Abstract
A number of poorly characterized genetic modifiers contribute to the extensive variability of von Willebrand disease, the most prevalent bleeding disorder in humans. We find that a genetic lesion inactivating the murine ST3Gal-IV sialyltransferase causes a bleeding disorder associated with an autosomal dominant reduction in plasma von Willebrand factor (VWF) and an autosomal recessive thrombocytopenia. Although both ST3Gal-IV and ST6Gal-I sialyltransferases mask galactose linkages implicated as asialoglycoprotein receptor ligands, only ST3Gal-IV deficiency promotes asialoglycoprotein clearance mechanisms with a reduction in plasma levels of VWF and platelets. Exposed galactose on VWF was also found in a subpopulation of humans with abnormally low VWF levels. Oligosaccharide branch-specific sialylation by the ST3Gal-IV sialyltransferase is required to sustain the physiologic half-life of murine hemostatic components and may be an important modifier of plasma VWF level in humans.
Von Willebrand factor (VWF) is a multimeric plasma sialoglycoprotein that stabilizes coagulation factor VIII and plays an essential role in hemostasis by mediating platelet aggregation to subendothelium at sites of vascular injury (reviewed in refs. 1 and 2). VWF levels in plasma vary widely among the human population, with particularly low VWF levels or dysfunctional VWF resulting in von Willebrand disease (VWD). VWD is the most common inherited bleeding disorder in humans. Inheritance is typically autosomal dominant with incomplete penetrance and highly variable expressivity. There are also a number of distinct variants of the disease, each with a specific qualitative functional defect, leading to a complex classification scheme (reviewed in ref. 3). A large number of mutations within the VWF gene have been identified in VWD individuals, particularly in patients with qualitative variants of VWD; however, the genetic basis for the wide variation in plasma VWF levels among both normal and VWD individuals remains largely unknown. VWF function appears conserved among mammalian species, indicating that animal models may be useful in identifying the molecular mechanisms and underlying genetic factors modifying VWF homeostasis and the severity of VWD (reviewed in ref. 4).
The glycosylation of VWF comprises a posttranslational mechanism capable of modulating VWF stability and function in circulation. Enzymatic elimination of multiple glycan structures, including sialic acid linkages, has been shown to alter VWF proteolytic degradation, platelet aggregation function, multimerization, and half-life in plasma (5–10). These findings suggest the possibility that inherited genetic lesions in the pathways of glycan synthesis could modify the severity of VWD and may be directly responsible for VWD in some cases. However, such lesions would likely be less severe in altering glycan structure, otherwise viability could be affected in mammalian gestation and postnatal development (11). Some of the variance in normal plasma VWF levels has been associated with the ABO blood group determinants, because humans with blood type O due to homozygosity for null alleles in the H-locus glycosyltransferase generally have reduced VWF levels (12, 13). The remarkable finding of an inherited genetic mutation altering cell type-specific expression of the Galgt2 glycosyltransferase in the RIIIS/J mouse, which resulted in an autosomal dominant VWF deficiency, revealed that enzymes in the glycosylation pathway could also play a dominant role in VWF homeostasis (14).
Sialic acids are negatively charged molecules existing in either α2–3, -6, or -8 linkages on various glycan types and are generated by a family of 18 sialyltransferase genes differentially expressed among tissues (15–18). Sialic acids are most commonly linked to the penultimate galactose (Gal) or N-acetylgalactosamine (GalNAc) on glycan branches and can be essential in the formation of ligands for endogenous sialic acid-specific lectins such as the Siglecs (reviewed in ref. 19), the Selectins (reviewed in ref. 20), or pathogen receptors (21). However, they can also mask ligands involving Gal or GalNAc recognition by the galectins and asialoglycoprotein receptors (ASGPRs) (reviewed in refs. 22 and 23).
The majority of sialic acids attached to plasma components are α2–3 linked and produced by up to six different ST3Gal genes encoding sialyltransferases ST3Gal-I–VI. Only the ST6Gal-I sialyltransferase has been implicated in hemostatic regulation, by producing α2–6 sialic acid linkages that can block recognition of VWF by ASGPRs (9). However, ST6Gal-I-deficient mice exhibit a B lymphocyte defect but otherwise appear normal (24). Studies herein of mice bearing distinct sialyltransferase lesions identify ST3Gal-IV as a modulator of hematologic components and provide mechanistic insights regarding sialic acid linkage-specific function in hemostasis.
Methods
Gene Targeting and Mutant Mouse Production.
Mouse ST3Gal-IV genomic DNA was isolated for use in gene-targeted mutagenesis procedures as previously described (25). In screening for homologous recombinants, the wild-type allele was detected by using PCR primers adjacent to the deleted region (W5′: 5′-GACGCCATCCACCTATGAG and W3′: 5′-GGCTGCTCCCATTCCACT-3′), resulting in a 260-bp fragment. The mutant allele was detected by using W5′ and a primer from the loxP region (M3′: 5′-GGCTCTTTGTGGGACCATCAG-3′), yielding a 450-bp fragment.
RNA Analysis.
Total RNA from indicated tissues was isolated and analyzed with a mouse ST3Gal-IV cDNA probe containing the entire protein-coding sequence, by procedures described (25).
Hematology.
Blood from the tail vein of methoxyfluorane-anethesized mice was collected into EDTA-containing polypropylene microtubes (Becton Dickinson). Analyses were carried out with a CELL-DYN 3500 (Abbott Labs, Abbott Park, IL) calibrated with normal mouse blood and microscopic examination with Wright–Giemsa stain. For clotting assays, plasma samples were prepared from whole blood collected by cardiac puncture in one-tenth of volume buffered citrate anticoagulant (0.06 mol/liter of sodium citrate/0.04 mol/liter of citric acid, pH 7.4).
Bleeding Time.
Mice were anesthetized and restricted horizontally. The tail was severed 2 mm from the tip with a razor blade and immersed vertically 1 cm below the surface of 37°C saline. Time until bleeding stopped was recorded. The tail was cauterized when bleeding times exceeded 10 min.
Coagulation Factor Analyses.
VWF levels were measured by an Elisa approach by using the anti-VWF antibody (DAKO), as previously described (14). Factor VIII and other coagulation factors were assayed as described (26).
Multimer Analysis.
Mouse plasma samples were diluted 1:25 and separated by SDS-agarose electrophoresis in standard SDS/PAGE running buffer by using a miniProtean II apparatus (BioRad). Gel and loading buffer conditions were as described (27). Gels were blotted onto Immobilon P membranes (Millipore) by capillary transfer overnight in PBS. Multimers were detected by incubating the membrane with horseradish peroxidase labeled anti-human VWF antibody (DAKO) at a 1:500 dilution and visualized by enhanced chemiluminescence (Amersham Pharmacia).
Lectin Binding.
Plates coated with anti-VWF antibodies (DAKO) and blocked with BSA were prepared. Plasma was added at 1:8 to 1:100 dilution. After washing, biotinylated Ricinus communis agglutinin-1 (RCA-I), Erythrina cristagalli (ECA), Sambucus nigra (SNA), or peanut agglutinin (PNA) lectins (Vector Laboratories) at 1 μg/ml was added. For VWF antigen determination in parallel anti-VWF, horseradish peroxidase-conjugated antibodies were added. Lectin binding was detected by using an avidin and biotinylated-horseradish peroxidase (ABC) detection system and developed with 3,3′,5,5′ tetramethylbenzidine (TMB, Bio-Rad). Binding was analyzed at 655 nm on a spectrophotometric plate reader (Molecular Devices) with softmax software. For VWF desialylation and lectin-binding analysis, whole plasma pooled from more than 20 wild-type mice was treated with 0.3 units Arthrobacter ureafaciens neuraminidase (Sigma) in the supplied buffer, pH 6.0, at 37°C for 90 min. Serial dilutions of 100 μl were analyzed as above.
VWF Clearance Measurements.
Blood was obtained by cardiac puncture into citrate from three to six wild-type or ST3Gal-IVΔ/Δ mice. Plasma proteins were biotinylated in fresh EDTA-anticoagulated plasma by using biotin-N-hydroxylsuccinimide (Calbiochem), followed by i.v. injection of 200 μl per mouse. Blood was collected from the tail vein at 0, 2, 4, and 6 h. VWF in these fractions was captured on anti-VWF antibody-coated plates, and biotinylated VWF was detected by using ABC/TMB as above. In experiments to detect ASGPR involvement, plasma obtained from ST3Gal-IVΔ/Δ mice was similarly injected with or without tissue-culture grade pyrogen-free fetuin preparations. Asialofetuin was prepared by mild acid hydrolysis as described (28). All fetuin preparations were tested for potential pyrogenic activity and contamination by a modified version of a blood mononuclear cell IL-6 secretion assay (29).
Platelet Cytometry and Clearance.
Whole blood from the tail vein was collected in EDTA microtubes and diluted in Tyrode's buffer. Platelets were stained with anti-CD41 FITC (Becton Dickinson) and RCA-I biotin (Vector Laboratories) followed by streptavidin-cytochrome (Becton Dickinson). Data from 10,000 platelet events determined by anti-CD41 binding as well as forward- and side-scatter were analyzed on a FACScan flow cytometer by using cellquest software (Becton Dickinson). In clearance studies, ST3Gal-IVΔ/Δ mice were injected i.p. with 10 mg of fetuin or asialofetuin at 0, 4, and 8 h. Blood was collected from the tail vein at 0, 4, 8, and 12 h into EDTA tubes and analyzed for platelet levels as above.
Statistical Analysis.
Data were analyzed by ANOVA and Scheffé's t test for unpaired samples.
Results
ST3Gal-IV Sialyltransferase Structure and Mutagenesis.
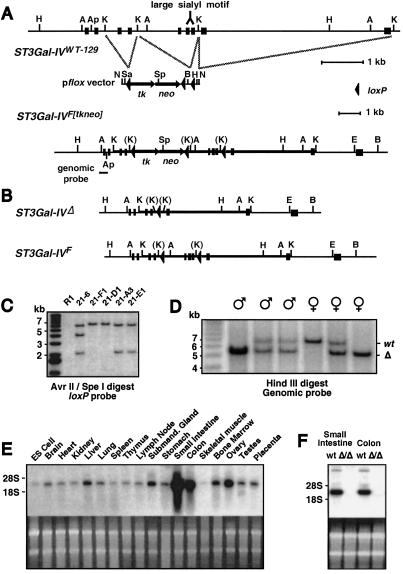
The murine ST3Gal-IV sialyltransferase is a type II transmembrane protein localized to the Golgi apparatus and encoded by a gene spanning 10 kb containing 10 exons (16). A genomic clone encompassing the first 9 exons was isolated and characterized for producing a mutant allele in embryonic stems cells by Cre-loxP gene-targeting approaches (Fig. 1A). All sialyltransferases share structural features that include the large sialyl motif shown to be essential for nucleotide sugar binding and enzymatic activity (30); therefore, we mutagenized ST3Gal-IV by flanking exons 5–7 containing the large sialyl motif with loxP sites (F allele), resulting in the deletion of these sequences on Cre recombination (Δ allele) (Fig. 1B) and a translational frameshift within exon 8 (data not shown). Targeted embryonic stem cells bearing the expected allelic structures (Fig. 1C) were used to generate mice as described (25). Mice homozygous for the Δ allele were produced in Mendelian ratios among heterozygous matings with both male and female mice fully fertile and appearing normal (Fig. 1D and data not shown).
Figure 1.
ST3Gal-IV mutation in the germline of mice ablates the large sialyl motif and results in loss of RNA. (A) An ST3Gal-IV genomic isolate in germline configuration was used in conjunction with the pflox vector to construct a targeting vector in which exons 5–7 containing the large sialyl motif (16) were flanked by loxP sites for deletion (ST3Gal-IVF[tkneo]). Restriction enzyme sites indicated are BamHI (B) AvrII (A), EcoRI (E), Hind III (H), KpnI (K), NotI (N), SalI (Sa), and SpeI (Sp). (B) Transient Cre expression in ST3Gal-IV gene-targeted R1 embryonic stem cells produces subclones heterozygous for the Δ (systemic deleted) or F (conditional) mutations illustrated. (C) Southern blot analysis of AvrII- and SpeI-digested embryonic stem cell DNA probed with a loxP probe confirmed the expected genomic structures. Three loxP sites are present in a targeted parental clone (21–6), whereas one loxP site is present in each of two ST3Gal-IVΔ/wt subclones (21-F1 and 21-D1), and two loxP sites are present in the ST3Gal-IVF/wt subclones (21-A3 and 21-E1). (D) Tail DNA derived from offspring of parents heterozygous for the Δ allele was digested with HindIII and probed with the indicated genomic probe to reveal the 6.8-kb wild-type allele and the 5.3-kb deleted (Δ) allele. (E) ST3Gal-IV RNA expression in normal wild-type mouse tissues. (F) ST3Gal-IV RNA expression is deficient among ST3Gal-IVΔ/Δ mice.
ST3Gal-IV RNA expression is widespread among tissues and cell types, with highest levels in the small intestine and colon (Fig. 1E). In mice homozygous for the Δ allele, ST3Gal-IV RNA levels were below detection by Northern blot analysis, indicating that the deletion of genomic sequences including exons 5–7 destabilizes ST3Gal-IV Δ RNA (Fig. 1F). The ST3Gal-IV Δ allele was bred into the C57BL/6 strain for more than five generations for the following studies.
An Autosomal Dominant Bleeding Disorder Associated with Reduced VWF and Factor VIII.
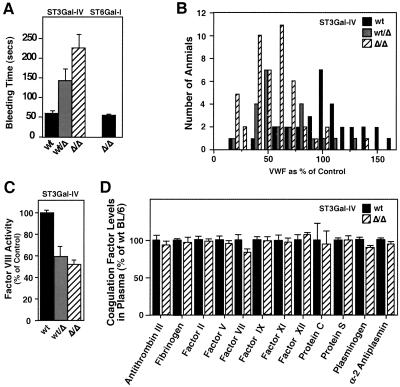
A determination of bleeding time after tail transection indicated a significant increase among mice bearing the ST3Gal-IV Δ allele (Fig. 2A). In contrast, mice lacking the ST6Gal-I sialyltransferase (24) exhibited normal bleeding times (Fig. 2A). Measurements of blood coagulation factor levels revealed a deficiency in plasma VWF in mice heterozygous and homozygous for the ST3Gal-IV Δ allele (Fig. 2B). VWF mRNA expression among various tissues was unaltered compared with wild-type littermates (data not shown).
Figure 2.
Increased bleeding time with decreased levels of VWF and Factor VIII occurs with ST3Gal-IV deficiency but not with ST6Gal-I deficiency. (A) Bleeding times were analyzed among 6- to 12-wk-old littermates of the indicated genotypes. Results are from over 30 mice of each ST3Gal-IV genotype and 11 of each ST6Gal-I genotype are expressed as means ± SEM. Significantly increased bleeding times for ST3Gal-IVΔ heterozygotes and homozygotes were observed (P < 0.05 and P < 0.001, respectively). (B) VWF levels in plasma were measured from over 30 mice of each genotype and plotted as a percentage of the mean value obtained from ST3Gal-IV wild-type littermates. A significant reduction in plasma VWF was observed in both heterozygous and homozygous ST3Gal-IV-deficient mice to a mean of 57 and 50%, respectively (P < 0.001). No decrease in plasma VWF levels was observed in mice lacking ST6Gal-I. (C) Factor VIII (F VIII) levels were also reduced in mice analyzed in B (P < 0.001). ST6Gal-I deficiency had no effect on F VIII levels in circulation (not shown). (D) Levels of blood coagulation factors were analyzed from 40 mice of indicated genotypes. No alterations of hemostatic significance were observed.
Factor VIII levels in plasma were reduced similarly compared with its carrier molecule VWF (Fig. 2C). Other coagulation factors were, however, present at normal levels in plasma (Fig. 2D), and analyses of serum chemistry indicated normal hepatic and renal function (data not shown). The cause of the increased bleeding times is not known but is not likely due to the degree of VWF deficiency observed, and analysis of VWF function in a collagen-binding assay indicated no change in this activity (data not shown).
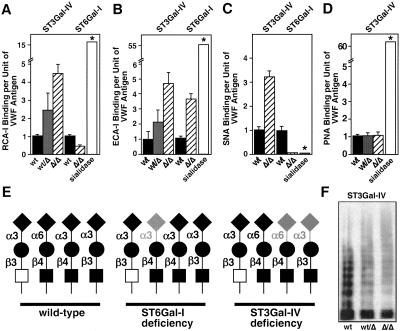
Deficient Sialylation of VWF in ST3Gal-IV and ST6Gal-I Deficiency with Normal VWF Multimer Formation.
Loss of either ST3Gal-IV or ST6Gal-I sialyltransferase activity would be expected to increase exposure of subterminal β-linked Gal on various glycan branches. Reactivity of the β-Gal-binding lectin RCA-I (ref. 31) to VWF was increased in ST3Gal-IV mutant plasma samples (Fig. 3A). Unexpectedly, reduced RCA-1 binding to VWF was observed in ST6Gal-I deficiency (Fig. 3A). The reason for this reduction is unclear but may reflect uncharacterized influences of other terminal glycan structures in RCA-1 binding. Analyses after neuraminidase (sialidase) treatment to remove all α2–3 and -6 sialic acids linkages indicated that sialic acids remained on VWF in the absence of either ST3Gal-IV or ST6Gal-I (Fig. 3A).
Figure 3.
VWF glycosylation deficiencies and multimer formation. Alterations in lectin binding are compared with results obtained by using purified sialidase treatment (*; see Methods). (A) RCA-I lectin binding to plasma-derived VWF from wild-type and ST3Gal-IV mutant mice indicates increased exposure of terminal β-linked Gal on VWF from both heterozygous and homozygous mutant samples. Reduced Ricin binding to VWF from ST6Gal-I-deficient mice was observed. (B) ECA-I lectin binding to Galβ1–4GlcNAc- termini on VWF is increased in mice heterozygous or homozygous for ST3Gal-IV gene mutation. Absence of ST6Gal-I also results as expected in increased ECA binding to VWF. (C) SNA lectin binding to VWF is eliminated in ST6Gal-I deficiency, as expected, but increased in ST3Gal-IV deficiency. (D) PNA lectin binding to Galβ1–3GalNAc- is unaltered on VWF glycans from ST3Gal-IV mutant mice. (E) Possible N-glycan branch termini structures of wild-type and mutant VWF. Wild-type VWF bears highly sialylated N-glycan branches with both α3- and α6-linked sialic acids. Absence of ST6Gal-I eliminates only α6-linked sialic acid, although a partial compensation by ST3Gal-IV may occur (lightly shaded sialic acid). Separately, ST3Gal-IV deficiency exposes ECA-reactive Galβ1–4 GlcNAc termini and may also expose the Galβ1–3GlcNAc- disaccharide. Partial compensation (shaded sialic acid linkage) by ST6Gal-I occurs, whereas no sialyltransferase exists that can add α6 linkages to Galβ1–3GlcNAc-. However, some degree of α3 sialylation of Galβ1–3GlcNAc- may occur (shaded sialic acid). No changes occur in the sialylation of the Core 1 O-glycan Galβ1–3GalNAc-. (F) Multimer analysis of plasma VWF from wild-type and ST3Gal-IV mutants indicates normal multimer formation in mutant samples. Data are representative of three separate experiments. Lectin binding in A–C was quantitated by ELISA and plotted as the ratio of lectin binding per unit of VWF antigen (see Methods). Data in A–C are means ± SEM as derived from analyses of 12 or more mice of each genotype in each assay. Saccharide symbols: GlcNAc, black square; GalNAc; open square; Gal, black circle; sialic acid, black diamond.
Gal exposure on the type II branch structure Galβ1–4GlcNAc- was detected by using ECA-I (refs. 32 and 33). An increase in ECA-I binding to VWF was observed among plasma samples from both ST3Gal-IV- and ST6Gal-I-deficient mice (Fig. 3B). ECA binding after sialidase treatment was greatly increased, suggesting that sialic acid linkages to the type II glycan branch structure remained with either sialyltransferase deficiency. Further evidence of ST6Gal-I sialylation of VWF was obtained by using the SNA lectin, which specifically binds to the sialylated type II glycan (Siaα2–6Galβ1–4GlcNAc-; ref. 34). Complete loss of SNA binding to VWF was observed in mice lacking the ST6Gal-I sialyltransferase or VWF treated with sialidase. However, increased SNA binding to VWF was evident among ST3Gal-IV-deficient mice suggesting compensation by ST6Gal-I (Fig. 3C). PNA lectin binds specifically to the unsialylated O-glycan Core 1 branch structure: Galβ1–3GalNAc- (35). No change in PNA binding to VWF occurred in mice deficient in either ST3Gal-IV or ST6Gal-I (Fig. 3D).
Lectin-binding profiles to VWF are consistent with the presence of glycan terminal branch sequences indicated in Fig. 3E (Left). These branches may also be modified in some cases by α1–2-linked fucose on the penultimate Gal, by α1–3-linked fucose on the N-acetylglucosamine proximal to the β-linked Gal, or in the mouse by terminal α1–3 Gal (not shown). In ST6Gal-I-deficient mice, a fraction of type II branch termini may be sialylated by other sialyltransferases bearing overlap in substrate specificity, such as ST3Gal-III, -IV, or -VI (Fig. 3E Center). However, such compensation would be incomplete as ECA-I binding to VWF is increased in ST6Gal-I-deficient mice. In contrast, VWF in ST3Gal-IV-deficient mice may have exposed Gal on types II and I (Galβ1–3GlcNAc-) glycans, should both chains be present on mouse VWF (Fig. 3E Right). Compensation by ST6Gal-I is evident from the increased SNA binding to VWF. However, this compensation is incomplete, as indicated by increased ECA-I binding. These alterations in glycan sialylation in ST3Gal-IV deficiency do not, however, result in a defect in VWF multimerization (Fig. 3F).
ST3Gal-IV Mutation Alters VWF Trafficking and Half-Life by ASGP Recognition and Clearance Mechanisms.
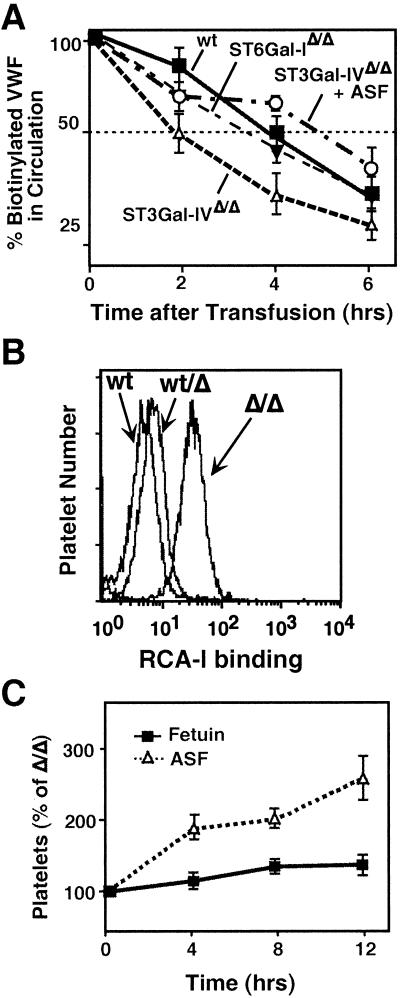
Elimination of sialic acids can reduce the half-life of some glycoproteins in circulation by ASGP clearance mechanisms (reviewed in ref. 36). ASGPRs exist among hepatocytes and macrophages in organs including the liver and spleen. We observed a significant increase in VWF antigen levels in the liver of ST3Gal-IV mutant mice (data not shown) and investigated whether the reduction in plasma VWF was due to ASGPR recognition and clearance.
Mouse plasma samples were biotinylated before i.v. injection into wild-type mice in the presence or absence of coinjected fetuin glycoprotein preparations. The levels of biotinylated VWF in plasma were measured over a time course to obtain the half-life of VWF in circulation (Fig. 4A). Wild-type VWF has a half-life of 4.5 h, whereas VWF from ST3Gal-IV homozygous mutant mice exhibits a half-life of 1.9 h. A half-life of 2.7 h was observed for heterozygote-derived VWF (data not shown). No change in VWF half-life was observed among ST6Gal-I-deficient plasma samples (Fig. 4A). Injection of native sialylated fetuin did not alter the rate of glycoprotein clearance from plasma (data not shown); however, coinjection of asialofetuin, which saturates ASPGRs, resulted in the restoration of normal half-life in circulation for VWF in ST3Gal-IV deficiency.
Figure 4.
Decreased VWF half-life and platelet homeostasis corrected by blocking ASGPR. (A) Plasma fractions from mice of indicated genotypes were biotinylated and injected into wild-type mice in the presence or absence of 10 mg of pyrogen-free asialofetuin. Biotinylated VWF levels in blood were analyzed at the indicated times. Data are derived from three mice per treatment group and are representative of two separate experiments. In additional experiments that included native fetuin, only asialofetuin was able to block ASGP clearance and increase half-life (data not shown). (B) RCA lectin binding to platelets was increased among blood samples from mice homozygous for the ST3Gal-IV mutation as detected by flow cytometric analysis. A slight increase in RCA-I binding to platelets is seen in heterozygous samples. Data are representative of three separate experiments. (C) Mice homozygous for the ST3Gal-IV gene deletion were injected with asialofetuin or fetuin at 0, 4, and 8 h. Platelet levels were analyzed by flow cytometry of whole blood at the timepoints indicated. Data are the means ± SEM from 10 mice per group.
Autosomal Recessive Thrombocytopenia Corrected by Blocking ASGPR in Vivo.
Both ST3Gal-IV and ST6Gal-I mutant mice were subjected to an extensive hematologic examination. No remarkable findings were noted among ST6Gal-I null mice; however, ST3Gal-IV deficiency resulted in a marked decrease in platelets to 30% of normal with a substantial increase in platelet volume among homozygotes (Table 1). This could reflect sequestration and degradation of platelets in the spleen; however, splenomegaly was not present, and no increase in platelet numbers occurred on splenectomy (data not shown). Megakaryocytes were not diminished among the bone marrow and spleen; in fact, there was a trend toward an increase in megakaryocyte numbers with normal cellular morphology (data not shown). We suspected that the thrombocytopenia results from an increase in platelet clearance by ASGPRs expressed among nonsplenic tissue.
Table 1.
Hematology
| Wild type | ST3Gal-IVwt/Δ | ST3Gal-IVΔ/Δ | ST6Gal-IΔ/Δ | |
|---|---|---|---|---|
| WBC, cells per μl | 6,239 ± 161 | 5,568 ± 542 | 5,455 ± 407 | 5,856 ± 235 |
| RBC, m/μl | 9.0 ± 0.07 | 8.9 ± 0.08 | 8.5 ± 0.09 | 8.8 ± 0.08 |
| HGB, g/dl | 14.1 ± 0.11 | 13.9 ± 0.13 | 13.3 ± 0.12 | 14.3 ± 0.12 |
| HCT, % | 45.7 ± 0.36 | 44.5 ± 0.55 | 42.5 ± 0.45 | 45.0 ± 0.41 |
| PLT, k/μl | 1,026 ± 56 | 1,117 ± 43 | 363 ± 25* | 1,012 ± 52 |
| MPV, fl | 4.8 ± 0.18 | 5.6 ± 0.26 | 8.4 ± 0.39* | 4.6 ± 0.20 |
| Sample size, n | 33 | 31 | 49 | 23 |
Values are presented as means ± SEM. WBC, white blood cells; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; PLT, platelet; MPV, mean platelet volume.
, P < 0.001 between wild-type and Δ/Δ genotypes.
A significant increase in β-linked Gal exposure was observed on the platelet cell surface of homozygous mutants in ST3Gal-IV measured by RCA-I binding in flow cytometric analyses (Fig. 4B). Platelets from heterozygotes had very slightly increased levels of β-linked Gal exposure. To determine whether ASGPRs were involved, i.p. injections of asialofetuin or sialylated fetuin were administered. ST3Gal-IV mutant mice receiving native sialylated fetuin continued to exhibit thrombocytopenia, whereas those receiving asialofetuin responded with an increase in circulating platelets to normal levels within 12 h (Fig. 4C).
Gal Exposure on VWF in a Patient Subpopulation with Abnormally Low VWF Levels.
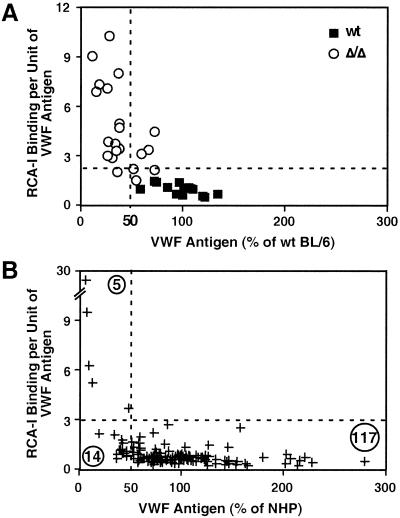
RCA-I lectin binds to VWF antigen from wild-type mice with an RCA-I/VWF ratio of 1.25:1. A substantial increase in this ratio to more than 2 SD above the mean occurs in mice lacking ST3Gal-IV (Fig. 5A). However, variation remained from 3:1 to 11:1, suggesting the presence of other genetic and stress modifiers that may contribute to VWF plasma levels.
Figure 5.
RCA/VWF ratios in ST3Gal-IV deficiency are similar to those in a subpopulation of humans bearing abnormally low plasma VWF levels. (A) Ratios of RCA-I binding to VWF antigen level in mouse plasma consist of low values in wild-type littermates. In ST3Gal-IV mutant mice, ratios are almost always increased to greater than 2 SD above the mean, as indicated by the dotted line. Data reflect more than 15 mice of each genotype. (B) A subpopulation of humans bearing abnormally low VWF levels shows increased RCA/VWF ratios more than 2 SD from the mean. No examples of similarly increased RCA/VWF ratios were found among samples bearing more than 50% the normal level of VWF.
Plasma from patients referred to the Special Coagulation Laboratory at the University of California at San Diego Hillcrest Hematology Clinic over a 2-yr period for a real or suspected bleeding disorder were analyzed for RCA-I/VWF ratios. VWF levels were expressed as a percentage of the value obtained from a pooled normal human plasma standard. Of 136 human patient samples analyzed, 117 contained VWF levels at 50% or more of the pooled normal human plasma standard. However, 19 patients were deficient in VWF by 50% or more. The VWF from 5 of these 19 patients had RCA-I/VWF ratios of more than 2–8 SD above the mean value. Moreover, four of those five patients had the lowest VWF levels recorded among all 136 patient samples (Fig. 5B). In no case did an increase in RCA-I/VWF ratio above 2 SD from the mean occur in human plasma samples bearing 50% or more of the normal level of VWF. These data reveal that a subpopulation of humans with abnormally low plasma levels of VWF have unusually high β-linked Gal or GalNAc exposure that may target VWF for ASGPR recognition and clearance.
Discussion
The ST3Gal-IV sialyltransferase is distinct from ST6Gal-I in the ability to modulate hemostasis by differential sialylation of VWF in the mouse. Previous studies of the stabilizing role of sialic acids in VWF homeostasis used “global” desialylation approaches in vitro followed by recombinant ST6Gal-I expression. Although sialic acids contributed by sialyltransferases other than ST3Gal-IV may also be involved in modulating VWF homeostasis, ST6Gal-I does not perform this function independently. Further, it is unlikely that asialo-VWF molecules with half-lives of a few minutes are produced in vivo, because this would require the absence of multiple sialyltransferases, a deficit of sialic acid in the Golgi that can be lethal, or perhaps abnormal induction of an endogenous or pathogen-derived sialidase. For these reasons, the physiologic properties ascribed to sialic acid linkages and their regulation by sialyltransferases cannot be empirically investigated by available desialylation schemes. By using a genetic approach to investigate sialyltransferase function in vivo, a high degree of functional specificity can be observed that identifies the ST3Gal-IV sialyltransferase as a discrete modifier of hemostasis that acts by concealing ASGPR ligands and thereby contributing to VWF homeostasis.
Most naturally occurring or experimentally derived genetic defects in the mouse yield autosomal recessive phenotypes. ST3Gal-IV mutations, however, result in an autosomal dominant phenotype with reductions in plasma VWF levels apparently due to haploinsufficiency. It is possible that the ST3Gal-IV mutation produced herein leads to the production of a truncated polypeptide bearing a dominant-negative function. This appears unlikely, however, as the catalytic deletion engineered destabilizes the RNA to the extent that no expression is observed. In addition, the thrombocytopenia is an autosomal recessive phenotype due to similar ASGPR clearance mechanisms without a significant increase in β-linked Gal exposure or a decrease in platelet levels in the heterozygote.
Sialyltransferases in Hemostasis.
VWF is a highly glycosylated and sialylated protein. Analyses in humans show that VWF contains approximately 24 glycosylation sites with 14 involved in N-glycan modification leading to various multiantennary N-glycan branch structures (37). In addition, VWF contains O-glycans that have sialic acid linked to the Core 1 Gal and the peptide-proximal GalNAc (38). Together, this glycan diversity encompasses two sialic acid linkage types (α2–3 and -6) distributed on at least five different glycan branch structures. Our data indicate that multiple sialyltransferases modify VWF in vivo. Besides ST6Gal-I and ST3Gal-IV, seven others may act, including ST3Gal-I, -III, -VI, and ST6GalNAc-I-IV. Discerning which may modulate VWF homeostasis has been unclear as all these exhibit substrate specificities for Gal and GalNAc linkages detected on human VWF, and their expression may overlap in VWF-producing endothelium.
A reproducible, quantitative, and low RCA/VWF ratio exists among most human plasma samples. However, a small subpopulation of individuals was identified with both abnormally low VWF levels and abnormally high Gal exposure detected by RCA-I lectin binding. Aberrantly low VWF levels in the presence of high RCA-I binding suggest the possibility of a defect in a sialyltransferase such as ST3Gal-IV, or perhaps a mutation in VWF that alters conformation and attenuates sialylation of VWF in the Golgi. The degree of plasma VWF deficiency in four of the five cases found was severe and only slightly higher than the VWF levels characteristic of type 3 VWD. However, the thrombocytopenia noted among ST3Gal-IV homozygous null mice is not a feature of human type 3 VWD. Although thrombocytopenia is characteristic of type 2B VWD, this variant exhibits enhanced VWF affinity for platelets leading to spontaneous platelet aggregation and a defect in circulating VWF multimers (39).
Systemic glycosyltransferase deficiencies may be expected to affect many different glycoproteins. Indeed, the inherited glycosyltransferase deficiencies responsible for the severe childhood syndromes termed the Congenital Defects in Glycosylation (CDG) include diagnostic blood coagulopathies involving multiple glycoproteins with reduced levels of antithrombin-III, Factors VII, XI, XII, and protein C (40). CDG type I is due to abnormal processing of N-glycans in the endoplasmic reticulum, which can widely attenuate glycoprotein folding, maturation, and expression. In a mouse model of CDG type II, the presence of a typical blood coagulopathy likely reflects widespread and essential roles involving multiple N-glycan branches on glycoproteins that are produced early in the biosynthetic process in the Golgi apparatus (26).
In contrast, sialyltransferase deficiencies yield less extensive structural changes and may involve fewer substrates dictated by expression patterns, substrate specificity, access to substrate, and access to substrate in the Golgi glycan biosynthetic pathway. In fact, the steady-state plasma levels of blood coagulation factors produced in the liver were unaltered in ST3Gal-IV deficiency. These glycoproteins may not be modified by ST3Gal-IV. Alternatively, Gal exposure per se may be insufficient to comprise an ASGPR ligand in vivo.
The Genetic Basis of ASGPR Ligand Masking in Vivo.
Multivalency and conformation appear as important elements of ASGPR function and lectin binding in general (reviewed in ref. 41). Although both ST6Gal-I and ST3Gal-IV sialylate VWF in vivo, ST6Gal-I prefers type II glycan branches residing on the α1–3 linked mannose of N-glycans (42). ST3Gal-IV, however, can sialylate both types II and I glycan branch termini without such preference for a subset of type II branches. Therefore, ST6Gal-I deficiency may not result in the exposure of multiple closely spaced β-linked Gal residues, which may be more important than the molecular abundance of types I or II branches. In these considerations, the different branch substrate specificity of ST3Gal-IV in vivo may effectively mask multivalent Gal-bearing glycan branches that comprise an ASGPR ligand.
ST3Gal-IV conceals ligands for multiple ASGPRs in altering ASGPR-dependent clearance of both VWF and platelets. The hepatic ASGPR can bind and initiate the phagocytosis of particles less than 8 nm in diameter (43). Large particles such as platelets could be bound and processed by the macrophage ASGPR, which differs in molecular weight, membrane anchorage, and receptor arrangement (43, 44). In particular, the arrangement of macrophage ASGPRs in clusters on the cell surface is thought to be critical for the binding and endocytosis of particulate ligands such as platelets. The identification of ASGPRs that modulate VWF and platelet levels in ST3Gal-IV deficiency should provide information useful in understanding further this endogenous hemostatic lectin-ligand system.
Plasma VWF levels are highly variable in normal mammalian physiology, as well as among human patients with VWD. This variability appears to be a major determinant of bleeding severity in VWD. We find that ST3Gal-IV acts as a limiting component in the masking of ASGPR ligands, achieving an equilibrium involving VWF and platelet levels in circulation that is susceptible to modulation. Intriguingly, reductions in sialic acid abundance on VWF and Factor VIII have been reported in some cases of VWD (45–47). Endogenous mechanisms that limit plasma expression of VWF may also be vital. High levels of VWF and Factor VIII appear to be a major risk factor for thrombosis in humans (48). Our findings suggest that complex interactions among glycosyltransferases are a critical factor in regulating the steady-state level of plasma constituents, including VWF and platelets, and identify the ST3Gal-IV sialyltransferase as an attractive candidate for a major VWF modifier gene in humans.
Acknowledgments
This research was funded by the National Institutes of Health program project Grant PO1-HL57345, Fellowship F32CA79130 (L.G.E.), and Grant R01-HL39693 (D.G.). J.D.M. and D.G. are supported as Investigators of the Howard Hughes Medical Institute.
Abbreviations
- VWF
von Willebrand factor
- VWD
von Willebrand disease
- ASGP
asialoglycoprotein
- ASGPR
ASGP receptor
- Gal
galactose
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ruggeri Z M, Ware J, Ginsberg D. In: Thrombosis and Hemorrhage. Loscalzo J, Schafer A I, editors. Baltimore: Williams & Wilkins; 1998. pp. 337–364. [Google Scholar]
- 2.Sadler J E. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 3.Nichols W C, Ginsburg D. Medicine (Baltimore) 1997;76:1–20. doi: 10.1097/00005792-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Denis C V, Wagner D D. Cell Mol Life Sci. 1999;56:977–990. doi: 10.1007/s000180050487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz S D, Federici A B. Blood. 1988;72:1790–1796. [PubMed] [Google Scholar]
- 6.Carew J A, Quinn S M, Stoddart J H, Lynch D C. J Clin Invest. 1992;90:2258–2267. doi: 10.1172/JCI116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federici A B, Elder J H, De Marco L, Ruggeri Z M, Zimmerman T S. J Clin Invest. 1984;74:2049–2055. doi: 10.1172/JCI111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sodetz J M, Pizzo S V, McKee P A. J Biol Chem. 1977;252:5538–5546. [PubMed] [Google Scholar]
- 9.Sodetz J M, Paulson J C, Pizzo S V, McKee P A. J Biol Chem. 1978;253:7202–7206. [PubMed] [Google Scholar]
- 10.Wagner D D, Mayadas T, Marder V J. J Cell Biol. 1986;102:1320–1324. doi: 10.1083/jcb.102.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marth J D. In: Essentials of Glycobiology. Varki A, Cummings R, Esko H, Freeze H, Hart G, Marth J, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 499–514. [Google Scholar]
- 12.Gill J C, Endres-Brooks J, Bauer P J, Marks W J, Jr, Montgomery R R. Blood. 1987;69:1691–1695. [PubMed] [Google Scholar]
- 13.Orstavik K H, Kornstad L, Reisner H, Berg K. Blood. 1989;73:990–993. [PubMed] [Google Scholar]
- 14.Mohlke K L, Purkayastha A A, Westrick R J, Smith P L, Petryniak B, Lowe J B, Ginsburg D. Cell. 1999;96:111–120. doi: 10.1016/s0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa H, Paulson J C. J Biol Chem. 1994;269:1394–1401. [PubMed] [Google Scholar]
- 16.Takashima S, Tsuji S. Cytogenet Cell Genet. 2000;89:101–106. doi: 10.1159/000015574. [DOI] [PubMed] [Google Scholar]
- 17.Lowe J B, Marth J D. In: Essentials of Glycobiology. Varki A, Cummings R, Esko H, Freeze H, Hart G, Marth J, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 211–252. [Google Scholar]
- 18.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi M, Samyn-Petit B, Julien S, Delannoy P. Biochimie. 2001;83:727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 19.Crocker P R, Varki A. Trends Immunol. 2001;22:337–342. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 20.Lowe J B. Kidney Int. 1997;51:1418–1426. doi: 10.1038/ki.1997.194. [DOI] [PubMed] [Google Scholar]
- 21.Gagneux P, Varki A. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 22.Stockert R J, Morell A G, Ashwell G. Targeted Diagn Ther. 1991;4:41–64. [PubMed] [Google Scholar]
- 23.Cooper D N, Barondes S H. Glycobiology. 1999;9:979–984. doi: 10.1093/glycob/9.10.979. [DOI] [PubMed] [Google Scholar]
- 24.Hennet T, Chui D, Paulson J C, Marth J D. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafi R, Iyer S P, Ellies L G, O'Donnell N, Marek K W, Chui D, Hart G W, Marth J D. Proc Natl Acad Sci USA. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Tan J, Sutton-Smith M, Ditto D, Panico M, Campbell R M, Varki N M, Long J M, Jaeken J, Levinson S R, et al. Glycobiology. 2001;11:1–20. doi: 10.1093/glycob/11.12.1051. [DOI] [PubMed] [Google Scholar]
- 27.Lawrie A S, Hoser M J, Savidge G F. Thromb Res. 1990;59:369–373. doi: 10.1016/0049-3848(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 28.Varki A, Diaz S. Anal Biochem. 1984;137:236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- 29.Taktak Y S, Selkirk S, Bristow A F, Carpenter A, Ball C, Rafferty B, Poole S. J Pharm Pharmacol. 1991;43:578–582. doi: 10.1111/j.2042-7158.1991.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 30.Datta A K, Paulson J C. J Biol Chem. 1995;270:1497–1500. doi: 10.1074/jbc.270.4.1497. [DOI] [PubMed] [Google Scholar]
- 31.Baenziger J U, Fiete D. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- 32.Debray H, Montreuil J, Lis H, Sharon N. Carbohydr Res. 1986;151:359–370. doi: 10.1016/s0008-6215(00)90355-0. [DOI] [PubMed] [Google Scholar]
- 33.Moreno E, Teneberg S, Adar R, Sharon N, Karlsson K A, Angstrom J. Biochemistry. 1997;36:4429–4437. doi: 10.1021/bi962231h. [DOI] [PubMed] [Google Scholar]
- 34.Shibuya N, Goldstein I J, Broekaert W F, Nsimba-Lubaki M, Peeters B, Peumans W J. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 35.Lotan R, Skutelsky E, Danon D, Sharon N. J Biol Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- 36.Weigel P H. BioEssays. 1994;16:519–524. doi: 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]
- 37.Matsui T, Titani K, Mizuochi T. J Biol Chem. 1992;267:8723–8731. [PubMed] [Google Scholar]
- 38.Samor B, Michalski J C, Mazurier C, Goudemand M, De Waard P, Vliegenthart J F, Strecker G, Montreuil J. Glycoconj J. 1989;6:263–270. doi: 10.1007/BF01047846. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri Z M, Zimmerman T S. J Clin Invest. 1980;65:1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeken J, Matthijs G, Caarchon J, Van Schaftingen E. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Vallee D, editors. New York: McGraw–Hill; 2001. pp. 1600–1622. [Google Scholar]
- 41.Rice K G, Lee Y C. Adv Enzymol Relat Areas Mol Biol. 1993;66:41–83. doi: 10.1002/9780470123126.ch2. [DOI] [PubMed] [Google Scholar]
- 42.Joziasse D H, Schiphorst W E, van den Eijnden D H, van Kuik J A, van Halbeek H, Vliegenthart J F. J Biol Chem. 1985;260:714–719. [PubMed] [Google Scholar]
- 43.Schlepper-Schafer J, Hulsmann D, Djovkar A, Meyer H E, Herbertz L, Kolb H, Kolb-Bachofen V. Exp Cell Res. 1986;165:494–506. doi: 10.1016/0014-4827(86)90602-6. [DOI] [PubMed] [Google Scholar]
- 44.Roos P H, Hartman H J, Schlepper-Schafer J, Kolb H, Kolb-Bachofen V. Biochim Biophys Acta. 1985;847:115–121. doi: 10.1016/0167-4889(85)90161-2. [DOI] [PubMed] [Google Scholar]
- 45.Gralnick H R, Coller B S, Sultan Y. Science. 1976;192:56–59. doi: 10.1126/science.1083071. [DOI] [PubMed] [Google Scholar]
- 46.Gralnick H R, Sultan Y, Coller B S. N Engl J Med. 1977;296:1024–1030. doi: 10.1056/NEJM197705052961802. [DOI] [PubMed] [Google Scholar]
- 47.Gralnick H R, Cregger M C, Williams S B. Blood. 1982;59:542–548. [PubMed] [Google Scholar]
- 48.Rosendaal F R. Thromb Haemostasis. 2000;83:1–2. [PubMed] [Google Scholar]