Abstract
Background
Phospholemman (PLM) is an abundant phosphoprotein in the plasma membrane of cardiac, skeletal, and smooth muscle. It is a member of the FXYD family of proteins that bind to and regulate the Na,K-ATPase. Protein kinase A (PKA) is known to phosphorylate PLM on serine 68 (S68), although the functional effect of S68 PLM phosphorylation is unclear. We therefore evaluated S68 PLM phosphorylation in swine carotid arteries.
Methods
Two phosphospecific anti-PLM antibodies were made to PLM peptides in rabbits and tested with purified PLM and PKA-treated PLM. Swine carotid arteries were mounted isometrically, contracted, relaxed with forskolin, and then homogenized. Proteins were separated on SDS gels and the intensity of immunoreactivity to the two PLM antibodies determined on immunoblots.
Results
An antipeptide antibody “C2” primarily reacted with unphosphorylated PLM. An antipeptide antibody “CP68” was specific for S68 PLM phosphorylation. Histamine stimulation of intact swine carotid artery induced a contraction, increased the CP68 PLM antibody signal, and reduced the C2 PLM antibody signal. High [K+]o depolarization induced a contraction without altering the C2 or CP68 PLM signal. Forskolin-induced relaxation of histamine or [K+]o contracted arteries correlated with an increased CP68 signal. Nitroglycerin-induced relaxation was not associated with changes in the C2 or CP68 PLM signal.
Conclusions
These data suggest that contractile agonists increase S68 PLM phosphorylation. Agents that increase [cAMP], but not agents that increase [cGMP], increased S68 PLM phosphorylation. S68 PLM phosphorylation may be involved in cAMP-dependent regulation of smooth muscle force.
Keywords: cyclic AMP, FXYD protein, phospholemman, phosphorylation, vascular smooth muscle
INTRODUCTION
Phospholemman (PLM, also known as FXYD1) is a 72 amino acid sarcolemmal protein that is abundant in heart and skeletal muscle and present in other tissues such as kidney [1]. Protein kinase A (PKA) induces PLM phosphorylation at serine 68 (S68) [2,3] while protein kinase C (PKC) induces phosphorylation at both serine 63 (S63) and S68 (S68) [2,3]. Initial studies suggested a role for PLM in osmolyte flux and cell volume regulation [4,5]. More recent findings suggest a physical and functional association of PLM with membrane ion transporters such as the Na,K-ATPase [6,7] and the Na-Ca exchanger (NCX1) [8,9]. These findings suggest that PLM may have functional roles similar to other members of the FXYD family of proteins [10]. For example, the γ (gamma) subunit of Na,K-ATPase (FXYD2) shares significant homology with PLM [10] that could explain the effect of PLM on Na,K-ATPase activity [11].
Very little is known about PLM in smooth muscle. Three papers describe a 16 to 17 kDa phosphoprotein in smooth muscle that may well have been PLM (PLM has an apparent MW of ~16kDa on SDS gels [1]). In 1985, Boulanger-Saunier, et al. [12] identified a 16 kDa phosphoprotein in a plasma membrane-enriched fraction of rat aortic smooth muscle cell membranes. This protein copurified with Na,K-ATPase and was phosphorylated by PKA in vitro and isoproterenol in vivo. Its dephosphorylation was inhibited by 10 mM NaF. Boulanger-Saunier, et al. subsequently found that a phorbol ester (TPA) in vitro and arginine vasopressin in vivo induced phosphorylation of the 16 kDa protein on a second site distinct from the PKA site [13]. In 1989, Sarevic et al [14] found that a 17 kDa membrane phosphoprotein, likely PLM, was phosphorylated by PKA but not by protein kinase G (PKG). PLM mRNA has been reported in canine aortic, esophageal, and gastric smooth muscle [1]. While these publications suggest that PLM is present and is phosphorylated in intact smooth muscle, they cannot be considered definitive as they predate the cloning of PLM in 1991 and the subsequent development of reagents to study it.
Contraction of smooth muscle is typically associated with increased [Ca2+]i, formation of a Ca2+4-calmodulin complex, activation of myosin light chain kinase (MLCK), and phosphorylation of myosin regulatory light chains (MRLC) on serine 19 [15]. Phosphorylation of MRLC enables crossbridge attachment to the thin filament thereby allowing crossbridge cycling and force generation [16]. In many cases, smooth muscle relaxation proceeds via a reversal of this contraction process: reduction of myoplasmic [Ca2+], inactivation of MLCK, and dephosphorylation of MRLC [17,18,19].
cAMP mediated smooth muscle relaxation occurs via binding of agonists to specific seven membrane spanning receptors (e.g. β 2 adrenergic) which activate adenylyl cyclase via G proteins (e.g. see [20]). Adenylyl cyclase can also be directly and relatively specifically activated by low concentrations of forskolin (e.g., 0.1 to 1 μM). It is well known that increases in [cAMP] can reduce [Ca2+]i and thereby inactivate MLCK and cause relaxation by MRLC dephosphorylation (see reviews [21,22]. Specific mechanisms for reduction in [Ca2+]i include i) inhibition of Ca2+ mobilization from the sarcoplasmic reticulum [21]; ii) hyperpolarization [23], iii) decreased Ca2+ influx through voltage-gated channels [24], and iv) activation of plasma membrane Ca2+ pumps [25]. There are also mechanisms other than reductions in [Ca2+]i where increased [cAMP] can cause relaxation: e.g., force suppression associated with serine 16 HSP20 phosphorylation [26].
Our general hypothesis is that cAMP-mediated phosphorylation of PLM on S68 phosphorylation causes vascular smooth muscle relaxation by increasing the activity of the Na,K ATPase. The goals of this study were 1) to determine if PLM is present in smooth muscle, 2) to determine if cAMP-mediated relaxation is associated with S68 PLM phosphorylation, the site phosphorylated by PKA in vitro, and 3) to infer whether cAMP-mediated relaxation is associated with a decrease in intracellular Na+ ([Na+]i). To accomplish these goals, we developed S68 phosphorylation-specific PLM antibodies. These antibodies were then tested in forskolin-induced relaxation of swine carotid artery.
MATERIAL AND METHODS
Tissues
Swine common carotid arteries were obtained from a slaughterhouse and transported at 0 °C in physiological salt solution (PSS). PSS contained (mM): NaCl, 140; KCl, 4.7; 3-[N-morpholino] propane sulfonic acid (MOPS) 5; Na2HPO4, 1.2; CaCl2, 1.6; MgSO4, 1.2; D-glucose, 5.6; pH adjusted to 7.4 at 37 °C. Zero Na+ PSS was PSS where CholineCl was substituted for NaCl and the Na2HP04 was omitted. Dissection of medial strips, mounting and determination of the optimum length (Lo) for stress development at 37 °C was performed as previously described [27]. The intimal surface was mechanically rubbed to remove the endothelium.
Antibodies
We developed two specific antibodies against PLM: C2 which was made to react with dephosphorylated PLM and CP68 which was made to react with PLM phosphorylated at S68, the site phosphorylated by PKA. Both were raised against C-terminal PLM peptides. To raise polyclonal antibodies against dephosphorylated PLM (C2), the 16-amino acid peptide NH2-C G T F R S S I R R L S T R R R-COOH was used as the antigen and the resulting rabbit polyclonal antibodies affinity purified as described [28]. To make S68 phosphorylation-specific antibodies (CP68), the 19-amino acid peptide NH2-D E E E G T F R S S I R R L Sp (68) T R R R-COOH was made with phosphoserine (Sp) at S68. This peptide were injected into rabbits and serum collected. For affinity-purification of CP68 (the phosphorylation specific antibody), the serum was processed over an agarose immunosorbent containing the unconjugated dephosphorylated peptide to remove antibody activity that would bind the peptide irrespective of phosphorylation status. PhosphoPLM-specific antibodies were then affinity-purified from the cleaned serum by passage over an immunosorbent containing the phosphoPLM peptide. ELISA showed less than 2% cross reactivity of the phosphoPLM-specific antibody with the dephosphoPLM peptide (Bethyl Labs, Montgomery, TX).
PLM standards
Purified recombinant dephosphorylated PLM was prepared as described [29]. A pig cardiac sarcolemmal vesicle preparation [30] was loaded on most blots since it contained dephosphorylated and phosphorylated PLM that was detected by both anti-PLM antibodies.
PLM in vitro phosphorylation
40 μg of purified recombinant dephosphorylated PLM (trifluorethanol removed by N2 evaporation) [29] was incubated in 200 μl of a buffer containing 50 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.1% Triton X-100, 25 mM NaF, and 0.1 mM ATP with γ-32P-ATP. Samples were removed both before and after addition of 1 μl (1.1 μg) of the catalytic subunit of PKA (specific activity 6.3 μmol/min/mg, a generous gift of Tom Lincoln PhD). The reaction was stopped by addition of 1% SDS. The stoichiometery of PLM phosphorylation was calculated from the 32P activity of the phosphorylated PLM (DPM/mol PLM) divided by the 32P specific activity of the ATP employed (DPM/mol γ-phosphate in ATP).
Measurement of PLM phosphorylation
Swine carotid arteries were pharmacologically treated and frozen in an acetone-dry ice slurry (20g/20ml) at −78° C [27]. The frozen tissue was allowed to slowly thaw to room temperature in the slurry (2 hours), the tissues were air dried, weighed, and homogenized in a buffer containing 1% SDS, 10% glycerol, and 20 mM dithiothreitol (20 mg wet weight/ml buffer). 20 μl of homogenates (concentration normalized to tissue weight) were then loaded identically onto two 12% SDS electrophoresis gels, blotted to nitrocellulose, and then incubated with the anti-phospholemman antibodies (1:10000 for C2 and 1:1000 for CP68). After washing, incubation with secondary antibodies (1:5000 of goat anti-rabbit), and detection with enhanced chemiluminescence, the blots were imaged and digitized with UnScanIt (Silk Scientific Inc.).
Two internal standards were loaded on every gel to allow comparison of immunoblotting intensity on different blots. One standard, “KF,” was a pooled homogenate of swine carotid tissues that had been activated with 109 mM [K+]o for 10 min followed by the addition of 10 μM forskolin for 30 min. The second standard, “K,” was a pooled homogenate of swine carotid tissues that had been activated with 109 mM [K+]o for 40 min. The intensity of the PLM antibody immunoreactivity was determined for the experimental samples and these standards on every blot. We found that the ratio of the intensity of experimental sample CP68 immunoblotting to the intensity of KF immunoblotting from the same blot was the most reproducible for CP68 (similar results were seen with other normalizations), therefore, we report the CP68/KF ratio as an index of S68-PLM phosphorylation (c.f. middle panel of Fig. 3). We found that the C2 blots were most reproducible when the intensity of sample C2 immunoblotting was normalized to the intensity of the C2 immunoblotting from the unstimulated control swine carotid artery tissue from the same blot, therefore, we report the C2/control ratio as an index of unphosphorylated PLM (c.f. top panel of Fig. 3).
Figure 3. Biochemical events occurring in forskolin relaxed swine carotid artery activated by histamine (left panels) or high [K+]o depolarization (right panels).
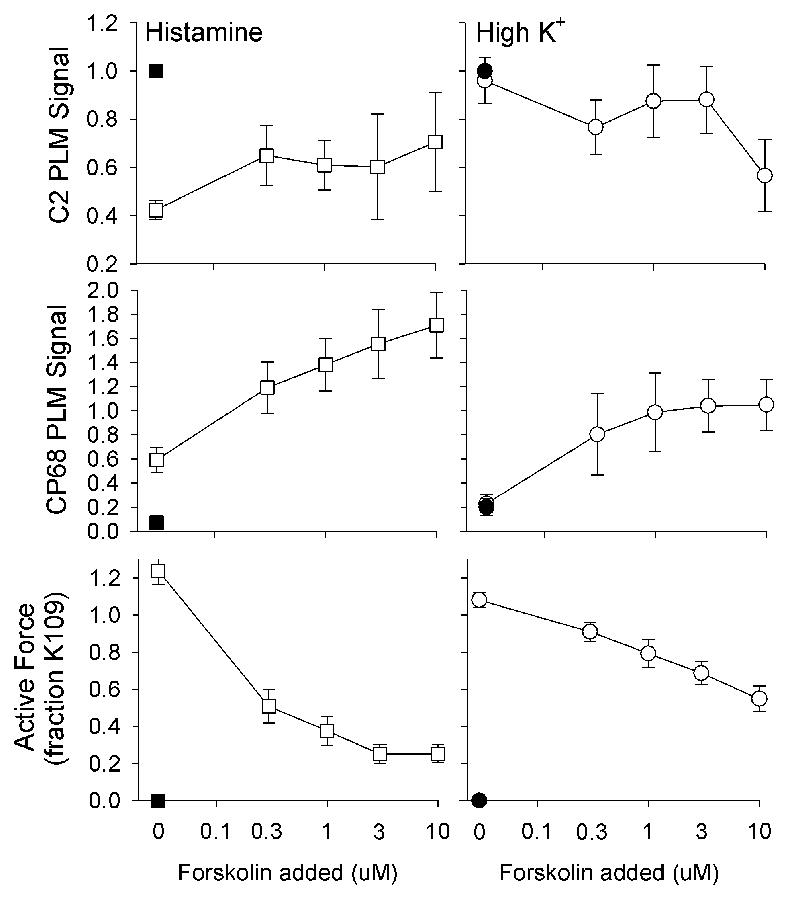
Immunoblot intensity was normalized as described in the methods and is presented as mean ± 1 SEM with n=4–5. Tissues were activated with 10 μM histamine (open squares) or 40 mM [K+]o (open circles) and then relaxed by addition of various [forskolin] as described on the abcissa. Unstimulated control tissues are the shown as filled squares or circles. Unphosphorylated PLM was estimated as the C2 signal (top panels), putative S68 PLM phosphorylation was estimated as the CP68 signal (middle panels), and force as percent of a prior 109 mM [K+]o contraction (bottom panels). Some error bars are obscured by the symbols.
Statistics
The significance of the correlation between the intensity of CP68 immunoblotting and contractile force shown in Figure 4 was tested using analysis of covariance (ANCOVA). The analysis showed no significant difference among the slopes for different experimental conditions, so the final model allowed intercepts but not slopes to vary. Significance was defined as p < 0.05.
Figure 4. Dependence of contractile force on the CP68 signal (putative S68 PLM phosphorylation).
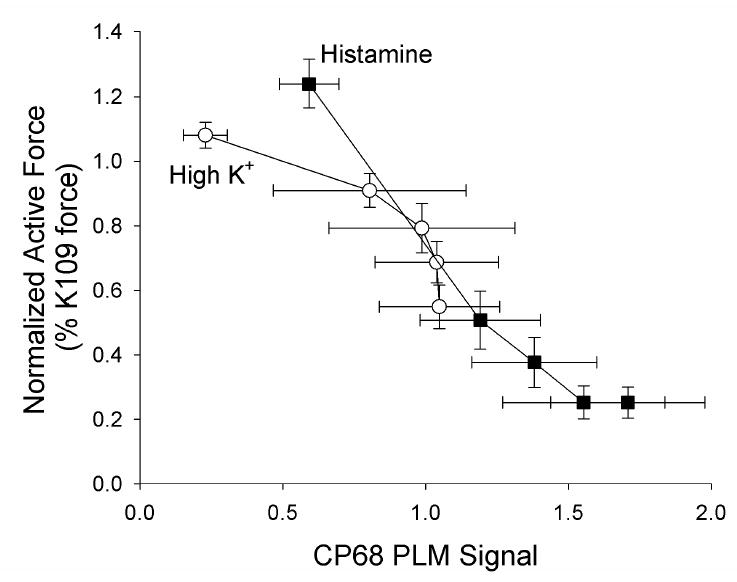
Data are replotted from Fig. 3. Histamine stimulated tissues are shown as filled squares and high [K+]o shown as open circles. There was a significant correlation between the the CP68 signal and force (top panel).
RESULTS
As detailed in the methods, two antibodies were made to C-terminal PLM peptides. To evaluate the specificity of these antibodies, purified recombinant PLM [29] (shown to be dephosphorylated by mass spectrometry) was phosphorylated with the catalytic subunit of PKA in the presence of γ-32P-ATP. Different amounts of phosphorylated and dephosphorylated PLM (exposed to γ-32P-ATP without PKA) were loaded on SDS gels, blotted, analyzed for 32P activity, and immunoblotted with both antibodies. Increasing 32P activity was observed with increasing amount of PKA-treated PLM (Fig. 1, top panel). Calculated stoichiometry revealed that ~50% of PLM was phosphorylated by PKA treatment. Dephosphorylated PLM was not detected by the CP68 antibody (Fig. 1, second panel). The intensity of the CP68 antibody signal was proportional to the amount of PKA treated PLM loaded on the immunoblot (Fig. 1, third panel). The intensity of the C2 antibody signal was proportional to the amount of dephosphorylated PLM loaded on the immunoblot (Fig. 1, fourth panel). The intensity of the C2 antibody signal was less intense on immunoblots loaded with PKA-treated PLM (Fig. 1, bottom panel) compared to that observed with dephosphorylated PLM.
Figure 1. Specificity of the anti-PLM antibodies.
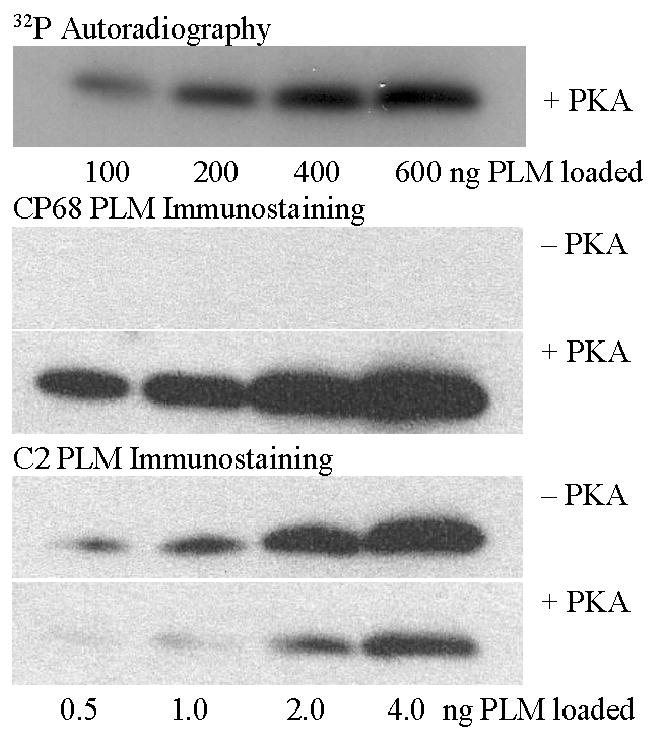
Purified recombinant PLM was either phosphorylated with or without PKA in the presence of γ-32P-ATP and then run on SDS gel electrophoresis. The top panel shows an autoradiogram of PLM treated with PKA (there was no 32P activity in PLM exposed to γ-32P-ATP without PKA). It shows increasing radioactivity with increasing amount of PLM loaded; calculated stoichiometry revealed that ~50% of PLM was phosphorylated. The second and third panel show immunoblots with the CP68 antibody. Dephosphorylated PLM was not detected by the CP68 antibody. PKA-treated PLM was detected by the CP68 antibody in proportion to the amount of PKA-treated PLM loaded. The fourth and fifth panels show immunoblots with the C2 antibody. Dephosphorylated PLM was detected by the C2 antibody in proportion to the amount of dephosphorylated PLM loaded. There was less C2 signal from PKA-treated PLM when compared to dephosphorylated PLM, suggesting the C2 antibody primarily immunoreacts with dephosphorylated PLM..
Swine carotid medial smooth muscle rings were first equilibrated and then contracted with 10 μM histamine or 40 mM [K+]o for 10 min. Some were then relaxed with 0.1, 0.3, 1, 3, or 10 μM forskolin, and others were left in the contraction solution. After an additional 30 min, tissues were frozen and homogenized. Proteins were separated on SDS gels and the intensity of immunoreactivity to the two PLM antibodies determined on immunoblots. As detailed in the methods, the intensity of both the C2 and CP68 antibodies were normalized to internal standards. For clarity the term “C2 signal” will refer to the intensity the C2 antibody immunoreactivity normalized to the intensity of C2 antibody immunoreactivity from the untreated control tissue homogenate loaded on the same blot. Similarly, the term “CP68 signal will refer to the intensity of the CP68 antibody immunoreactivity normalized to the intensity of the CP68 antibody immunoreactivity from the KF pooled homogenate loaded on the same blot. This normalization procedure allows comparison of changes in the C2 and CP68 antibody intensity on different immunoblots. Fig. 2 shows a representative immunoblot of tissues stimulated with histamine, and Fig. 3 shows aggregate data. PLM was present in swine arterial smooth muscle, and its phosphorylation status depended on the treatment.
Figure 2. Changes in the CP68 (top panel) and C2 (bottom panel) PLM signals in swine carotid artery tissue homogenates.
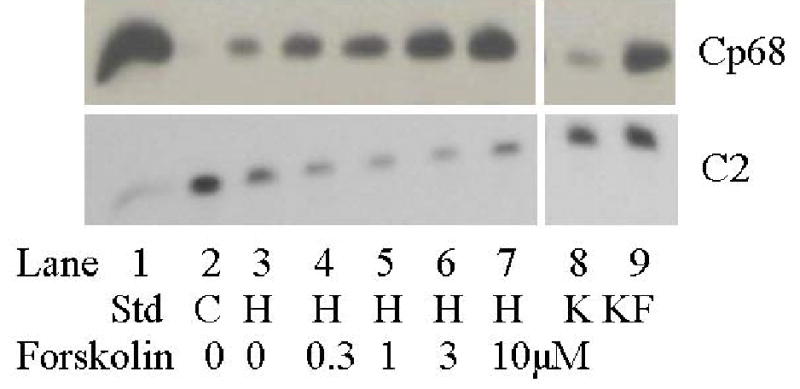
Lane 1 (from left) is the heart sarcolemmal PLM standard that is detected by all antibodies. Lane 2 is from unstimulated swine carotid tissues, i.e the control (which is used to normalize the C2 signals in Fig. 3 as detailed in the methods). Lane 3 is from 10 μM histamine stimulated tissues. Lanes 4–7 are from tissues first stimulated with 10 μM histamine followed by addition of 0.3, 1, 3, or 10 μM forskolin to induce relaxation, respectively. Lanes 8–9 are the K and KF standards (see methods). The CP68 signal (reflecting S68 PLM phosphorylation, top panel) revealed small increases with histamine alone and larger dose dependent increases with forskolin. The C2 signal (reflecting unphosphorylated PLM, lower panel), showed decreases with histamine treatment and no further change with addition of forskolin.
Unstimulated (control) tissues had low levels of CP68 signal and force (lane 2 in Fig. 2 and the filled symbols in Fig. 3). 10 μM histamine induced a large decrease in the C2 signal, a small increase in the CP68 signal, and a maximal contraction (lane 3 in Fig. 2 and open squares in Fig. 3). Adding forskolin to histamine stimulated tissues was associated with no change in the C2 signal, dose dependent increases in the CP68 signal (lanes 4–7 in Fig. 2), and dose dependent reduction in force (Fig. 3).
40 mM [K+]o depolarization induced a maximal contraction without significant changes in the C2 or CP68 signal (open circles in Fig. 3). Adding forskolin to high [K+]o depolarized tissues was associated with a reduced C2 signal, dose dependent increases in the CP68 signal, and dose dependent reduction in force. When relaxed with forskolin, the histamine stimulated tissues exhibited a greater CP68 signal and lower force than that observed in the 40 mM [K+]o-depolarized tissues.
The relation between the CP68/KF ratio and active force is shown in Fig. 4. There was a highly significant negative correlation between the CP68 signal and force (r2 = 0.5, p<0.0001 ANCOVA). There was no significant difference in the slopes or intercept of the regression lines for tissues stimulated with histamine or high [K+]o, suggesting a simple inverse relation between the degree of forskolin-induced relaxation and the degree of serine 68 PLM phosphorylation.
We evaluated whether agents that increase [cGMP] also induce PLM phosphorylation. 10 μM histamine induced a small increase in the CP68 signal and a maximal contraction. Addition of 100 μM nitroglycerin (to increase [cGMP]) induced a relaxation without changing the CP68 signal from the values observed with histamine alone. Addition of 1 μM forskolin (to increase [cAMP]) further increased the CP68 signal.
If S68 PLM phosphorylation were to cause relaxation by increasing the activity of the Na,K ATPase, then forskolin should reduce intracellular [Na+] ([Na+]i). [Na+]i is difficult to measure in intact smooth muscle, but can be inferred by the response to removal of extracellular [Na+] ([Na+]o) [31,32]. If L-type Ca2+ channels are blocked, contraction induced by removal of [Na+]o should result from the increase in [Ca2+]i that occurs from Ca2+ influx through reversal of the Na-Ca exchanger. If forskolin were to reduce [Na+]i, then there would be less Na+ for the Na-Ca exchanger, less increase in [Ca2+]i, and less contraction. We evaluated the effect of forskolin pretreatment on swine carotid artery contraction induced by a zero Na+ saline in the presence of 10 μM diltiazem (Figure 6). Pretreatment with 1 μM forskolin for 15 min significantly reduced the zero Na+ contraction compared to a control that was not treated with forskolin.
Figure 6. Forskolin dependent contractile response to removal of extracellular [Na+].
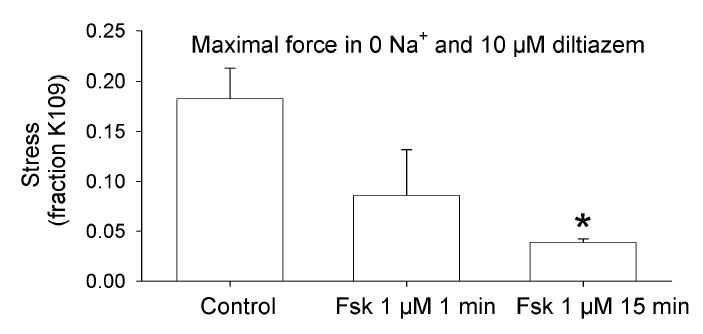
Four sets of tissues were incubated in 10 μM diltiazem for 15 min prior to removal of [Na+]o by changing to a Na+ free PSS which had choline+ substituted for Na+. Forskolin (1 μM) was either not added (control), added 1 min prior to removal of [Na+]o, or added 15 min prior to to removal of [Na+]o. The resulting peak contraction is shown as mean ± 1 SEM and analyzed by an ANOVA with Student-Newman-Keuls testing with * indicating a significant difference compared to no forskolin. Forskolin pretreatment for 15 min significantly reduced the zero Na+ contraction.
DISCUSSION
We found PLM immunoreactivity in smooth muscle (Fig. 2). These data suggest that the 16/17 kDa phosphoprotein identified in smooth muscle plasma membrane preparations [12,13,14] was likely PLM. We found that the CP68 PLM signal correlated with cAMP, but not cGMP mediated relaxation (Figs. 3 & 5). These data suggest a potential role for S68 PLM phosphorylation in cAMP-, but not cGMP-mediated relaxation. The CP68 PLM signal was increased by histamine stimulation but not high [K+]o depolarization (Fig. 3), suggesting that histamine stimulation, but not high [K+]o depolarization, also increases S68 PLM phosphorylation, either via histamine induced activation of PKC or PKA. Pretreatment with 1 μM forskolin for 15 min significantly reduced swine carotid contraction induced by zero Na+o (Fig. 6). This result is consistent with the hypothesis that forskolin reduced [Na+]i.
Figure 5. Biochemical events occurring with forskolin or nitroglycerin induced swine carotid artery relaxation.
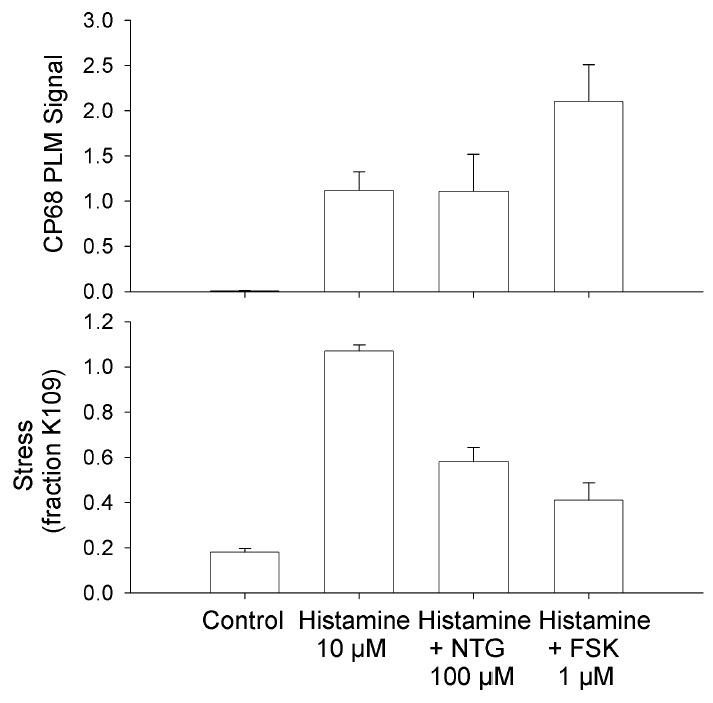
Immuoblot intensity was normalized as described in the methods and is presented as mean ± 1 SEM with n=4–5. Tissues were unstimulated (control), contracted with 10 μM histamine for 60 min, contracted with 10 μM histamine for 10 min and relaxed by addition of 100 μM nitroglycerin for 50 min, or contracted with 10 μM histamine for 10 min and relaxed by addition of 1 μM forskolin for 50 min. Putative S68 PLM phosphorylation was estimated as the CP68 signal (top panel) and force as percent of a prior 109 mM [K+]o contraction (bottom panel).
PLM antibody specificity
Our results suggest that the CP68 antibody is specific for S68 PLM phosphorylation and that the C2 antibody is at least mostly specific for dephosphorylated PLM. PKA treatment of recombinant PLM increased the CP68 signal and decreased the C2 signal (Fig. 1). Since dephosphorylated PLM did not produce a CP68 signal, we suggest that the CP68 antibody is specific for S68 phosphorylation. The specificity of the C2 antibody could not be clearly delineated since PKA treatment induced only ~50% phosphorylation. Nevertheless, the C2 signal decreased by ~50% with PKA treatment, suggesting that the C2 antibody is at least mostly specific for dephosphorylated PLM. Similar results were seen in intact swine carotid: forskolin treatment increased the CP68 signal and decreased the C2 signal (Fig. 2). We found it important to use large dilutions (1:10,000) of the C2 antibody to detect the fall in the C2 signal when PLM was phosphorylated. These results confirm and extend those of Silverman et al. [33] who evaluated the C2 and CP68 immunoreactivity in forskolin and phorbol ester treated rat cardiac myocytes. Both studies agree that PKA activation leads to an increase in the CP68 signal and a decrease in the C2 signal.
Histamine alone induced a large decrease in the C2 signal with only a small increase in the CP68 signal (Fig. 3). This suggests that histamine alone may have caused phosphorylation of PLM on sites other than S68. These sites may include S63 phosphorylated by PKC [2,3] or other sites phosphorylated by other kinases. Supporting this contention was the finding that addition of forskolin to histamine increased in the CP68 signal without further decreases in the C2 signal. Perhaps forskolin induced S68 PLM phosphorylation (detected by CP68) on PLM already phosphorylated at other sites (S63?) so that there was no change in the C2 signal. The results with high K+ depolarized tissues support this contention: there was no change in the C2 signal with high K+ alone, consistent with a lack of PKC activation with high K+. After high K+ activation, forksolin decreased the C2 signal and increased the CP68 signal, consistent with the C2 antibody detecting the decline in unphosphorylated PLM and CP68 detecting the S68 phosphorylation. These data suggest that forskolin can induce S68 PLM phosphorylation regardless of PLM phosphorylation at other sites.
Physiology of PLM phosphorylation in smooth muscle
Addition of forskolin to high [K+]o increased S68 PLM phosphorylation, but at any given [forskolin], there was less S68 PLM phosphorylation and less relaxation in tissues depolarized with high [K+]o than in tissues stimulated with histamine (Fig. 3). This may be related to the increase in S68 PLM phosphorylation induced by histamine alone. There was a highly significant inverse correlation between S68 PLM phosphorylation and force (Fig. 4). This suggests that S68 PLM phosphorylation could be involved in cAMP-dependent relaxation. However, such a correlation does not definitively establish PLM as a determinant of the resulting relaxation. Further experiments are planned to evaluate whether S68 PLM phosphorylation is a regulator of smooth muscle force.
We also evaluated whether cGMP mediated relaxation involves S68 PLM phosphorylation. Many substrates for PKA are also substrates for protein kinase G (PKG). We find that nitroglycerin induced relaxation was not associated with changes in S68 PLM phosphorylation (Fig. 5). This suggests that PLM can only be involved in cAMP-mediated relaxation.
There is a physical and functional association of PLM with membrane ion transporters such as the Na,K-ATPase [6,7] and the Na-Ca exchanger (NCX1) [8,9]. Based on our data and these studies, we hypothesize that PLM may be involved in smooth muscle regulation via regulation of Na,K-ATPase. β2 adrenergic stimuli, presumptively via increasing [cAMP], increased Na,K-ATPase activity in smooth muscle [34]. Fay and Moore [35] found that β2 adrenergic stimuli in smooth muscle reduced [Na+]i suggesting an increase in Na,K-ATPase activity. However, Borin found that cAMP increased [Na+]i in isolated smooth muscle cells [36]. Na,K-ATPase knockout mice have been made, but are lethal, with death occurring just after birth. Neonatal aortae from α2-Na,K-ATPase −/− mice were less sensitive to forskolin-induced relaxation when compared to α2-Na,K-ATPase +/+ mice [37]. We also find that forskolin inhibited the swine carotid contraction induced by removal of [Na+]o (Fig. 6), suggesting that forskolin reduced [Na+]i, possibly by stimulation of Na,K-ATPase activity. It must be noted that zero Na+o studies are only suggestive that [Na+]i is reduced. We plan to study more direct measures of Na,K-ATPase activity in the future. Nevertheless, these data support the hypothesis that cAMP-mediated relaxation at least partially involves Na,K-ATPase, likely by increasing its activity.
Specifically, we hypothesize that PKA-mediated phosphorylation of PLM on S68 increases the activity of Na,K-ATPase. Since Na,K-ATPase moves 3 Na+ outward and 2 K+ inward for every ATP consumed, it will produce a small hyperpolarization from the excess outward Na+ flux (reviewed in [38]). This hyperpolarization reduces Ca2+ influx through L-type Ca2+ channels, and is one method whereby increases in Na,K-ATPase activity could reduce [Ca2+]i and cause smooth muscle relaxation. A second mechanism is that increases in Na,K-ATPase activity, via decreases in [Na+]i, also could increase Ca2+ extrusion via forward Na-Ca exchange. This would also reduce [Ca2+]i and cause smooth muscle relaxation, however, this would be associated with increased Ca2+ efflux rather than the decrease in Ca2+ influx expected with hyperpolarization. We found that forskolin induced a hyperpolarization in rat tail artery [23] and decrease in Mn2+ influx in swine carotid artery [39], suggesting that hyperpolarization may be the dominant mechanism in some smooth muscles.
PLM has a role in regulation of cardiac contractility by [Ca2+] as well. In cultured rat heart myocytes, the myocytes near an area of infarction have an abnormally blunted contractile response to extracellular Ca2+ ([Ca2+]o) [40]. Overexpression of PLM produces myocytes with a blunted [Ca2+]o response similar to those observed after myocardial infarction [28]. Intriguingly, PLM is one of a few genes whose expression level rises after myocardial infarction [41], suggesting that PLM could be the mediator of the blunted [Ca2+]o response. If NCX1, the cardiac Na-Ca exchanger, is over expressed in peri-infarction myocytes, a normal [Ca2+]o response is observed [42]. A unifying explanation would be that PLM inhibits NCX1. Supporting this were findings that PLM overexpression reduced currents through NCX1 and slowed the decline in [Ca2+]i after release of SR stores with caffeine [28]. Co-overexpression of NCX1 with PLM restored the currents and Ca2+ dynamics to normal [8]. Moreover, reduction of PLM expression using anti-sense oligonucleotides increased NCX1 currents and sped the decline in [Ca2+]i after caffeine [9]. Most recently, Ahlers et al. [43] found a physical and functional interaction between PLM and NCX1 co-expressed in HEK293 cells. The importance of S68, the site of PKA phosphorylation, was underscored by the finding that a phosphodeficient PLM mutant S68A lost the ability to inhibit Na-Ca exchange despite persistent physical association.
These data demonstrate that PLM is functional and present in smooth muscle and is phosphorylated by physiologic stimuli. We propose that S68 PLM phosphorylation may have a role in cAMP-, but not cGMP-mediated smooth muscle relaxation possibly by activation of the Na,K-ATPase activity. The data also demonstrate relative specificity for the C2 and CP68 PLM antibodies for dephosphorylated and S68 phosphorylated PLM, respectively.
Acknowledgments
Smithfield Co., Smithfield, VA donated the swine carotid arteries and Tom Lincoln PhD (University of Alabama) donated the PKA. Grants from the NIH (HL071191 (C.R.), HL070548 (R.M), CA082864 (F.M.), HL058672 (J.C)) and the American Heart Association (Mid-Atlantic and Pennsylvania Affiliates) supported this research. We thank DE Lake for help with the statistical analysis.
References
- 1.Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991;266:11126–11130. [PubMed] [Google Scholar]
- 2.Walaas SI, Czernik AJ, Olstad OK, Sletten K, Walaas O. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochemical Journal. 1994;304( Pt 2):635–640. doi: 10.1042/bj3040635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mounsey JP, Lu KP, Patel MK, Chen ZH, Horne LT, John JE, III, Means AR, Jones LR, Moorman JR. Modulation of Xenopus oocyte-expressed phospholemman-induced ion currents by co-expression of protein kinases. Biochim Biophys Acta. 9211999;1451:305–318. doi: 10.1016/s0167-4889(99)00102-0. [DOI] [PubMed] [Google Scholar]
- 4.Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'Brian JJ, Szabo G, Jones LR. Unitary anion currents through phospholemman channel molecules. Nature. 1995;377:737–740. doi: 10.1038/377737a0. [DOI] [PubMed] [Google Scholar]
- 5.Morales-Mulia M, Pasantes-Morales H, Moran J. Volume sensitive efflux of taurine in HEK293 cells overexpressing phospholemman. Biochim Biophys Acta. 2000;1496:252–260. doi: 10.1016/s0167-4889(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 6.Crambert G, Fuzesi M, Garty H, Karlish S, Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc Natl Acad Sci USA. 2002;99:11476–11481. doi: 10.1073/pnas.182267299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feschenko MS, Donnet C, Wetzel RK, Asinovski NK, Jones LR, Sweadner KJ. Phospholemman, as single-span membrane protein, is an accessory protein of the Na,K-ATPase in cerebellum and choroid plexus. J Neurosci. 2003;23:2161–2169. doi: 10.1523/JNEUROSCI.23-06-02161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X-Q, Quershi A, Song J, Carl LL, Tian R, Stahl RC, Carey DJ, Rothblum LI, Cheung JY. Phospholemman modulates Na+/Ca2+ exchange in adult rat myocytes. Am J Physiol Cell Physiol. 2003;284:H225–H233. doi: 10.1152/ajpheart.00698.2002. [DOI] [PubMed] [Google Scholar]
- 9.Mirza MA, Zhang X-Q, Ahlers BA, Qureshi A, Carl LL, Song J, Tucker AL, Mounsey JP, Moorman JR, Rothblum LI, Zhang TS, Cheung JY. Effects of phospholemman downregulation on contractility and [Ca2+](i) transients in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H1322–H1330. doi: 10.1152/ajpheart.00997.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- 11.Mercer RW, Biemesderfer D, Bliss DP, Jr, Collins JH, Forbush B., III Molecular cloning and immunological characterization of the gamma polypeptide, a small protein associated with the Na,K-ATPase. J Cell Biol. 1993;121:579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulanger-Saunier C, Kattenburg DM, Stoclet J-C. Cyclic AMP-dependent phosphorylation of a 16 kDa protein in a plasma membrane-enriched fraction of rat aortic myocytes. FEBS Letters. 1985;193:283–288. doi: 10.1016/0014-5793(85)80169-1. [DOI] [PubMed] [Google Scholar]
- 13.Boulanger-Saunier C, Stoclet J-C. A 16kDa protein substrate for protein kinase C and its phosphorylation upon stimulation with vasopressin receptors in rat aortic myocytes. Biochem Biophys Res Commun. 1987;143:517–521. doi: 10.1016/0006-291x(87)91384-2. [DOI] [PubMed] [Google Scholar]
- 14.Sarcevic B, Brookes V, Martin TJ, Kemp BE, Robinson PJ. Atrial natriuretic peptide-dependent phosphorylation of smooth muscle cell particulate fraction proteins is mediated by cGMP-dependent protein kinase. J Biol Chem. 1989;264:20648–20654. [PubMed] [Google Scholar]
- 15.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiological Reviews. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- 16.Hai C-M, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- 17.Rembold CM. Relaxation, [Ca2+]i, and the latch-bridge hypothesis in swine arterial smooth muscle. Am J Physiol Cell Physiol. 1991;261:C41–C50. doi: 10.1152/ajpcell.1991.261.1.C41. [DOI] [PubMed] [Google Scholar]
- 18.Gerthoffer WT, Murphy RA. Ca2+, myosin phosphorylation, and relaxation of arterial smooth muscle. American Journal of Physiology. 1983;245:C271–C277. doi: 10.1152/ajpcell.1983.245.3.C271. [DOI] [PubMed] [Google Scholar]
- 19.Wingard CJ, Murphy RA. Inhibition of Ca2+-dependent contraction in swine carotid artery by myosin kinase inhibitors. Gen Pharmacol. 1999;32:483–494. doi: 10.1016/s0306-3623(98)00289-4. [DOI] [PubMed] [Google Scholar]
- 20.Kume H, Mikawa K, Takagi K, Kotlikoff MI. Role of G proteins and KCa channels in the muscarinic and β-adrenergic regulation of airway smooth muscle. American Journal of Physiology Lung Cellular and Molecular Physiology. 1995;268:L221–L229. doi: 10.1152/ajplung.1995.268.2.L221. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln, TM, Cornwell, TL, Komalavilas, P, MacMillan-Crow, LA, Boerth, NJ: The nitric oxide-cyclic gmp signaling system. 1996; 257–268.
- 22.Rembold, CM: Electromechanical and Pharmacomechanical Coupling. 1996; 227–239.
- 23.Rembold CM, Chen XL. Mechanisms responsible for forskolin-induced relaxation of rat tail artery. Hypertension. 1998;31:872–877. doi: 10.1161/01.hyp.31.3.872. [DOI] [PubMed] [Google Scholar]
- 24.Knot, HJ, Brayden, JE, Nelson, MT: Calcium channels and potassium channels. 1996; 203–219.
- 25.Raeymaekers, L and Wuytack, F: Calcium pumps. 1996; 241–253.
- 26.Rembold CM, O'Connor MJ, Clarkson M, Wardle RL, Murphy RA. HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid media tone. Journal of Applied Physiology. 2001;91:1460–1466. doi: 10.1152/jappl.2001.91.3.1460. [DOI] [PubMed] [Google Scholar]
- 27.Rembold CM, Murphy RA. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circulation Research. 1988;63:593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Zhang X-Q, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alters contractility and [Ca(2+)](i) transients in adult rat myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H576–H583. doi: 10.1152/ajpheart.00197.2002. [DOI] [PubMed] [Google Scholar]
- 29.Crowell KJ, Franzin CM, Koltay A, Lee S, Lucchese AM, Snyder BC, Marassi FM. Expression and characterization of the FXYD ion transport regulators for NMR structural studies in lipid miscelles and lipid bilayers. Biochim Biophys Acta. 2003;1645:15–21. doi: 10.1016/s1570-9639(02)00473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones LR. Rapid preparation of canine cardiac sarcolemmal vesicles by sucrose flotation. Methods in Enzymology. 1988;157:85–91. doi: 10.1016/0076-6879(88)57070-2. [DOI] [PubMed] [Google Scholar]
- 31.Rembold CM, Richard HL, Chen X-L. Na+/Ca2+ exchange, myoplasmic [Ca2+], and contraction of arterial smooth muscle. Hypertension. 1992;19:308–313. doi: 10.1161/01.hyp.19.4.308. [DOI] [PubMed] [Google Scholar]
- 32.Rembold CM, Chen XL. The buffer barrier hypothesis, [Ca2+]i homogeneity, and sarcoplasmic reticulum function in swine carotid artery. Journal of Physiology. 1998;513:477–492. doi: 10.1111/j.1469-7793.1998.477bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman BD, Fuller W, Eaton P, Deng JL, Moorman JR, Cheung JY, James AF, Shattock MJ. Serine 68 phosphorylation of phospholemman: acute isoforrn-specific activation of cardiac Na/K ATPase. Cardiovascular Research. 2005;65:93–103. doi: 10.1016/j.cardiores.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Limas CJ, Cohn JN. Stimulation of vascular smooth muscle sodium potassium adenotriphosphatase by vasodilators. Circ Res. 1974;35:601–607. doi: 10.1161/01.res.35.4.601. [DOI] [PubMed] [Google Scholar]
- 35.Moore EDW, Fay FS. Isoproterenol stimulates rapid extrusion of sodium from isolated smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8058–8062. doi: 10.1073/pnas.90.17.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borin ML. cAMP evokes a rise in intracellular Na+ mediated by Na+ pump inhibition in rat aortic smooth muscle cells. Am J Physiol Cell Physiol. 1995;269:C884–C891. doi: 10.1152/ajpcell.1995.269.4.C884. [DOI] [PubMed] [Google Scholar]
- 37.Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, Lingrel J, Paul RJ. The Na+-pump alpha2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene targeted mice. Am J Physiol Cell Physiol. 2003 eprint nov;19:0. doi: 10.1152/ajpcell.00389.2003. [DOI] [PubMed] [Google Scholar]
- 38.Rose AM, Valdes R. Understanding the sodium pump and its relevance to disease. Clinical Chemistry. 1994;40:1674–1685. [PubMed] [Google Scholar]
- 39.Chen X-L, Rembold CM. Cyclic nucleotide dependent regulation of Mn2+ influx, [Ca2+]i, and arterial smooth muscle relaxation. Am J Physiol Cell Physiol. 1992;263:C468–C473. doi: 10.1152/ajpcell.1992.263.2.C468. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. Journal of Applied Physiology. 1999;87:2143–2150. doi: 10.1152/jappl.1999.87.6.2143. [DOI] [PubMed] [Google Scholar]
- 41.Sehl PD, Tai JT, Hillan KJ, Brown LA, Goddard A, Yang R, Jin H, Lowe DG. Application of cDNA microarrays in determining molecular phenotype in cardiac growth, development, and response to injury. Circulation. 2000;101:1990–1999. doi: 10.1161/01.cir.101.16.1990. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X-Q, Song J, Quershi A, Rothblum LI, Carl LL, Tian R, Cheung JY. Rescue of contractile abnormalities by Na+/Ca2+ exchanger overproduction in postinfarction rat myocytes. J Appl Physiol. 2002;93:1925–1931. doi: 10.1152/japplphysiol.00583.2002. [DOI] [PubMed] [Google Scholar]
- 43.Ahlers,BA, Zhang,X-Q, Moorman,JR, Rothblum,LI, Carl,LL, Song,J, Wang,J, Geddis,LM, Tucker,AL, Mounsey,JP, Cheung,JY: Identification of an endogenous inhibitor of the Na+/Ca+ exchanger: phospholemman. Journal of Biological Chemistry 2005; in press: [DOI] [PubMed]


