Abstract
The ability of weeds to proliferate into nonindigenous habitats has been attributed to escape from their native natural enemies, allowing reallocation of resources from chemical defense into growth and reproduction. Many invasive weeds, however, eventually encounter their native, coevolved enemies in areas of introduction. Examination of herbarium specimens of an invasive phototoxic European weed, Pastinaca sativa, through 152 years reveals phytochemical shifts coincident in time with the accidental introduction of a major herbivore, the parsnip webworm, Depressaria pastinacella. Plants collected before the introduction of webworms in North America and during the earliest stages of establishment (1850–1889) are lower in toxic furanocoumarins than all plants subsequently collected in North America and lower than European plant samples collected before 1889. Thus, introduction of a major specialist herbivore can increase noxiousness of a species in its area of introduction, illuminating a potential consequence of classical biocontrol programs involving insect herbivores and poisonous weeds.
Keywords: enemy release hypothesis, furanocoumarin, herbivory, invasive species, Lepidoptera
Among the more insidious forms of anthropogenic global change is the establishment of invasive plant species in communities within which they have not evolved, where they cause economic damage by reducing crop yields and livestock growth and ecological damage by altering community composition by means of displacement or genetic alteration of native species (1, 2). Because the rate at which invasive species become established is increasing in many parts of the world, understanding the dynamics of invasion is critical (2, 3). Transport of a species out of its native habitat is thought to result in a reduction in herbivory because of the absence of coevolved specialist insects (4, 5); this reduction is postulated to divert investment of resources from chemical defense and toward increased competitive ability (5, 6). Indeed, the idea that plant populations in areas of indigeneity are regulated by herbivores underlies the practice of classical biological control of weeds (7, 8). Evidence in support of this hypothesis is equivocal (5, 9, 10). Although phytochemical traits may differ in plants in their native and introduced ranges, identifying natural enemies as the selective agents underlying these changes has proved difficult because other evolutionary forces, including genetic drift and founder effects, can generate similar outcomes (11).
Importantly, little information is available on phytochemical changes that ensue when coevolved specialist herbivores resume interacting with a host plant in a nonindigenous area. This scenario is of no small consequence in that classical biological control involves reconstructing such plant–herbivore associations in the area of introduction. Understanding the selective impact of reassociated herbivores on the chemistry of their host plants in areas of introduction is thus important in predicting potential dynamics of classical weed biological control programs.
Museum specimens provide extraordinary access to the dynamics of historical interactions (12, 13). Whereas molecular methods have been used to reconstruct patterns of genetic change over time based on historical material (13), relevant phenotypic changes may be directly assessed rather than inferred (14). In this study, we examined herbarium specimens of an invasive European weed, the wild parsnip, Pastinaca sativa, spanning a 152-year period to document phytochemical shifts in response to the introduction of a major herbivorous selective agent, the parsnip webworm, Depressaria pastinacella (Lepidoptera: Oecophoridae). Consequent postintroduction changes in plant chemistry may have contributed to increases in noxiousness of this species.
Brought to North America by the earliest colonists as a source of food, the parsnip was cultivated in Virginia by 1609 and established as “common” by 1630 (15). Escaped from cultivation, it has long been regarded as noxious because all aerial parts produce furanocoumarins, phototoxic secondary compounds that, on contact with human skin, cause reddening, blistering, and hyperpigmentation (16). The weed rapidly colonizes prairie restorations and encroaches on native prairie preserves (17) and is reported as invasive in Michigan, Ohio, Pennsylvania, Tennessee, Virginia, and Wisconsin (18). Contemporary populations of wild parsnip in North America now exist along roadsides and in waste places in all but five states in the United States (Florida, Mississippi, Alabama, Georgia, and Hawaii) (19). In Wisconsin (and elsewhere throughout its distribution), P. sativa appears to be increasing in abundance (17), possibly due in part to changes in roadside-mowing practices.
Very few native insects have colonized P. sativa (20); currently, the principal herbivore throughout its range in North America is the parsnip webworm (D. pastinacella), an accidental introduction from Europe, first reported in North America in 1869 from Ontario, Canada (21). D. pastinacella webs together and feeds on the reproductive structures of species in the closely allied genera Pastinaca and Heracleum (22). Since their introduction, webworms have become established widely in North America, ranging in the north from Nova Scotia to British Columbia, in the south to Washington DC, and westward to Arizona at high elevations (22). Thus, the contemporary interaction between webworms and wild parsnip resembles many interactions resulting from classical biological control: a specialized herbivore on a narrow range of plants in a community within which it did not originally evolve.
The furanocoumarins in wild parsnip are known to function as resistance factors against webworms (23, 24). As a specialist that consumes the reproductive structures of its monocarpic biennial host plant, D. pastinacella has been shown to act as a selective agent on plant chemistry and increased concentrations of three furanocoumarins, xanthotoxin, bergapten, and sphondin, are associated with webworm herbivory (23, 24). Genotypes with high levels of furanocoumarins experience lower fitness in the absence of herbivores than genotypes with lower furanocoumarin content (25), indicative of a metabolic cost of producing these defense compounds.
In an earlier study involving a limited sample of Midwestern herbarium specimens (26), we found no indication that furanocoumarins in P. sativa seeds are unstable over 140 years; there was no correlation between seed furanocoumarin content and time of collection. Here, we report the results of an analysis of furanocoumarin content of an extensive number of herbarium specimens of wild parsnip collected in North America over the last 152 years, spanning the period from introduction of its principal herbivore to the present, as well as collections of European specimens from the 19th century. To determine whether changes in chemistry are associated specifically with the presence of webworms, we also scored a large number of North American specimens for the presence of damage unique to parsnip webworms.
Fig. 1.
Photograph of a 6-cm2 area of an inflorescence of a P. sativa specimen collected along a railroad in McLean County, IL, by R. A. Evers in June 1958 (Illinois Natural History Survey, no. 77885). Note the presence of a larva of D. pastinacella together with its webbing. For the purposes of the survey, a specimen was scored as having been attacked by webworms if both webbing and feeding damage were present.
Methods
Specimens of wild parsnip (536 in all) from six North American herbaria that held large collections of the species were scored for presence of webworm damage (Chrysler Herbarium, New Brunswick, NJ; University of Illinois at Urbana–Champaign, IL; Illinois Natural History Survey, Champaign; University of Massachusetts, Amherst, MA; Academy of Natural Sciences, Philadelphia; Yale University, New Haven, CT). Because webworms feed by webbing together reproductive parts of their host plant, the damage they inflict is distinctive. Nonetheless, the presence of feeding damage as well as silk was requisite for scoring a specimen as having webworm damage (Fig. 1) because feeding alone could have been caused by other herbivores (e.g., Papilio polyxenes, black swallowtails, which occasionally consume fruits but do not produce webbing), and because silk alone could have been deposited by spiders, which do not feed on the plant. Changes in furanocoumarin chemistry during and after the North American introduction were documented by analyzing furanocoumarin content of ripe seed samples of 342 specimens from 25 herbaria (in addition to the six herbaria listed above: Boston University, Boston; Cleveland Museum of Natural History, Cleveland; Carnegie Museum, Pittsburgh; Duke University, Durham, NC; Field Museum of Natural History, Chicago; Gray Herbarium and Economic Herbarium of Oakes Ames, Harvard University, Cambridge, MA; Kent State University, Kent, OH; University of Kentucky, Lexington, KY; University of Michigan, Ann Arbor, MI; Michigan State University, East Lansing, MI; Kansas State University, Manhattan, KA; North Carolina State University, Raleigh, NC; University of North Carolina, Chapel Hill, NC; Greene–Nieuwland Herbarium and Nieuwland Herbarium, University of Notre Dame, IN; University of New Hampshire, Durham, NH; New York Botanical Garden, Bronx, NY; Smithsonian Institution, Washington, DC). Two ripe seeds from each specimen were cut in half to open the furanocoumarin-bearing oil tubes. The furanocoumarins were then extracted with ethyl acetate and analyzed by high-pressure liquid chromatography (27). With the exceptions of the earliest and latest time periods, data were grouped into 20-year increments. To balance sample sizes during time intervals, the earliest time period spanned 39 years from 1850 to1889, a period that includes the earliest report of parsnip webworms in North American (1869). Because of diminished collecting activity in recent years, the last period comprised samples collected from 1970 to 2002. To avoid regional discrepancies between webworm survey data and chemical data, care was taken to ensure that both types of data were obtained from the same region; thus, if only one type of data were available from a state, no data from that state were included in the analyses. States or provinces within Canada that provided both types of data were Alaska, Arkansas, Colorado, Connecticut, District of Columbia, Delaware, Iowa, Illinois, Indiana, Kansas, Kentucky, Massachusetts, Maryland, Maine, Michigan, North Carolina, North Dakota, New Hampshire, New Jersey, Nevada, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Utah, Virginia, Vermont, Washington, Wisconsin, West Virginia, and Ontario. Moreover, imbalances in sample sizes between the two types of data coming from states were minimized; the correlation between the number of webworm samples and the number of chemical samples taken from states providing both types of data was 0.905. The bulk of the samples (78% of the chemical samples and 95% of the webworm samples) came from the states circumscribed by the Mississippi River to the west, the Ohio and Potomac rivers to the south, the Atlantic Ocean, and Ontario, Canada. For comparison with the early U.S. samples, 58 seed samples from European herbaria (Jardin Botanique National de Belgique, Meise, Belgium; Royal Botanic Garden, Edinburgh, Scotland; National Herbarium Netherlands, Leiden University, Leiden, Netherlands; Université Claude Bernard, Lyon, France; Jardin Botanique, Lyon, France; Botanische Staatssammlung München, München, Germany; Muséum National, Paris; Université de la Mediterranee Aix-Marseille III, Marseille Cedex, France) were also analyzed for furanocoumarin content. Webworm data were analyzed by Fisher's exact tests and chemical data were analyzed by t tests.
Results and Discussion
From 1850 to 1889, webworm damage was nonexistent in our U.S. samples (Fig. 2). During the same time period, four of five furanocoumarins were present in significantly lower amounts in seeds of North American specimens than in specimens collected in Europe, where the webworm is native. Because there was no significant difference between continents in seed mass (t = 1.14, df = 94, and P = 0.257), this difference is not attributable to seed size. A regression model of the fitness cost of furanocoumarin allocation to seeds in wild parsnip has previously been estimated from field data (total seed biomass = 0.779–0.0597 furanocoumarin concentration + 0.296 vegetative biomass) (25). Using this model, the range in plant size from the data used to generate the regression (25), and the difference in total furanocoumarin concentration between European and North American samples before 1898, the fitness differential attributable to furanocoumarin concentration is on the order of 0.127 g of seed or ≈23% of total seed biomass for small plants and 3% for large plants. Thus, absent a specialist herbivore exerting selective pressure for high levels of furanocoumarin investment, it is likely that the high cost of furanocoumarin defense led to selection for reduced investment before reassociation with parsnip webworms.
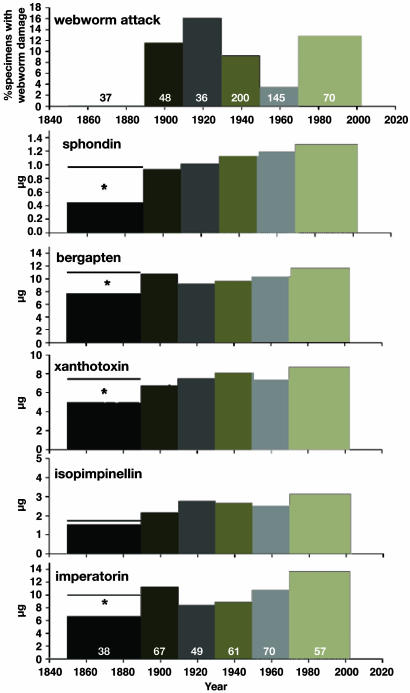
Fig. 2.
Webworm attack and chemical defenses in wild parsnip herbarium specimens. (Top) The percentage of North American wild parsnip specimens exhibiting webworm damage (numbers are sample sizes). In the sample period of 1850–1889, none of the 37 specimens displayed webworm damage. During the period of 1890–1909, the frequency of specimen damage increased marginally (one-sided Fisher's exact test, P = 0.052). By the period 1910–1929, specimens damaged by webworms increased significantly compared with specimens collected during the period 1850–1889 (one-sided Fisher's exact test, P = 0.025). Seed content (in μg per seed, for five furanocoumarins) is presented in the lower five histograms. The sample sizes for all of the furanocoumarins are the same as those listed for imperatorin. Mean seed mass did not vary across time periods (one-way ANOVA, P = 0.574) and cannot account for the observed changes in furanocoumarin content. For the earliest period, a line above the bar represents mean content of specimens collected in Europe between 1820 and 1889 (n = 58). *, Significant difference between the European and North American samples (all t test P values < 0.05). For all five furanocoumarins, there was a significant difference in furanocoumarin content between the earliest period and the subsequent period from 1890–1909 (all t test P values < 0.05).
Concomitant with the rise in webworm infestation between 1890 and 1909, levels of all five furanocoumarins increased significantly (Fig. 2) and continued to increase thereafter. Given the temporal congruence between webworm frequency of attack and furanocoumarin content, it is likely that webworms were responsible for the changes in chemistry. An alternative explanation for the apparent increase in furanocoumarin content postreassociation is that furanocoumarins gradually break down over time; this explanation cannot, however, account for the sharp increase in furanocoumarins between the pre-1889 and post-1889 periods. On the other hand, the steady decrease in amounts of several furanocoumarins as a function of time since collection between 1889 and 2000 might be consistent with a mechanism of gradual decay; significant negative regressions between content and number of years since collection were found for all but one furanocoumarin, bergapten. However, this decay scenario does not comport well with the additional finding that furanocoumarin content is not correlated with collection year in a sample of 163 herbarium samples, including those incorporated in Fig. 2, that were collected between 1819 and 2000 in Europe where the webworm is native (all P values for regressions were >0.466).
Levels of webworm infestation post-1889 displayed statistically significant variation; infestation levels declined, for example, between 1930 and 1969. This decline is consistent with enhanced plant resistance to attack due to higher furanocoumarin content (24). The principal mode of resistance to furanocoumarins in parsnip webworms is cytochrome P450-mediated detoxification; high levels of activity promote survival in the presence of high concentrations of furanocoumarins in wild parsnips (24). Because variation in detoxification rates is heritable at least in some webworm populations (28), the possibility exists that the post-1969 increase in webworm infestation levels was due to coevolved resistance to elevated furanocoumarin chemistry in the principal host plant for this species.
Inferences gained from herbarium specimens carry certain restrictions. Infestation estimates are undoubtedly biased downward because collectors are likely to avoid damaged plants. Over the last decade, surveys throughout the Midwest have revealed that the percentage of parsnips exhibiting webworm damage varies from 22% to 97% among populations, with typical infestations ranging from 60% to 80% (29, 30). These rates are far greater than those indicated by herbarium specimens collected at any period during the last 152 years; herbarium infestations never exceeded 16%. Whether the bias is likely to have varied over the time period as a result of change in collector preferences is an open question. We doubt that this was the case, as very few of the specimens exhibited easily detectable webworm damage. In most specimens, damage is visible only with the aid of a dissecting microscope; consequently, the damage most likely would have escaped detection by the collector. Another potential problem is that collector bias for largely damage-free plants led to overrepresentation of resistant phenotypes. However, the mean total furanocoumarin content for herbarium samples from the most recent period (1970–2002) is 38.4 μg per seed, a value that does not differ from the mean of 37.2 μg per seed based on 133 samples that we collected from four Illinois populations in 2004 for another study (data not shown).
At present, the oldest weed biological control programs in the continental United States are now ≈60 years old (dating back to the introduction of Chrysolina species for control of Hypericum perforatum in 1944) (31). By contrast, the North American interaction between D. pastinacella and P. sativa has been in place for at least 130 years (21), approximately twice as long. Thus, this interaction, brought about by an accidental introduction that antedated the formal development of weed biological control, can provide insight into the potential long-term fate of classical biocontrol programs in which a specialist herbivore is introduced to control a chemically noxious weed. If the genetic variation exists in weed populations to respond to selection pressure from their coevolved associate, enhanced chemically based resistance may evolve. In the case of wild parsnip, elevated levels of furanocoumarins in reproductive structures resulting from interactions with their specialist herbivore may make these plants more phototoxic to humans and other vertebrates (32). In that furanocoumarins are potent germination inhibitors associated with allelopathy (33, 34), it is even conceivable that increased furanocoumarin content in the seeds may increase the ability of this weed to spread and invade new locations and habitats in North America (35).
Acknowledgments
The cooperation of the following herbarium personnel is appreciated: Bonnie Isaac, Debra Trock, Alexander Krings, Barbara J. Hellenthal, Emilie Bess, Christine Niezgoda, Frédéric Danet, Franz Schuhwerk, Sasha Eisenman, Elmar Robbrecht, Leo Vanhecke, Garrett E. Crow, Carol McCormick, James Macklin, Karen Searcy, Kenneth Robertson, Stephen Downie, Mark Mayfield, Rusty Russell, Emily Wood, Nico Cellinese, Richard K. Rabeler, Richard Primack, Rob Paratley, Sherri Herndon, Gerard Thijsse, G. Aymonin, Cecile Aupic, Georges Barale, G. Guignard, Andrea Schwarbach, Adele Smith, J. Frederic Medail, and Renee Boronka. We thank Evan DeLucia (University of Illinois at Urbana–Champaign Department of Plant Biology), David Holway (University of California at San Diego), and Robert Marquis (University of Missouri, St. Louis) for comments on the manuscript; from our laboratory, we thank Jennifer McGovern for collecting samples at the Carnegie Museum and Lauren Chambers for sample preparation. This work was supported by National Science Foundation Grant 0235773 (to M.R.B. and A.R.Z.).
Author contributions: A.R.Z. and M.R.B. designed research and wrote the paper; and A.R.Z. performed research and analyzed data.
References
- 1.Pimentel, D., Lach, L., Zuniga, R. & Morrison, D. (2000) Bioscience 50, 53–65. [Google Scholar]
- 2.Mooney, H. A. & Cleland, E. E. (2001) Proc. Natl. Acad. Sci. USA 98, 5446–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rejmanek, M. & Richardson, D. M. (1996) Ecology 77, 1655–1665. [Google Scholar]
- 4.Carpenter, D. & Cappuccino, N. (2005) J. Ecol. 93, 315–321. [Google Scholar]
- 5.Blossey, B. & Notzold, R. (1995) J. Ecol. 83, 887–889. [Google Scholar]
- 6.Hierro, J. L., Maron, J. L. & Callaway, R. M. (2005) J. Ecol. 9, 5–15. [Google Scholar]
- 7.Huffaker, C. B., Dahlsten, D. L., Janzen, D. H. & Kennedy, G. G. (1984) in Ecological Entomology, eds. Huffaker, C. B. & Rabb, R. L. (Wiley, New York), pp. 659–691.
- 8.Willis, A. J., Memmott, J. & Forrester, R. I. (2000) Ecol. Lett. 3, 275–283. [Google Scholar]
- 9.Willis, A. J., Thomas, M. B. & Lawton, J. H. (1999) Oecologia 120, 632–640. [DOI] [PubMed] [Google Scholar]
- 10.Maron, J. L., Vila, M., Bommarco, R., Elmendorf, S. & Beardsley, P. (2004) Ecol. Monogr. 74, 261–280. [Google Scholar]
- 11.Müller-Schärer, H., Schaffner, U. & Steinger, T. (2004) Trends Ecol. Evol. 19, 417–422. [DOI] [PubMed] [Google Scholar]
- 12.Suarez, A. V. & Tsutsui, N. D. (2004) Bioscience 54, 66–74. [Google Scholar]
- 13.Ristaino, J. B., Groves, C. T. & Parra, G. R. (2001) Nature 411, 695–697. [DOI] [PubMed] [Google Scholar]
- 14.Bouzat, J. L., Lewin, H. A. & Paige, K. N. (1998) Am. Nat. 152, 1–6. [DOI] [PubMed] [Google Scholar]
- 15.Sturtevant, E. L. (1890) Am. Nat. 24, 30–49. [Google Scholar]
- 16.Berenbaum, M. R. & Zangerl, A. R. (1996) in Phytochemical Diversity and Redundancy in Ecological Interactions, eds. Romero, J. T., Saunders, J. A. & Barbosa, P. (Elsevier, New York), pp. 1–24.
- 17.Eagan, D. (1999) Wis. Nat. Resour. Mag., June. Available at www.wnrmag.com/stories/1999/jun99/parsnip.htm.
- 18.United States Department of Agriculture (2005) Alien Plant Invaders of Natural Areas (USDA). Available at www.nps.gov/plants/alien/map/pasa1.htm.
- 19.United States Department of Agriculture (2005) Plants Database (USDA). Available at http://plants.usda.gov/cgi_bin/topics.cgi?earl=plant_profile.cgi&symbol=PASA2.
- 20.Berenbaum, M. (1981) Ecology 62, 1254–1266. [Google Scholar]
- 21.Bethune, C. J. S. (1869) Can. Entomol. 2, 1–4. [Google Scholar]
- 22.Hodges, R. (1974) Moths of America North of Mexico (E. W. Classey and R. B. D. Publications, London), Fasc. 6.2.
- 23.Berenbaum, M. R., Zangerl, A. R. & Nitao, J. K. (1986) Evolution 40, 1215–1228. [DOI] [PubMed] [Google Scholar]
- 24.Zangerl, A. R. & Berenbaum, M. R. (1993) Ecology 74, 47–54. [Google Scholar]
- 25.Zangerl, A. R. & Berenbaum, M. R. (1997) Am. Nat. 150, 491–504. [DOI] [PubMed] [Google Scholar]
- 26.Berenbaum, M. R. & Zangerl, A. R. (1998) Proc. Natl. Acad. Sci. USA 95, 13743–13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitao, J. K. & Zangerl, A. R. (2004) Phytochem. Anal. 15, 262–266. [DOI] [PubMed] [Google Scholar]
- 28.Berenbaum, M. R. & Zangerl, A. R. (1992) Evolution 46, 1373–1384. [DOI] [PubMed] [Google Scholar]
- 29.Zangerl, A. R. & Berenbaum, M. R. (2003) Evolution 57, 806–815. [DOI] [PubMed] [Google Scholar]
- 30.Zangerl, A. R. & Rutledge, C. E. (1996) Am. Nat. 147, 599–608. [Google Scholar]
- 31.McFadyen, R. E. C. (1998) Annu. Rev. Entomol. 43, 369–393. [DOI] [PubMed] [Google Scholar]
- 32.Murray, R. D. H, Mendez, J. & Brown, S. A. (1982) The Natural Coumarins (Wiley, New York).
- 33.Friedman, J., Rushkin, E. & Wallace, G. R. (1982) J. Chem. Ecol. 8, 55–65. [DOI] [PubMed] [Google Scholar]
- 34.Garcia, C., Moyna, P., Fernandez, G. & Heinzen, H. (2002) Chemoecology 12, 107–111. [Google Scholar]
- 35.Bais, H. P., Vepachedu, R., Gilroy, S., Callaway, R. M. & Vivanco, J. M. (2003) Science 301, 1377–1380. [DOI] [PubMed] [Google Scholar]