expression in several organs of CA-AhR mice was compared with the induction response produced in wild-type mice after oral exposure to TCDD. In the thymus and liver of homozygous CA-AhR mice, the levels of CYP1A1 mRNA were comparable to those observed in wild-type mice treated with a single dose of TCDD of 3 and 0.3 μg of TCDD/kg of body weight, respectively (Fig. 2 A and B). In the stomach, CYP1A1 mRNA expression levels were higher than in the wild-type mice that had received the highest dose of TCDD tested, i.e., 3 μg/kg of body weight (Fig. 2C). In fact, these expression levels were matched by treating wild-type mice with 10 μg of TCDD/kg of body weight (Fig. 2D). After exposure to these relatively low doses of TCDD no acute toxic effects (e.g., lethality or the wasting syndrome) are observed in mouse models (2). Furthermore, no effect on body weight was observed in either male or female CA-AhR mice between the first and third month of age.
Figure 2.
Functional activity of CA-AhR. (A–C) RNA blots showing expression of CYP1A1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in thymus, liver, and stomach of 6-month-old female wild-type, heterozygous, and homozygous CA-AhR mice treated orally 3 days earlier with vehicle or TCDD as indicated. bw, body weight. (D) RNA blot showing expression of AhR, CA-AhR, CYP1A1, and glyceraldehyde-3-phosphate dehydrogenase in forestomach (fs) or glandular stomach (gs) from 3-month-old CA-AhR (CA) or wild-type male mice treated orally with vehicle or TCDD 1 day earlier. (E and F) Alteration of the relative weights of thymus and liver in homozygous CA-AhR animals. The closed bars represent wild-type animals, and open bars represent CA-AhR animals. Thymuses from at least four female animals of each genotype and age and livers from at least five males of each genotype and age were examined. *, P < 0.05, **, P < 0.01, and ***, P < 0.005 as assessed by two-tailed Student's t test. (G) Ages of the homozygous CA-AhR mice found dead stratified by sex (closed symbols for males, open for females) and independent founder lines (triangles for line A3 and circles for line Y8).
Well characterized adverse effects of dioxin are involution of the thymus and enlargement of the liver (1). In comparison to wild-type mice the thymus weight of CA-AhR mice was decreased gradually up to 6 months of age (Fig. 2E), and the livers of CA-AhR mice show a statistically significant weight increase (Fig. 2F) consistent with low-dose exposure to TCDD (17). These results indicate that CA-AhR mimicked biological effects that normally are elicited by the dioxin-activated form of the AhR. CA-AhR mice showed a significantly reduced life span where only very few homozygous animals survived after 12 months (Fig. 2G). Several mice were found dead, beginning already at 6 months of age, often without any preceding clinical symptoms other than a decrease in body weight immediately before dying (≈1 day). There was a striking sex difference in that male mice died earlier than females. In addition, a difference in survival time between two independent homozygous founder lines of mice was observed (Fig. 2G).
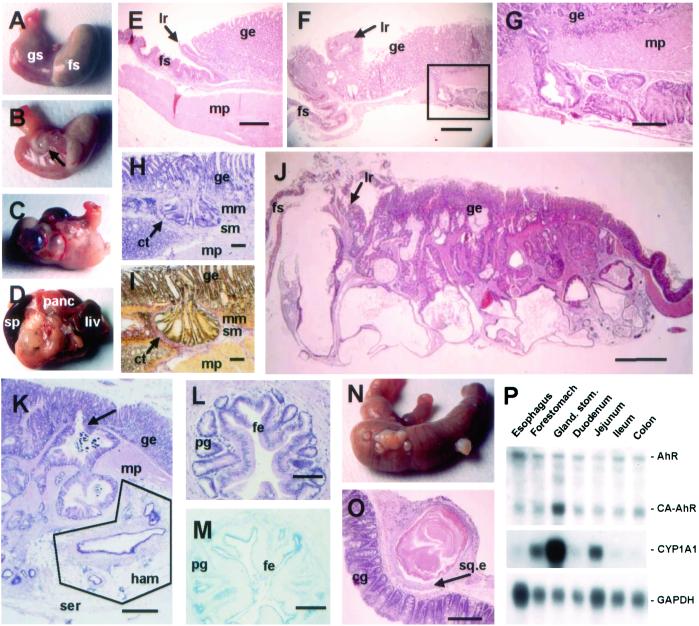
At necropsy, dramatic stomach lesions were observed in CA-AhR mice. In contrast to stomachs from wild-type mice (Fig. 3A), CA-AhR mice demonstrated grossly visible cysts at 3–4 months of age in the lesser curvature of the stomach (Fig. 3B). The cysts became more numerous with age (Fig. 3C), and in the most severe cases (around 12 months of age) the growths adhered to surrounding organs such as liver, pancreas, and abdominal fat (Fig. 3D). In many cases, the stomach wall was thickened throughout the cardia and corpus regions of the glandular stomach. In the limiting ridge, which defines the border between the forestomach and the glandular part of the rodent stomach (Fig. 3E), invading glandular structures were found in CA-AhR mice (Fig. 3F). These elements expanded from the mucosa into the wall of the stomach at the level of the limiting ridge, resulting in a substantial thickening that also was visible at gross inspection. The expansive growth of the cystic glandular structures in the mucosa also showed penetration of dysplastic crypts through the muscularis mucosa into the submucosa, muscularis propria, and subserosa (Fig. 3 F and G). Despite the aggressive behavior of the invading tumor cells, they retained a remarkably well differentiated appearance (Fig. 3G). Analysis of the penetrating epithelial glands showed that these cells were not surrounded by the muscularis mucosa layer (Fig. 3 H and I), suggesting that mucosal herniation is not the cause of penetration but the result of an invasive growth behavior. The tumor development progressed over time resulting in a bizarre, distorted tissue architecture before lethality (12 months of age, Fig. 3J).
Figure 3.
Neoplastic lesions and intestinal metaplasia in the stomach of CA-AhR mice. (A) Normal stomach from a 12-month-old wild-type male showing the forestomach (fs) and glandular stomach (gs). (B) At 3–4 months of age single small cysts close to the limiting ridge were seen in CA-AhR mice (arrow). (C) In older CA-AhR animals (6–12 months old) the number of cystic tumors increased and occupied a larger area of the stomach. (D) In the most severe cases (9–12 months of age), the stomach was adherent to adjacent organs such as spleen (sp), pancreas (panc), and liver (liv). (E) Normal stomach from a 6-month-old wild-type male mouse showing the muscularis propria layer (mp) and limiting ridge (lr) constituting the border between the squamous epithelium of the forestomach and the glandular epithelium (ge, HE staining). (Bar, 0.5 mm.) (F) Close to the limiting ridge a rupture of the submucosa by neoplastic crypts is seen in a 3.5-month-old CA-AhR male. Note glands within the stroma of the limiting ridge (HE). (Bar, 0.5 mm.) (G) Larger magnification of the boxed area in F (HE). (Bar, 0.15 mm.) (H and I) Invading crypts surrounded by connective tissue (ct) invade the submucosa (sm) by penetrating through the muscularis mucosa (mm) and submucosa layers and further into the muscularis propria in a 9-month-old CA-AhR female (H, HE staining; I, van Gieson staining). (Bars, 0.1 mm.) (J) Stomach from a 12-month-old CA-AhR male with severely distorted tissue architecture (HE). (Bar, 1.25 mm.) (K) Glands underlying the serosa (ser) in a 12-month-old CA-AhR female with characteristics of a hamartoma (ham), i.e., lymphatic tissue, vessels, and fat (HE). (Bar, 0.5 mm.) Note also invasion (arrow) of glands from the glandular epithelium into the muscularis propria. (L and M) Glandular structures located in the muscularis propria with cells resembling foveolar epithelium (fe) and pyloric glands (pg) showing intestinal metaplasia in a 9-month-old CA-AhR male (L, HE staining; M, Alcian blue pH 2.5 staining). (Bars, 0.1 mm.) (N and O) Squamous cysts on the caecum showing colonic glands (cg) and squamous epithelium (sq.e) of a 9-month-old CA-AhR male (HE). (Bar, 0.5 mm.) (P) The expression and activity of CA-AhR in the gastrointestinal tract is highest in the glandular stomach. RNA blot showing expression of CA-AhR, endogenous AhR (AhR), CYP1A1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in different parts of the alimentary tract of homozygous CA-AhR mice 3 months of age.
In the subserosa of CA-AhR mice, starting at 3–4 months of age, glandular structures could be detected surrounded by fibroblast-rich stroma containing lymphatic tissue, fatty tissue, vessels, and nerves (Fig. 3K). In human pathology, similar lesions are classified as hamartomas. However, whereas hamartomas in humans are regarded as inborn disorders in differentiation occurring during embryonic development, they evolved during early adult life of CA-AhR mice. Intestinal metaplasia was common in most cysts of the tumors demonstrating staining of intestinal-type mucous not normally observed in the corpus of the stomach (Fig. 3 L and M). Focally located squamous cysts were found in the caecum and occasionally in the ileum in several CA-AhR mice 6 months of age or older (Fig. 3 N and O). CA-AhR mRNA was expressed at the highest level in the glandular part of the stomach, resulting in strong CYP1A1 induction (Fig. 3P).
Proliferation in the normal glandular gastric mucosa occurs in a narrow zone in the isthmus region of the gastric pits, as demonstrated by staining for PCNA (Fig. 4 A and D). The proliferative zone was expanded basally to include the parietal and chief cell region after exposure to 10 μg of TCDD/kg of body weight (Fig. 4 B and E) or expression of CA-AhR (Fig. 4 C and F). Moreover, the tumor tissue in the CA-AhR stomach showed PCNA expression. Weak expression of CYP1A1 was observed in the most luminal cells of control animals (Fig. 4G), whereas TCDD treatment increased CYP1A1 expression mainly in interstitial cells (Fig. 4H). Significant CYP1A1 expression was observed in glandular cells as well as in the tumors of CA-AhR mice (Fig. 4I), which also was demonstrated by in situ hybridization (data not shown). Positive staining for the AhR was observed throughout the stomach in both wild-type and CA-AhR mice (Fig. 4 J–L), detecting expression of both receptor types in the CA-AhR tissue. In wild-type animals, the subcellular localization was both cytoplasmic and nuclear (Fig. 4 M and N), whereas nuclear staining predominated in CA-AhR tissue (Fig. 4O), indicating constitutive nuclear import of the active CA-AhR (9). Taken together with the target-gene induction, these results suggest that the CA-AhR is expressed in the tumor tissue.
Figure 4.
Staining for proliferation, target-gene induction, and AhR expression in glandular stomachs from control or TCDD-treated wild-type mice and CA-AhR mice. Nine-month-old wild-type (wt) or CA-AhR males were treated orally with vehicle (oil) or 10 μg of TCDD/kg of body weight, and tissues were removed and fixed in formaldehyde 4 days later. Paraffin-embedded serial sections were stained with HE (A–C) or immunostained with antibodies specific for PCNA (D–F), CYP1A1 (G–I), or AhR (J–O). Incubation without primary antibody was used as negative control. (Bars in A–L, 0.25 mm; bars in M–O, 0.015 mm.) The brackets, arrows, and star indicate reference points in the serial sections. (D–F) The proliferative zone shown by PCNA staining (brackets) is expanded basally into the parietal/chief cell region in stomachs from TCDD-treated wild-type animals and CA-AhR animals. The arrow indicates proliferation in submucosal tumors in the CA-AhR stomach. (G–I) Examples of CYP1A1 staining are shown by arrows. The star indicates strong CYP1A1 expression in the submucosal tumor. (J–O) AhR is expressed throughout the glandular stomach of both wild-type and CA-AhR mice. The arrow in L indicates a submucosal tumor. The arrow in O indicates mainly nuclear staining in the stomach of CA-AhR mice.
The gastric tumors were not observed in any wild-type mice (n > 200) but were present in more than 200 transgenic animals. Moreover, the tumors appeared in three independent founder lines of CA-AhR mice, indicating that neoplasia was not an effect of random integration of the expression construct into the genome. Heterozygous mice showed less severe stomach tumors than homozygous mice, indicating a gene-dosage effect (Fig. 5A). Moreover, the severity of the gastric tumors increased with age, and males were affected more severely (Fig. 5 B and C), further illustrating the sex difference in susceptibility to CA-AhR.
Figure 5.
Severity of the tumors correlated with CA-AhR gene dosage and sex. Tissue sections were used to classify in arbitrary units the cellular alterations from 0 (for no cystic/dysplastic histological changes) to 5 (for severe cystic/dysplastic histological changes). (A) The histological classification of stomach tumors in 12-month-old wild-type, heterozygous (+/−), and homozygous (+/+) CA-AhR male mice demonstrates a gene-dosage effect. The stomach lesions became more severe with increasing age of both sexes, with homozygous male mice (B) being more susceptible than homozygous female mice (C).
Discussion
This study shows that expression of CA-AhR in transgenic mice induces lethality beginning at 6 months of age. This effect correlated with the development of severe tumors in the stomach, demonstrating an oncogenic potential of the AhR. It has been difficult to interpret the histopathology of the stomach tumors in the CA-AhR mice unambiguously. The well organized glandular structures and low levels of cellular atypia argue for a benign phenotype. On the other hand, the reduced life span, the aggressive, expanding invasion of all stomach layers, and the adherence to surrounding organs point toward a more malignant phenotype. Intestinal metaplasia, which is regarded as a precancerous lesion (18), was widespread in the CA-AhR tumors. Furthermore, a subgroup of human intestinal-type gastric carcinoma contains highly differentiated cells (19). Given the striking gastric oncogenic phenotype of the CA-AhR mice it is interesting to note that several physiological candidates of receptor ligands are indole derivatives, most notably indolo[3,2-b]carbazole, which are generated in the acidic environment of the stomach from dietary precursors, e.g., indolo-3-carbinol (20). Moreover, certain food-born heterocyclic amines that are generated during the food-cooking process also constitute AhR ligands (21). Thus, the correlation between presence of putative dietary ligands and a possible role of the AhR in homeostatic control of cells of the gastric mucosa presents an intriguing biological scenario that remains to be scrutinized in closer molecular detail. In support of a potential role of the AhR in regulation of gastric epithelial cell homeostasis, focal proliferative lesions have been observed in the gastric epithelium of AhR-deficient mice (7).
Stomach cancer is the second most common human malignancy in the world (22). The role (if any) of the AhR in this cancer form is not known. Interestingly, stomach cancer is found more commonly in men than in women (22, 23), a sex difference that is reflected in the CA-AhR mice. Some epidemiological studies show an increased incidence of stomach cancer in human populations exposed to herbicides (24) or fatty fish (25) contaminated with TCDD or other dioxins. A more commonly discussed risk factor for stomach cancer is a diet containing mutagenic nitrosating compounds as well as infection with Helicobacter pylori (23). However, the CA-AhR animals in this study received conventional rodent feed, and no infection by Helicobacter was detected by selective culture of tissue homogenates (data not shown). Given the absence of any known carcinogen, it is unlikely that induction of drug-metabolizing enzymes and ensuing bioactivation of mutagens can explain the oncogenic effect of the CA-AhR. A more intriguing hypothesis is that a network of critical growth-control genes is dysregulated by the CA-AhR. In this context it is interesting to note that AhRR mRNA was constitutively expressed in the stomach of untreated wild-type mice. The AhRR functions as a dominant negative regulator of AhR-mediating signaling events (16), suggesting that a possible AhR function may be repressed normally in the stomach. This mode of regulation possibly could be restricted to glandular cells in the stomach, which could explain the lack of CYP1A1 induction in these cells after TCDD treatment, an effect that seems to be bypassed by the CA-AhR.
There exist seemingly contradictory reports on the role of the AhR in cell-cycle control. TCDD has been reported to stimulate growth of human keratinocytes (26), and mutant cells that express reduced levels of AhR display decreased growth rates in comparison to wild-type cells (27, 28). On the other hand, TCDD has been reported to induce expression of the cyclin/cyclin-dependent kinase inhibitor p27 (Kip1) in certain cells (29). Interestingly, mice develop adenocarcinomas in the glandular stomach after expression of viral oncoproteins binding the retinoblastoma protein Rb (30, 31). Notably, the AhR has been reported recently to interact physically with Rb (32, 33) via an as-yet-unresolved mechanism. It remains to be investigated whether this effect is relevant for the phenotype of CA-AhR mice or whether CA-AhR in fact sequesters another transcriptional coregulatory protein relevant for cell-cycle control in the gastric epithelium. Therefore, it will be important now to identify the network of genes that are dysregulated after expression of the CA-AhR.
Interestingly, several species of laboratory animals treated with AhR ligands have been reported to develop lesions in the glandular stomach mucosa that resemble those in CA-AhR mice. For instance, hyperplasia of the gastric mucosa and cysts in the submucosa of rhesus monkeys (34) and adenocarcinoma of rat glandular stomach (35) have been observed after exposure to dietary mixtures of polychlorinated biphenyls that can activate the AhR. Moreover, we observed significant proliferation in the parietal/chief cell region after TCDD treatment, indicative of an effect of TCDD on cell proliferation in the stomach epithelium. Taken together, these observations are consistent with an important role of the AhR in gastric tumorigenesis and thus also in the control of growth and proliferation of gastric epithelial cells.
Acknowledgments
We are grateful to Dr. Lennart Philipson for helpful comments on the manuscript and Dr. Sven Reiland for valuable histopathological analysis. We also thank Dr. Farzad Olfat for help with the H. pylori cultures, Gunilla Scheu for excellent technical assistance, and Sara Brunnberg for fruitful discussions. This work was supported by the Swedish Cancer Society and the Swedish Council for Working Life Research.
Abbreviations
- AhR
aryl hydrocarbon (dioxin) receptor
- CA-AhR
constitutively active AhR
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- HE
hematoxylin/eosin
- PCNA
proliferating cellular nuclear antigen
- AhRR
AhR repressor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gu Y Z, Hogenesch J B, Bradfield C A. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Pohjanvirta R, Tuomisto J. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- 3.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Salguero P, Pineau T, Hilbert D M, McPhail T, Lee S S, Kimura S, Nebert D W, Rudikoff S, Ward J M, Gonzalez F J. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J V, Su G H, Reddy J K, Simon M C, Bradfield C A. Proc Natl Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi T N, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Salguero P M, Ward J M, Sundberg J P, Gonzalez F J. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 8.Lahvis G P, Lindell S L, Thomas R S, McCuskey R S, Murphy C, Glover E, Bentz M, Southard J, Bradfield C A. Proc Natl Acad Sci USA. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuire J, Okamoto K, Whitelaw M L, Tanaka H, Poellinger L. J Biol Chem. 2001;276:41841–41849. doi: 10.1074/jbc.M105607200. [DOI] [PubMed] [Google Scholar]
- 10.Bodrug S E, Warner B J, Bath M L, Lindeman G J, Harris A W, Adams J M. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 12.Gradin K, Toftgård R, Poellinger L, Berghard A. J Biol Chem. 1999;274:13511–13518. doi: 10.1074/jbc.274.19.13511. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 14.Whitelaw M L, Göttlicher M, Gustafsson J Å, Poellinger L. EMBO J. 1993;12:4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenuwein T, Grosschedl R. Genes Dev. 1991;5:932–943. doi: 10.1101/gad.5.6.932. [DOI] [PubMed] [Google Scholar]
- 16.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher N, Hanberg A, Håkansson H. Toxicol Sci. 2001;62:166–175. doi: 10.1093/toxsci/62.1.166. [DOI] [PubMed] [Google Scholar]
- 18.Stemmermann G N. Cancer. 1994;74:556–564. doi: 10.1002/1097-0142(19940715)74:2<556::aid-cncr2820740205>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H. Hum Pathol. 1999;30:826–832. doi: 10.1016/s0046-8177(99)90144-2. [DOI] [PubMed] [Google Scholar]
- 20.Kleman M I, Poellinger L, Gustafsson J Å. J Biol Chem. 1994;269:5137–5144. [PubMed] [Google Scholar]
- 21.Kleman M I, Övervik E, Mason G G, Gustafsson J Å. Carcinogenesis. 1992;13:1619–1624. doi: 10.1093/carcin/13.9.1619. [DOI] [PubMed] [Google Scholar]
- 22.Parkin D M, Pisani P, Ferlay J. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Stadtländer C T, Waterbor J W. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 24.Ekström A M, Eriksson M, Hansson L E, Lindgren A, Signorello L B, Nyrén O, Hardell L. Cancer Res. 1999;59:5932–5937. [PubMed] [Google Scholar]
- 25.Svensson B G, Mikoczy Z, Strömberg U, Hagmar L. Scand J Work Environ Health. 1995;21:106–115. doi: 10.5271/sjweh.17. [DOI] [PubMed] [Google Scholar]
- 26.Milstone L M, LaVigne J F. J Invest Dermatol. 1984;82:532–534. doi: 10.1111/1523-1747.ep12261149. [DOI] [PubMed] [Google Scholar]
- 27.Elizondo G, Fernandez-Salguero P, Sheikh M S, Kim G Y, Fornace A J, Lee K S, Gonzalez F J. Mol Pharmacol. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 28.Ma Q, Whitlock J P. Mol Cell Biol. 1996;16:2144–2150. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolluri S K, Weiss C, Koff A, Göttlicher M. Genes Dev. 1999;13:1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceci J D, Kovatch R M, Swing D A, Jones J M, Snow C M, Rosenberg M P, Jenkins N A, Copeland N G, Meisler M H. Oncogene. 1991;6:323–332. [PubMed] [Google Scholar]
- 31.Searle P F, Thomas D P, Faulkner K B, Tinsley J M. J Gen Virol. 1994;75:1125–1137. doi: 10.1099/0022-1317-75-5-1125. [DOI] [PubMed] [Google Scholar]
- 32.Ge N L, Elferink C J. J Biol Chem. 1998;273:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 33.Puga A, Barnes S J, Dalton T P, Chang C, Knudsen E S, Maier M A. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 34.Allen J R, Norback D H. Science. 1973;179:498–499. doi: 10.1126/science.179.4072.498. [DOI] [PubMed] [Google Scholar]
- 35.Morgan R W, Ward J M, Hartman P E. Cancer Res. 1981;41:5052–5059. [PubMed] [Google Scholar]