Abstract
The present study emphasizes the importance of cell surface expression and secretion of heparanase (endo-β-d-glucuronidase) in tumor angiogenesis and metastasis. For this purpose, nonmetastatic Eb mouse lymphoma cells were transfected with the predominantly intracellular human heparanase or with a readily secreted chimeric construct composed of the human enzyme and the chicken heparanase signal peptide. Eb cells overexpressing the secreted heparanase invaded a reconstituted basement membrane to a much higher extent than cells overexpressing the intracellular enzyme. Cell invasion was inhibited in the presence of laminaran sulfate, a potent inhibitor of heparanase activity and experimental metastasis. The increased invasiveness in vitro was reflected in vivo by rapid and massive liver colonization and accelerated mortality. In fact, mice inoculated with cells expressing the secreted enzyme succumb because of liver metastasis and dysfunction, as early as 10 days after s.c. inoculation of the cells, when their tumor burden did not exceed 1% of body weight. Cell surface localization and secretion of heparanase markedly stimulated tumor angiogenesis, as demonstrated by a 4–6-fold increase in vessel density and functionality evaluated by MRI of tumors produced by cells expressing the secreted vs. the nonsecreted heparanase, consistent with actual counting of blood vessels. Altogether, our results indicate that the potent proangoigenic and prometastatic properties of heparanase are tightly regulated by its cellular localization and secretion. The increased potency of the secreted enzyme makes it a promising target for anticancer drug development.
Tumor spread involves degradation of macromolecules in the extracellular matrix (ECM) and blood vessel wall. Among these are heparan sulfate proteoglycans, which play a key role in the self-assembly, insolubility, and barrier properties of basement membranes (BM) and ECM (1–4). Mammalian endoglycosidase (heparanase), capable of partially depolymerizing heparan sulfate (HS) chains, has been identified in highly invasive normal and malignant cells, including cytotrophoblasts, activated cells of the immune system, lymphoma, melanoma, and carcinoma cells (5–9). In fact, expression of heparanase has long been correlated with the metastatic potential of tumor cells and treatment with heparanase inhibitors markedly reduced the incidence of experimental metastasis and autoimmunity (6–13). The significance of heparanase in tumor progression is emphasized further in recent studies performed since the cloning and expression of a single gene encoding heparanase (14–18). Preferential expression of the heparanase mRNA and protein was evident in human specimens derived from a variety of tumors (7, 8, 19–23). Moreover, there is a significant correlation between enhanced heparanase mRNA expression and shorter postoperative survival of patients with cancer (21, 23).
Apart from its involvement in the egress of cells from the vasculature, heparanase is tightly involved in angiogenesis, primarily by means of releasing heparin-binding angiogenic factors sequestered by HS in BM and ECM (8, 24, 25). The enzyme also releases accessory HS-degradation fragments that promote receptor binding and signaling of basic fibroblast growth factor (bFGF) and possibly other endothelial cell growth factors (8, 24, 26). Enzymatic degradation of HS has therefore been implicated in tumor angiogenesis (8, 26, 27). The human heparanase cDNA encodes a latent enzyme of 543 amino acids, which is then cleaved at the N terminus, yielding the mature highly active 50-kDa enzyme (14, 15, 18). Only a single heparanase cDNA sequence encoding a functional enzyme was identified (14–18), indicating that this enzyme is the dominant HS-degrading endoglycosidase in mammalian tissues.
The mammalian heparanase protein is localized primarily in perinuclear acidic endosomal and lysosomal cellular granules and in the tertiary granules of human neutrophils (28, 29). Unlike the human enzyme, the recently cloned chicken heparanase is localized primarily in association with the cell membrane and is readily secreted (30). The most prominent difference between the human and chicken heparanase sequences resides in the predicted signal peptide region, showing a marked difference in hydrophobicity and length (30). Cells transfected with a chimeric construct composed of the human enzyme and the chicken heparanase signal sequence exhibited cell surface localization and secretion of heparanase, similar to cells transfected with the full-length chicken enzyme (30).
The present study was undertaken to investigate the effect of cell surface expression and secretion of heparanase on tumor progression. For this purpose, Eb mouse lymphoma cells transfected with the human, chicken, or chimeric heparanase cDNAs were compared for their invasiveness in vitro and for tumor vascularization, metastasis, and survival of the tumor-bearing mice. Our results indicate that cell surface expression and secretion of heparanase are major determinants in the control of tumor angiogenesis and metastasis.
Materials and Methods
Cells.
The methylcholanthrene-induced nonmetastatic Eb T-lymphoma cells were grown in RPMI medium 1640 as described (14, 31). Cultures of bovine corneal endothelial cells were established from steer eyes and maintained as described (31, 32).
Plasmids and Transfections.
Chicken-, human-, and chimeric-heparanase cDNAs (Chk-hpa, H-hpa, and Chimeric-hpa, respectively), prepared as described (30), were subcloned into the eukaryotic expression plasmid pcDNA3 (Invitrogen) at the EcoRI site. Eb cells (0.5 × 106 cells per ml) were transfected (48–72 h, 37°C) with 1–2 μg of DNA by using 6 μl of FuGene transfection reagent (Roche Molecular Biochemicals) in 94 μl of Optimem (GIBCO/BRL), and stable pooled populations of heparanase-expressing cells were maintained in growth medium containing 150 μg/ml of G418 to avoid the overgrowth of nontransfected cells (30). Expression of heparanase was evaluated by reverse transcription–PCR, activity measurements, and immunostaining (14, 30).
Matrigel Invasion Assay.
Cells were assayed for Matrigel invasion at 37°C in a 5% CO2 incubator for 6 h, with blind-well chemotaxis chambers and polycarbonate filters (13 mm in diameter, 8 μm pore size) (Costar) as described (33). Medium conditioned by 3T3 fibroblasts was applied as a chemoattractant and placed in the lower compartment of the Boyden chamber (33). Cells on the lower surface of the filter were stained and counted by examination of five microscopic fields as described (33).
Animal Protocol.
Male CD1 nude mice (2 months old, 25 g of body weight) were inoculated s.c. in the lower back with 1 × 106 Eb-lymphoma cells stable transfected with each of the hpa expression plasmids (Chk-hpa, H-hpa, and Chimeric-hpa) or with insert-free pcDNA3 control plasmid (n = 11–15 mice per group). Mice were monitored for tumor size, survival rate, and histology of the primary tumor, lungs, and liver. Metastatic dissemination into the lungs and liver was evaluated macroscopically and by microscopic examination of tissue sections. Animal experiments were approved by the animal care committee of the Hebrew University.
Histology.
Tissue samples were fixed with 4% formaldehyde in PBS, embedded in paraffin, and sectioned (5-μm sections). After deparaffinization and rehydration, sections were washed (3 times) with PBS and stained with hematoxylin/eosin or with anti-smooth muscle actin (αSMA) Abs (Sigma) as described (19, 34).
Vascular Density.
For vessel density analysis, 5-μm-thick sections of tumor tissue, processed and stained as described previously, were examined. The microvessel density was determined in tumor areas with the highest vascularization. Individual vessels were counted by light microscopy on a ×200 microscopic (0.785-mm2) field. A total of nine fields per tumor (three sections × three fields) were analyzed and the mean value ± SD was determined as described (35).
MRI Analysis of Blood Vessel Density, Functionality, and Maturation.
MRI experiments were performed on a horizontal 4.7 T Biospec spectrometer (Bruker Medical, Ettlingen, Germany), with an actively radio frequency (RF)-decoupled surface coil, 2 cm in diameter, and a bird-cage transmission coil (34). Mice (4 mice of each of the previously described groups; 10 days after cell inoculation) were anesthetized (75 mg/kg ketamine + 3 mg/kg xylazine, i.p.) and placed supine with the tumor located at the center of the surface coil. Tumor vascularity was reflected by reduction of the mean signal intensity inside the tumor in gradient echo T*2-weighted images (repetition time = 130 ms; echo time = 10 ms). Data are reported as the apparent vessel density [AVD = −ln(s(t)/s (0))], in which s(t) is the mean intensity in the tumor, and s (0) is the mean intensity of a distant muscle, as described (36). Vascular density was evaluated with the intrinsic contrast originating from deoxygenated hemoglobin. We have shown a good correlation between the AVD determined by gradient echo MRI and the density of blood-containing vessels (34, 36).
Functionality and maturation of the neovasculature were determined from gradient echo images acquired during the inhalation of air, air-CO2 (95% air and 5% CO2), and oxygen-CO2 (95% oxygen and 5% CO2; carbogen) as described (34). Four images were acquired at each gas mixture (65 s per image; slice thickness = 0.6 mm; repetition time = 130 ms; echo time = 10 ms; spectral width = 25,000 Hz; field of view = 3 cm; 256 × 128 pixels; in plane resolution = 110 μm; 4 averages). Other experimental details were as reported (34, 36).
Vascular function (VF) was derived from images acquired during inhalation of carbogen and air-CO2, and vasodilatation (VD) was derived from air and air-CO2 images, with the described equation (34, 36). The rational for this approach is that vessels coated with pericytes and smooth muscle cells, in contrast with immature endothelial capillaries, respond to hypercapnia (elevated CO2) by VD, whereas all functional blood vessels show a change in hemoglobin saturation in response to hyperoxia (elevated O2), reflecting the capacity of erythrocytes to deliver inhaled oxygen to the tumor vasculature. Both VD and changes in hemoglobin saturation are detected by gradient echo imaging with the intrinsic contrast generated by changes in deoxyhemoglobin, blood volume, and blood flow (34, 36). MRI data were analyzed on a PC computer with idl software (Research Systems, Boulder, CO). Data are presented in color maps overlaid on the original baseline image for absolute values of VD and VF >0.005 (34).
Results
Invasion Through Matrigel.
Nonmetastatic Eb lymphoma cells do not express the heparanase gene and activity (14, 31). In a recent study (30), we have demonstrated that viable Eb cells transfected with the chicken-hpa or with a chimeric construct (Chimeric-hpa) composed of the human enzyme and the chicken heparanase signal sequence, degraded the ECM′ HS to a much higher extent than cells transfected with the human enzyme (30). There was, however, no difference in heparanase activity when lysates of the same cells were compared. Moreover, partially purified human and chicken heparanases exhibited a similar apparent specific activity (30). Both the chicken and chimeric heparanases, unlike the human enzyme, are expressed on the cell surface and are readily secreted into the incubation medium (30). These results indicate that cell surface expression and secretion of heparanase is determined by the Chk-hpa signal peptide and markedly potentiate the ability of cells to degrade HS in a complex substrate such as the subendothelial ECM.
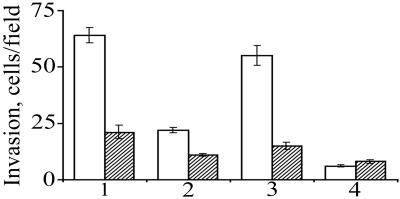
The Matrigel invasion assay was applied to evaluate whether cells transfected with secreted and nonsecreted species of heparanase differ in their ability to invade a reconstituted BM substrate. For this purpose, the various hpa-transfected cells were incubated on top of filters coated with Matrigel, in the absence and presence of laminaran sulfate, a 1–3 β-glucan, which inhibits heparanase activity (37). As shown in Fig. 1, overexpression of either the Chk-hpa or Chimeric-hpa resulted in a 3–10-fold increase in Matrigel invasion compared with cells transfected with either H-hpa or control insert-free plasmid. Cell invasion was inhibited in the presence of laminaran sulfate (Fig. 1). The four cell types did not differ in their ability to traverse through filters coated with collagen type IV, a process which dose not involve enzymatic degradation, indicating that they did not differ in cell motility (not shown). Evaluation of gelatinolytic activity by zymography, applying gelatin-impregnated gels, revealed no difference among the various hpa-transfected cells (not shown). These results indicate that cell surface expression and secretion of heparanase markedly facilitate lymphoma cell invasion through reconstituted BM.
Figure 1.
Matrigel invasion of lymphoma cells overexpressing secreted vs. nonsecreted heparanase. Eb-lymphoma cells transfected with (1) Chk-hpa, (2) H-hpa, or (3) Chimeric-hpa were incubated (1 × 106 cells per ml, 6 h, 37°C, pH 6.6) in RPMI medium supplemented with 0.1% BSA on top of Matrigel-coated filters in the absence (□) or presence (▨) of 10 μg/ml of laminaran sulfate. Mock-transfected cells (4) were used as control. The number of cells per microscopic field in the lower surface of the filter (cellular invasion) was determined as described in Materials and Methods. Each data point is the mean ± SD of triplicate filters (3 fields per filter).
Metastatic Dissemination and Survival Rate.
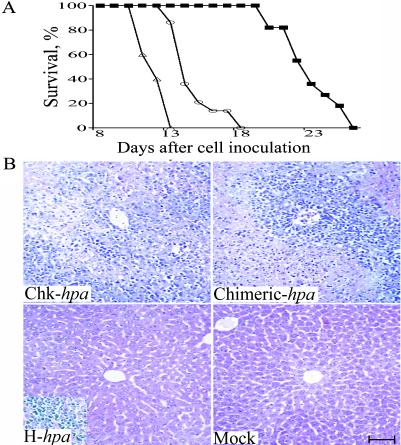
In view of the observed differences in heparanase activity (30) and cell invasiveness, we compared the metastatic potential of cells expressing the secreted vs. the nonsecreted forms of heparanase. For this purpose, CD1 nude mice were inoculated s.c. with Eb-lymphoma cells stable-transfected with Chk-hpa, H-hpa, Chimeric-hpa, or control pcDNA3 plasmid alone (n = 11, 15, 13, and 11 mice, respectively) (Fig. 2). All mice injected with cells overexpressing the chicken heparanase died on days 11–13 after cell inoculation (Fig. 2A). A slight delay (2–5 days) in mortality was observed in mice inoculated with Eb cells overexpressing the chimeric heparanase. Nearly 80% of these mice died on days 15–17 after cell inoculation (Fig. 2A). With both groups, the size of the primary tumors did not exceed 1% of the body weight. In contrast, a marked delay in mortality was observed in mice injected with cells transfected with the human hpa cDNA (Fig. 2A). These mice survived the first 3 weeks after cell inoculation and died during the 4th week, when the size of their s.c. tumors was about 10% of the body weigh. All mice injected with mock-transfected Eb cells were killed on day 30 after cell inoculation, when the primary tumor mass reached a size exceeding 15% of the body weight, to avoid excess pain and suffering. All mice were dissected and examined for the size and gross vascularity of the primary tumor and the extent of liver and lung colonization. Four extra mice of each group were killed on days 10 (2 mice) and 13 (2 mice) for histological examination of tissue sections derived from the liver tissue (Fig. 2B) and primary tumor (Fig. 3B). These mice were excluded from the survival curve. On these days, gross macroscopic examination of the liver revealed numerous liver metastases in all mice inoculated with Chk-hpa- or Chimeric-hpa-transfected cells vs. few or no visible metastatic nodules in the liver of mice injected with H-hpa- or mock-transfected Eb cells, respectively (not shown). The lungs of all mice seemed free of metastatic nodules, consistent with the preferential liver colonization of these lymphoma cells (38). Microscopic examination of the respective tissue sections revealed massive infiltration of Eb cells into the liver of mice inoculated with cells overexpressing the chicken- and chimeric-heparanase enzymes vs. little or no infiltration of the H-hpa- or mock-transfected cells (Fig. 2B). These differences were noted already when the mice were first killed, 10 days after cell inoculation (not shown), and were highly pronounced on day 13 when the entire liver was severely damaged and fully infiltrated with lymphoma cells overexpressing the secreted heparanase (Fig. 2B Upper). Primary tumors excised on days 10 and 13 were small (≈0.2 g) and had a similar size and weight, regardless of the type of inoculated Eb cells. This observation indicates that the accelerated mortality of mice injected with cells expressing the secreted forms of heparanase is the result of liver metastasis rather than the primary tumor mass. Later on (day 23), infiltration into the liver of H-hpa-transfected lymphoma cells was prominent (Fig. 2B Inset, Lower Left) but there was no infiltration of the mock-transfected cells. The various hpa- and mock-transfected Eb cells exhibited a similar proliferation rate in culture. Cell viability was nearly 100% and there were no signs of apoptosis. Altogether, these results emphasize the importance of cell surface expression and secretion of heparanase in accelerating liver colonization and mortality of the tumor-bearing mice.
Figure 2.
Overexpression of secreted heparanase in Eb-lymphoma cells promotes liver metastasis and accelerated mortality. (A) Survival rate. CD1 nude mice were inoculated s.c. with 1 × 106 Eb-lymphoma cells stable-transfected with Chk-hpa (▵), Chimeric-hap (○), H-hpa (■), or cells transfected with control pcDNA3 plasmid alone (not shown) (n = 11, 15, 13, and 11 mice, respectively). Survival of the mice was monitored daily. (B) Infiltration of hpa-transfected lymphoma cells into the liver. Two extra mice of each group were killed on day 13, and the liver tissue was processed and stained with hematoxylin/eosin for histological examination. Eb-lymphoma cells overexpressing the Chk-hpa or Chimeric-hpa infiltrated the entire liver tissue, as opposed to little or no infiltration of H-hpa- or mock-transfected cells, respectively. Infiltration of the liver by H-hpa-transfected cells was noted on day 23 (Inset, Lower Left) after cell inoculation. (Bar = 100 μM.)
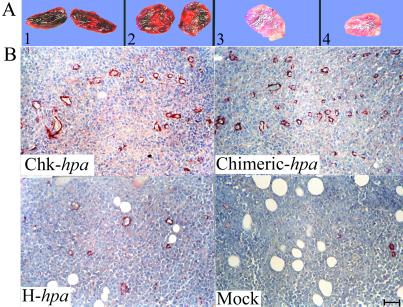
Figure 3.
Overexpression of secreted heparanase promotes tumor angiogenesis. (A) Macroscopic examination. CD1 nude mice were inoculated (s.c.) with transfected Eb-lymphoma cells as described in the legend to Fig. 2. Primary tumors were excised on day 13 after cell inoculation and photographed. Tumors produced by cells overexpressing the Chk-hpa (1) or Chimeric-hpa (2) appeared dark-reddish, as opposed to a pale appearance of tumors generated by H-hpa- (3), or mock-transfected (4) cells, reflecting a marked difference in vascularity, blood content, and hemorrhage. (B) Microvessel density. Primary tumors produced by the various transfected Eb cells (legend to Fig. 2) were excised on day 10, processed for histological examination, and 5 μm sections were stained with anti-smooth muscle actin (αSMA) Abs. (Upper) Tumors produced by cells overexpressing secreted heparanase. (Lower) Tumors produced by H-hpa- and mock-transfected cells. (Bar = 50 μM.)
Vascular Density and Functionality.
Despite their similar size, primary tumors produced by cells expressing the secreted and nonsecreted forms of heparanase showed a marked difference in blood content and hemorrhage. Whereas tumors produced by Chk-hpa- and Chimeric-hpa-transfected cells were dark-reddish, tumors generated by H-hpa- or mock-transfected Eb lymphoma cells appeared pale (Fig. 3A). The high vascularity of tumors produced by cells expressing the secreted enzyme was confirmed by histological examination of the respective tissue sections stained with anti-smooth muscle actin (αSMA) Abs (Fig. 3B). Representative tumor tissue sections derived from each of the four experimental groups are shown in Fig. 3B. Actual counts of microvessels revealed that in tumors produced by mock- or H-hpa-transfected Eb cells, the mean microvessel count was 2.7 ± 0.6 and 5.0 ± 1.7 vessels per ×200 microscopic field, respectively, vs. 13.0 ± 4.3 and 16.7 ± 5.8 microvessels per ×200 microscopic field in sections derived from tumors produced by Eb cells overexpressing the Chk-hpa and Chimeric-hpa, respectively (Student's t test, P = 0.003) (Fig. 3B).
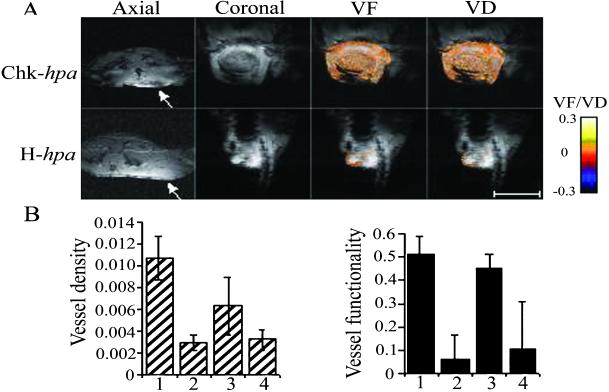
In subsequent experiments, primary tumors produced by Eb cells expressing the secreted and nonsecreted species of heparanase were compared in real time for their vascular density, functionality, and maturation. For this purpose, tumor-bearing mice were subjected to MRI on day 10 after cell inoculation. A markedly increased vascularity in tumors produced by Chk-hpa- vs. H-hpa-transfected cells was reflected by a decrease in the mean signal intensity inside the tumor [Fig. 4A, axial and coronal; dark area (Upper arrow) vs. bright area (Lower arrow)]. The AVD derived from transversal sections through the tumor demonstrated approximately five-fold higher vessel density in tumors expressing the secreted vs. the nonsecreted species of heparanase (Student's t test, P = 0.002) (Fig. 4B Left).
Figure 4.
MRI analysis of vessel density, functionality, and vasodilatation. Tumor-bearing mice were imaged on day 10 after cell inoculation as described in Materials and Methods. (A) Gradient echo imaging. Representative gradient echo images of primary tumors produced by (Upper) Chk-hpa- and (Lower) H-hpa-transfected cells are presented (Left, axial and coronal). Increased tumor vascularity is reflected by reduction of the mean signal intensity (dark area inside the tumor, Upper arrow). Functionality and maturation of the vasculature were derived from gradient echo images acquired during inhalation of air, air-CO2, and carbogen. Color-coded VF and VD maps were derived and overlaid (VF > 0.005; VD > 0.005, see color scale) on original images. Note the darker appearance and increased vessel functionality (VF) and maturation (VD) in tumors produced by (Upper) Chk-hpa- vs. (Lower) H-hpa-transfected Eb cells. (Bar = 1 cm.) (B) AVD and VF. The mean ± SD values of AVD (Left) and VF (Right) were calculated from the region of interest containing the whole tumor, applying 4 mice per group and 5 slices per mouse. The AVD (Left) was derived from transversal T*2-weighted MRI images through the tumor.
Functionality and maturation of the neovasculature were determined from gradient echo images acquired during the inhalation of air, air-CO2, and oxygen-CO2 (34). As shown in Fig. 4, there was a significant elevation in VF in tumors produced by cells overexpressing the Chimeric-hpa and Chk-hpa vs. those produced by H-hpa- and mock-transfected Eb cells (Student's t test, P = 0.001) (Fig. 4 A, VF and B Right). The extent of VF in the images was highest at the tumor periphery (Fig. 4A, VF). Maturation of the tumor vasculature appeared to proceed from the margins of the tumor inward, regardless of the inoculated cell type. However, an exceptionally high degree of vessel maturation (VD) was observed in the center of tumors generated by Chimeric-hpa (not shown) and Chk-hpa cells (Fig. 4A, VD), which was in agreement with actual staining of the corresponding histological sections with anti-smooth muscle actin (αSMA) Abs (Fig. 3B). Altogether, these results clearly indicate that cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and vascular maturation.
Discussion
Heparanase seems to function in implantation (5) and embryonic development of the vascular and nervous systems (30). In the adult, the enzyme functions in wound repair, tissue remodeling, HS turnover, and immune surveillance (8, 24). Taking into account the normal functions of the HS substrate and the heparanase enzyme and the potential tissue damage that could result from inadvertent cleavage of HS, tight regulation and balance of heparanase expression are essential. Our results emphasize the significance of cellular localization and secretion of the enzyme in the control of heparanase biological functions. We have identified a chicken-derived heparanase and demonstrated the significance of its signal peptide sequence in determining the cellular localization and secretion properties of the enzyme (30). Cells transfected with a chimeric construct, encoding the chicken-signal peptide preceding the human heparanase sequence, acquired cell surface localization and secretion of a functional enzyme (30). Applying the chicken- and chimeric-heparanase constructs, we have now demonstrated the essential involvement of cell surface expression and secretion of heparanase in promoting tumor angiogenesis and metastases formation. Nonmetastatic Eb mouse lymphoma cells overexpressing the secreted enzyme acquired a high invasiveness in vitro as revealed by their increased ability to traverse a reconstituted BM (Matrigel). In fact, both mock- and H-hpa-transfected Eb cells hardly invaded the Matrigel substrate, indicating that BM invasion by Eb lymphoma cells depends on cell surface expression and/or secretion of heparanase. HS side chains bind to various constituents of the ECM and hence play an important role in maintaining the integrity of the BM and ECM (1–4). It seems that degradation of the HS polysaccharide side chains enables accelerated cell invasion through Matrigel, as inhibition of heparanase activity by laminaran sulfate markedly suppresses cell invasion. The increased invasiveness of cells overexpressing the secreted enzyme was clearly reflected in vivo by a rapid and massive liver colonization and accelerated mortality of mice inoculated with these cells relative to cells expressing mostly the intracellular human enzyme. Whereas the delayed mortality of the latter mice was primarily the result of the primary tumor burden, mice inoculated with cells expressing the secreted enzyme succumb because of liver metastases and dysfunction.
The significance of cell surface localization and secretion of ECM-degrading enzymes in cancer metastasis was demonstrated by showing a correlation between the metastatic potential of breast and bladder carcinoma cells and translocation of cathepsins D and B from within lysosomes to the plasma membrane (39, 40). In support of these observations are our recent results showing a markedly increased lung colonization of B16-F1 mouse melanoma cells that were first incubated with recombinant pro-heparanase, washed free of the unbound enzyme, and then inoculated into the tail vein of mice. Another feature that may regulate the expression of a functional enzyme resides in the proteolytic activity that converts the latent proheparanase into an active enzyme (18). Processing of the enzyme at the N terminus occurs on the cell surface (14), again emphasizing the significance of cell surface localization of heparanase, although the nature of the involved proteolytic activity was not elucidated. Applying both histological analysis of vascular density in tissue sections and noninvasive MRI for in vivo mapping of vascular density, maturation, and functionality in the primary tumor (34, 36), we have demonstrated a remarkable proangiogenic response to cells overexpressing the secreted forms of heparanase. In comparison, lymphoma cells expressing the enzyme mostly intracellularly elicited a modest angiogenic response. Tumor cells overexpressing heparanase were shown to elicit neovascularization, as also reflected by stimulated tissue vascularity, granulation, and wound repair in response to exogenously added recombinant heparanase (24). We have recently generated homozygous transgenic mice overexpressing the heparanase cDNA and protein in all tissues (20). MRI analysis of a wound area made in the skin of these transgenic mice revealed an increased vascular density and functionality, again demonstrating the proangiogenic activity of heparanase (unpublished observations). These effects are attributed to heparanase-mediated release of active HS-bound basic fibroblast growth factor and vascular endothelial growth factor (VEGF), release of accessory HS degradation fragments that promote FGF-receptor binding and activation, and to stimulation of endothelial cell invasion and degradation of the subendothelial basal lamina (8, 24). In support of these results, microscopic examination of resected human bladder tumors revealed 6-fold higher microvessel counts in cancers with heparanase expression than in cancers without heparanase. There was no correlation with MMP-2 and MMP-9 expression in the same specimens (23). In other studies, analysis of pancreatic islets during tumor progression indicated that MMP-9 is a specific component of the angiogenic switch, primarily by rendering VEGF more accessible to its receptors (41).
Our findings emphasize the need for tight regulation and balance of heparanase expression, focusing on the critical significance of the compartmentalization, routing, and cellular localization of the enzyme. It is conceivable that malignant transformation is associated, in part, with overexpression of heparanase on the cell surface and/or its stimulated secretion. In fact, secreted heparanase was detected in wound fluid and in the urine of patients suffering from diabetes or aggressive metastatic disease (our unpublished observations). Moreover, human heparanase is secreted by activated platelets and cells of the immune system (i.e., neutrophils) (6). Also, expression of the heparanase protein on the cell surface was observed by immunostaining in a fraction of human tumor cells in tissue sections from carcinomas of the colon, breast, prostate, and ovary (19–22). Cell surface localization of the enzyme was also noted in endothelial cells of small angiogenic vessels of human tumors (24) and normal placenta (42).
Overexpression of heparanase on the cell surface may elicit cellular responses that are not directly related to increased degradation of HS. For example, we have demonstrated that at a physiological pH where little or no heparanase activity is detected, exogenously added heparanase binds to the cell surface, most likely to HS, and facilitates leukocyte adhesion (43). Our recent experiments indicate that cell surface expression and secretion of heparanase promote firm adhesion to ECM and endothelial cells of otherwise floating lymphoma cells (O.G., E.Z., M. Cohen, H.A., L. Nadav, I. Cohen, B.-Z. Katz, B. Gieger, and I.V., unpublished work). We propose that depending on the local pH, cells expressing the secreted and membrane-bound heparanase gain increased invasiveness and proangiogenic properties when situated at the slightly acidic pH of the tumor mass vs. increased adhesion and flattening at a physiological pH (pH > 7.2). Cell adhesion and ECM degradation are closely associated with both tumor angiogenesis and metastasis (44). Although cell adhesion precedes cell invasion, it seems that heparanase enzymatic activity is the principal factor in cell invasion, because its inhibition by laminaran sulfate inhibited Matrigel invasion by over 80% but had little or no effect on cell adhesion (O.G., E.Z., M. Cohen, H.A., L. Nadav, I. Cohen, B.-Z. Katz, B. Gieger, and I.V., unpublished work).
There are several ways by which the heparanase protein may be retained on the cell surface. Sequence analysis suggests that the heparanase enzyme may be a glycosylphosphatidylinositol-anchored protein and hence possibly located on specific membrane domains such as rafts. It may also exist as a putative transmembrane protein through a highly conserved hydrophobic region (residues 515–534) at the C terminus (7, 15). It has also been reported that in activated T-lymphocytes the enzyme can be displaced by mannose-6-phosphate, suggesting an interaction with cell surface receptors for mannose-6-phosphate (13). Interestingly, the signal peptide of the human heparanase resembles (≈65% homology) that of the mannose-6-phosphate receptor, responsible for lysosomal trafficking of enzymes, whereas that of the chicken enzyme exhibits less than 30% homology.
We propose that as long as the enzyme is confined to the intracellular compartment, its proangiogenic and prometastatic activities are highly restricted. The potential capacity of heparanase in promoting tumor cell dissemination and vascularization is fully expressed provided that the enzyme is first translocated to the cell surface and/or secreted. Understanding the control of heparanase routing, secretion, and retention on the cell surface is needed to better elucidate the significance of heparanase as a promising target for anticancer drug development and eventual inhibition of its hazardous proangiogenic and prometastatic effects. Factors affecting heparanase sorting, cellular localization, and secretion may similarly regulate its normal functions in embryonic development, inflammation, immune surveillance, and tissue repair.
Acknowledgments
We thank Dr. Tamara San for excellent technical assistance and Dr. R. Reich (Hebrew University) for his helpful advice. This work was supported by the Belfer Foundation, the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ), Israel's Ministry of Science (MOS), by Grant 503/98 from the Israel Science Foundation, and by the Association for International Cancer Research (U.K.).
Abbreviations
- HS
heparan sulfate
- ECM
extracellular matrix
- BM
basement membranes
- VF
vascular functionality
- VD
vasodilatation
- AVD
apparent vessel density
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kjellen L, Lindahl U. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 2.David G. FASEB J. 1993;7:1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park P W, Reizes O, Fitzgerald M L, Lincecum J, Zako M. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo R V. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey L A, Brunn G J, Platt J L. Trends Biochem Sci. 2000;25:349–351. doi: 10.1016/s0968-0004(00)01619-4. [DOI] [PubMed] [Google Scholar]
- 6.Vlodavsky I, Eldor A, Haimovitz-Friedman A, Matzner Y, Ishai-Michaeli R, Lider O, Naparstek Y, Cohen I R, Fuks Z. Invasion Metastasis. 1992;12:112–127. [PubMed] [Google Scholar]
- 7.Parish C R, Freeman C, Hulett M D. Biochim Biophys Acta. 2001;1471:M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Friedmann Y. J Clin Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima M, Irimura T, Nicolson G L. J Cell Biochem. 1988;36:157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- 10.Vlodavsky I, Mohsen M, Lider O, Svahn C M, Ekre H P, Vigoda M, Ishai-Michaeli R, Peretz T. Invasion Metastasis. 1994;14:290–302. [PubMed] [Google Scholar]
- 11.Parish C R, Coombe D R, Jakobsen K B, Bennett F A, Underwood P A. Int J Cancer. 1987;40:511–518. doi: 10.1002/ijc.2910400414. [DOI] [PubMed] [Google Scholar]
- 12.Parish C R, Freeman C, Brown K J, Francis D J, Cowden W B. Cancer Res. 1999;59:3433–3441. [PubMed] [Google Scholar]
- 13.Bartlett M R, Cowden W B, Parish C R. J Leukocyte Biol. 1995;57:207–213. doi: 10.1002/jlb.57.2.207. [DOI] [PubMed] [Google Scholar]
- 14.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, et al. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 15.Hulett M D, Freeman C, Hamdorf B J, Baker R T, Harris M J, Parish C R. Nat Med. 1999;5:803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 16.Kussie P H, Hulmes J D, Ludwig D L, Patel S, Navarro E C, Seddon A P, Giorgio N A, Bohlen P. Biochem Biophys Res Commun. 1999;261:183–187. doi: 10.1006/bbrc.1999.0962. [DOI] [PubMed] [Google Scholar]
- 17.Toyoshima M, Nakajima M. J Biol Chem. 1999;274:24153–24160. doi: 10.1074/jbc.274.34.24153. [DOI] [PubMed] [Google Scholar]
- 18.Fairbanks M B, Mildner A M, Leone J W, Cavey G S, Mathews W R, Drong R F, Slightom J L, Bienkowski M J, Smith C W, Bannow C A, et al. J Biol Chem. 1999;274:29587–29590. doi: 10.1074/jbc.274.42.29587. [DOI] [PubMed] [Google Scholar]
- 19.Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, Pecker I, Pappo O. Am J Pathol. 2000;157:1167–1175. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zcharia E, Metzger S, Chajek-Shaul T, Friedmann Y, Pappo O, Aviv A, Elkin M, Pecker I, Peretz T, Vlodavsky I. J Mammary Gland Biol Neoplasia. 2001;6:311–322. doi: 10.1023/a:1011375624902. [DOI] [PubMed] [Google Scholar]
- 21.Koliopanos A, Friess H, Kleeff J, Shi X, Liao Q, Pecker I, Vlodavsky I, Zimmermann A, Buchler M W. Cancer Res. 2001;61:4655–4659. [PubMed] [Google Scholar]
- 22.Ginath S, Menczer J, Friedmann Y, Aingorn H, Aviv A, Tajima K, Dantes A, Glezerman M, Vlodavsky I, Amsterdam A. Int J Oncol. 2001;18:1133–1144. doi: 10.3892/ijo.18.6.1133. [DOI] [PubMed] [Google Scholar]
- 23.Gohji K, Hirano H, Okamoto M, Kitazawa S, Toyoshima M, Dong J, Katsuoka Y, Nakajima M. Int J Cancer. 2001;95:295–301. doi: 10.1002/1097-0215(20010920)95:5<295::aid-ijc1051>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I. FASEB J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 26.Vlodavsky I, Miao H Q, Medalion B, Danagher P, Ron D. Cancer Metastasis Rev. 1996;15:177–186. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- 27.Vlodavsky I. In: Tumour Angiogenesis. Lewis C E, Bicknell R, Ferrara N, editors. Oxford: Oxford Univ. Press; 1997. pp. 125–140. [Google Scholar]
- 28.Bame K J. Glycobiology. 2001;11:91R–98R. doi: 10.1093/glycob/11.6.91r. [DOI] [PubMed] [Google Scholar]
- 29.Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, Fabra A. Biochem J. 1997;327:917–923. doi: 10.1042/bj3270917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldshmidt O, Zcharia E, Aingorn H, Guatta-Rangini Z, Atzmon R, Michal I, Pecker I, Mitrani E, Vlodavsky I. J Biol Chem. 2001;276:29178–29187. doi: 10.1074/jbc.M102462200. [DOI] [PubMed] [Google Scholar]
- 31.Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Cancer Res. 1983;43:2704–2711. [PubMed] [Google Scholar]
- 32.Vlodavsky I. Current Protocols in Cell Biology. Vol. 1. New York: Wiley; 1999. pp. 10.4.1–10.4.14. [Google Scholar]
- 33.Elkin M, Reich R, Nagler A, Aingorn E, Pines M, de-Groot N, Hochberg A, Vlodavsky I. Clin Cancer Res. 1999;5:1982–1988. [PubMed] [Google Scholar]
- 34.Abramovitch R, Dafni H, Smouha E, Benjamin L E, Neeman M. Cancer Res. 1999;59:5012–5016. [PubMed] [Google Scholar]
- 35.Elkin M, Ariel I, Miao H Q, Nagler A, Pines M, de-Groot N, Hochberg A, Vlodavsky I. Cancer Res. 1999;59:4111–4118. [PubMed] [Google Scholar]
- 36.Abramovitch R, Frenkiel D, Neeman M. Magn Reson Med. 1998;39:813–824. doi: 10.1002/mrm.1910390519. [DOI] [PubMed] [Google Scholar]
- 37.Miao H Q, Elkin M, Aingorn E, Ishai-Michaeli R, Stein C A, Vlodavsky I. Int J Cancer. 1999;83:424–431. doi: 10.1002/(sici)1097-0215(19991029)83:3<424::aid-ijc20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 38.Cheingsong-Popov R, Robinson P, Altevogt P, Schirrmacher V. Int J Cancer. 1983;32:359–366. doi: 10.1002/ijc.2910320316. [DOI] [PubMed] [Google Scholar]
- 39.Sloane B F, Moin K, Sameni M, Tait L R, Rozhin J, Ziegler G. J Cell Sci. 1994;107:373–384. doi: 10.1242/jcs.107.2.373. [DOI] [PubMed] [Google Scholar]
- 40.Rochefort H, Liaudet E, Garcia M. Enzyme Protein. 1996;49:106–116. doi: 10.1159/000468620. [DOI] [PubMed] [Google Scholar]
- 41.Bergers G, Brekken R, McMahon G, Vu T H, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haimov-Kochman R, Friedmann Y, Prus D, Goldman-Wohl D, Greenfield C, Anteby E, Aviv A, Vlodavsky I, Yagel S. Mol Hum Reprod. 2002;8:566–573. doi: 10.1093/molehr/8.6.566. [DOI] [PubMed] [Google Scholar]
- 43.Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O. J Exp Med. 1995;181:1929–1934. doi: 10.1084/jem.181.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varner J A, Cheresh D A. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]