Abstract
The Fas/FasL system provides the major apoptotic mechanism for many cell types, participating in cell turnover in hormone-dependent tissues. In the present study we localized both Fas and FasL in anterior pituitary cells, mainly in lactotropes and somatotropes. The percentage of anterior pituitary cells showing immunoreactivity for Fas or FasL was higher in cells from rats killed in proestrus than in diestrus. Also, the proportion of pituitary cells from ovariectomized (OVX) rats expressing Fas or FasL increased in the presence of 17β-estradiol (10−9 M). This steroid increased the percentage of lactotropes with immunoreactivity for Fas or FasL and the percentage of somatotropes expressing Fas. Activation of Fas by an agonist anti-Fas antibody (Mab-Fas) decreased the viability (MTT assay) of anterior pituitary cells from OVX rats cultured in the presence of 17β-estradiol. Also, membrane-bound FasL decreased cell viability (MTS assay) only when anterior pituitary cells from OVX rats were incubated with 17β-estradiol. Moreover, FasL increased the percentage of hypodiploid anterior pituitary cells (flow cytometry). Mab-Fas increased the percentage of TUNEL-positive pituitary cells and lactotropes from OVX rats only when cells were incubated in the presence of 17β-estradiol. Also, Mab-Fas triggered apoptosis of anterior pituitary cells from rats killed at proestrus but not at diestrus. Our results show that 17β-estradiol upregulates the expression of the Fas/FasL system in anterior pituitary cells and increases Fas-induced apoptosis in lactotropes, suggesting that Fas-induced apoptosis could be involved in the pituitary cell renewal during the estrous cycle.
Keywords: Fas, Fas ligand, estrogen, estrous cycle, pituitary, lactotropes, apoptosis
INTRODUCTION
Apoptosis plays a critical role in the maintenance of tissue homeostasis and is a physiological mechanism that eliminates excess or damaged cells. The induction of apoptosis in many cell types is achieved through the activation of the Fas/FasL system (1, 2). Fas receptor (Fas, CD95) is a transmembrane glycoprotein belonging to the Tumor Necrosis Factor (TNF) receptor family, whose members have been extensively reported to initiate signaling cascades leading to cell death. However, there is also a solid body of evidence demonstrating that Fas can transduce activation, proliferation and differentiation signals as well as trigger apoptotic pathways. Fas is activated by trimerization elicited by the binding of its natural ligand (FasL, CD95L). FasL can interact with Fas as a transmembrane protein or can be cleaved to a soluble trimer. After trimerization, the intracellular domain of Fas binds to specific adaptor proteins, such as FADD, leading to the activation of initiator caspases, caspase 8 and caspase 10 (2–5). Caspase-8 activates effector caspases, such as caspase 3, directly or via the mitochondrial release of cytochrome c, which activates caspase 9, thus amplifying the death signal (6). Fas activation also triggers alternative signal transduction pathways by recruiting other adaptor proteins different to FADD. Subsequent activation of transduction signals such as MAPK and NF-κB pathways may confer resistance to Fas-mediated cell death (7).
Fas is expressed in a variety of cell types such as ovary, lung, heart and immune cells (8). FasL is expressed in immune cells, immunoprivileged tissues such as eye or testis, and in several tissues including brain and hormone-dependent tissues (7–10). Also, some tumor cell types express FasL allowing cell survival through apoptosis of Fas-positive lymphocytes (11).
Reproductive organs undergo cycles of cell proliferation and cell death in response to cyclic changes in circulating sex hormone levels (9, 12, 13). Several reports suggest that gonadal steroids affect cell renewal in hormone-dependent tissues such as the ovary, endometrium and mammary gland, through modulation of Fas/FasL expression (9, 14–16). The anterior pituitary gland undergoes a process of cell renewal during the estrous cycle in the female rat. Cell proliferation and death in the anterior pituitary gland was suggested to respond to cyclic changes in circulating sex hormone levels (17–19). Previous results from our laboratory showed that TNF-α-induced apoptosis of anterior pituitary cells is predominant at proestrus and estrogen dependent (20, 21). We also reported that estrogens sensitize the anterior pituitary gland to the proapoptotic effect of endotoxin, which is higher at proestrus than at other stages of the estrous cycle (22). Immunohistochemical analysis of FasL expression revealed the presence of this ligand in non identified cells within the human anterior pituitary gland (23). It has also been reported that the mouse corticotroph cell line AtT20 expresses both FasL and Fas (24). However, as far as we know, the expression of Fas has not been studied in normal anterior pituitary cells, in which the role of the Fas/FasL system also remains to be determined. Therefore, the aim of the present study was to identify the anterior pituitary cell types expressing Fas and FasL and to explore whether the expression of these proteins is affected by estrogens. Also, in order to investigate whether the activation of Fas promotes apoptosis in anterior pituitary cells and whether estrogens modulate this proapoptotic effect, we examined Fas-induced apoptosis in anterior pituitary cells from intact rats killed at selected stages of the estrous cycle or from ovariectomized rats cultured in the presence of 17β-estradiol.
MATERIALS AND METHODS
All drugs, media and supplements were obtained from Sigma Chemical Co. (St. Louis, MO, USA), except Minimum Essential Medium Eagle (United States Biological, Swampscott, MA, USA), fetal bovine serum (GenSa, Buenos Aires, Argentina), membrane bound Fas Ligand, (Upstate, Lake Placid, NY, USA), anti-Fas antibody (R&D Systems Inc., Minneapolis, MN, USA), anti-FasL antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), MTS reagents Promega, WI, USA) all TUNEL reagents (Roche Molecular Biochemicals, Mannheim, Germany), primary antibodies against anterior pituitary hormones (Dr. A. Parlow, National Hormone and Pituitary Program; Torrance, CA, USA), anti-guinea pig fluorescein isothiocyanate secondary antibody (Chemicon International, Temecula, CA, USA), avidin and biotin blocking solutions, rhodamine avidin and biotynilated donkey anti-mouse or anti-rabbit IgG (Vector Laboratories Inc., CA, USA).
Animals
Adult female Wistar rats were kept in controlled conditions of light (12 h light-dark cycles) and temperature (20–25 oC). Rats were fed standard lab chow and water ad libitum and kept in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Rats were monitored by daily vaginal smears over 3 consecutive cycles and killed in proestrus or diestrus. Groups of rats were ovariectomized under ketamine (75 mg/Kg, i.p.) and xylazine (10 mg/Kg, i.p.) anesthesia two weeks before the experiments. Anterior pituitary glands were removed within minutes after decapitation.
Cell culture
A pool of anterior pituitary cells from 5 to 8 OVX rats or from 3 rats per stage of the estrous cycle was used for each culture. Anterior pituitary glands were washed several times with Dulbecco's Modified Eagle's Medium (DMEM) and cut into small fragments. Sliced fragments were dispersed enzymatically by successive incubations in DMEM supplemented with 3 mg/ml bovine serum albumin (BSA), containing 2.5 mg/ml trypsin (Type I from bovine pancreas), 1 mg/ml DNase (Deoxyribonuclease II, Type V from bovine spleen) and 1 mg/ml trypsin inhibitor (Type II-S from soybean), and finally dispersed by extrusion through a Pasteur pipette in Krebs buffer without Ca++ and Mg++. Dispersed cells were washed twice and resuspended in DMEM or Minimum Essential Medium Eagle (MEM-D-valine) supplemented with 10 μl/ml MEM aminoacids, 2 mM glutamine, 5.6 μg/ml amphotericin B and 25 μg/ml gentamicin (DMEM-S or MEM-D-valine-S). Cell viability as assessed by trypan blue exclusion was over 90 %. The cells were seeded onto coverslides in 24-well tissue culture plates (10 x 104 cells/0.5 ml/well) for the TUNEL method or immunocytochemistry, and onto 24-well tissue culture plates (25 x 104 cells /0.5 ml/well) for flow cytometry (FACS) or onto 96-well tissue culture plates (10 x 104 cells/0.2 ml/well) for cell viability determination.
Anterior pituitary cells from intact rats were cultured for 2 days (37 oC, 5 % CO2 in air) in DMEM-S or MEM-D-valine-S with 10% fetal bovine serum previously treated with 0.025 % dextran - 0.25 % charcoal (FBS-DCC) in order to remove steroids. After this period, cells were fixed for immunocytochemistry or incubated in DMEM-S (0.1–0.5 % BSA) or MEM-D-valine-S without FBS-DCC supplemented with 10 μg/ml insulin, 6.7 ng/ml sodium selenium, 5.5 μg/ml transferrin, 0.02 ng/ml triiodothyronin and 10 μl/ml MEM vitamins (MEM-D-valine-SS) containing mouse anti-Fas antibody (Mab-Fas, 1 μg/ml). This antibody was reported to have agonistic activity (25).
In the case of ovariectomized (OVX) rats, cells were cultured in DMEM-S or MEM-D-valine-S with 10 % FBS-DCC for 2 days and then for 2 more days in the same fresh medium containing 17β-estradiol (10−9 M) or vehicle (ethanol, final concentration 1 μl/l). After this period, cells were fixed for immunocytochemistry or washed twice, the medium replaced by serum free DMEM-S (0.1–0.5 % BSA) or MEM-D-valine-SS containing 17β-estradiol or vehicle and incubated with Mab-Fas (0.1–10 μg/ml) or recombinant Fas Ligand (FasL, 5–20 ng/ml).
Immunolocalization of Fas and FasL
We localized the presence of the Fas/FasL system in anterior pituitary cells by double indirect immunofluorescent staining (26, 27). After the culture period, cells were fixed with 4% formaldehyde in PBS for 30 min. Fixed cells were incubated with 10 % normal donkey serum in PBS-1% BSA for 1 h. Then, slides were sequentially incubated with avidin and biotin blocking solutions for 15 and 20 minutes respectively and incubated overnight with mouse anti-Fas antibody (1:50) or rabbit anti-FasL antibody (1:25) in PBS-1 % BSA. After rinsing, slides were incubated for 1 h with the corresponding biotinylated donkey anti-mouse or anti-rabbit IgG at a 1:200 dilution in the same buffer. Slides were washed and incubated with 2 μg/ml rhodamine-conjugated avidin in 10 mM HEPES buffer, pH 7.5, for 25 min. To detect prolactin, GH, βLH or ACTH immunoreactivity, slides were incubated with 10 % normal donkey serum in PBS with 0.2 % Triton X-100 (vol/vol) for 30 min and then with guinea pig anti-rat PRL (NHPP-IC, 1:2500), anti-rat GH (NHPP-IC, 1:2000), anti-rat βLH (NHPP-IC, 1:5000) or anti-rat ACTH (NHPP-IC, 1:400) in PBS containing 0.2 % Triton X-100 (vol/vol) and 1 % normal donkey serum for 1 h. After rinsing, slides were incubated for 1 h with donkey anti-guinea pig fluorescein isothiocyanate at a 1:200 dilution in the same buffer. Finally, slides were mounted with mounting medium for fluorescence (Vectashield, Vector Laboratories, Inc., Burlingame, CA) containing DAPI for DNA staining, and visualized in a fluorescence microscope (Axiophot, Carl Zeiss, Jena, Germany). Control slides were incubated with the corresponding normal serum or IgG subtype instead of primary antibody.
Microscopic determination of DNA fragmentation by the TUNEL method
Briefly, after the culture period, cells were fixed with 4 % formaldehyde in PBS for 30 min and permeabilized by microwave irradiation (28). DNA strand breaks were labeled with digoxigenin-dUTP using terminal deoxynucleotidyl transferase (0.18 U/μl) according to the manufacturer’s protocol. In some experiments, after an incubation with blocking buffer, cells were incubated with anti-rat prolactin (1:2500). Then, slides were incubated with anti-digoxigenin-fluorescein antibody (1:7) to detect incorporation of nucleotides into the 3′-OH end of damaged DNA and rhodamine-conjugated anti-guinea pig secondary antibody (1:200). Slides were mounted as described above. The percentage of apoptotic lactotropes was calculated as [(TUNEL+ PRL+)/total PRL+] x 100.
Assessment of the metabolic activity of viable cells
The metabolic activity of viable cells was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay or by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. For the MTT assay, cells were washed twice and incubated for 4 h in 100 μl Krebs buffer plus 50 μg MTT reagent dissolved in 10 μl phosphosaline buffer (PBS) at 37 ° C. The developed crystals were dissolved in 100 μl 0.04 N HCl in isopropanol, and the OD was read in a microplate spectrophotometer at a wavelength of 600 nm. For the MTS assay, 20 μl of reaction solution containing MTS (final concentration 333 μg/ml) and an electron coupling reagent (phenazine ethosulfate, final concentration 25 μM) were added to each well containing 100 μl of culture medium. After 2 h at 37ºC, the optical density (OD) was read at a wavelength of 490 nm. The quantity of formazan product in both methods is directly proportional to the number of living cells in culture.
Flow cytometric analysis (FACS)
Cultured cells were harvested with trypsin-EDTA and fixed with 70% ice-cold ethanol. Then DNA was stained with propidium iodide (50 μg/ml) in PBS containing RNAse (10 μg/ml) and 0.1% sodium azide. The fluorescence intensity was analysed using a FACScan (Becton Dickinson). Analysis of hypodiploid DNA content in anterior pituitary cells was done with WinMDI 98 software.
Statistical analysis
Cell viability data (from MTT or MTS assay) and percentage of hypodiploid cells (obtained by FACS) were expressed as mean ± SE and evaluated by Student t test, one-way ANOVA (followed by Dunnet multiple comparison test) or two-way ANOVA (followed by Tukey test). The number of immunoreactive (as identified by immunocytochemistry) or apoptotic (as identified by the TUNEL method) cells was analyzed in duplicate slides from at least two independent experiments. Total anterior pituitary cell number in each slide was evaluated by DAPI nuclear staining. The percentage of immunoreactive cells for either Fas or FasL was referred to the total cell number in each condition. The percentage of immunoreactive cells for either Fas or FasL in lactotropes or somatotropes was referred to the number of cells of each subpopulation in each condition and was calculated as [(FH)/H ] x100 (considering F as Fas+ or FasL+ cells, H as prolactin+ or GH+ cells and FH as cells with colocalization of F and H. Distribution of Fas and FasL immunoreactivity among different anterior pituitary cell types was calculated from [(FH)/F] x 100. Results were expressed as the percentage of immunoreactive or apoptotic cells ± 95 % confidence limits (CL) of the total number of cells counted in each specific condition. Confidence intervals for proportions were analyzed by the χ 2 test. p< 0.05 was used as the cut-off point for significance. All experiments were performed at least twice.
RESULTS
Expression of FasL and Fas in anterior pituitary cells
To explore whether the Fas/FasL system is involved in the physiological cell turnover of the anterior pituitary gland, we first evaluated the expression of FasL and Fas using immunocytochemistry, in cultures of anterior pituitary cells from female rats sacrificed at different stages of the estrous cycle. Cells from rats killed at diestrus or proestrus showed immunoreactivity for FasL and Fas (Fig. 1). No substantial difference in intensity of immunoreactivity was observed between cells from diestrus and proestrus. However, the percentage of FasL and Fas immunoreactive cells was significantly higher in cultures of anterior pituitary cells from rats killed at proestrus than at diestrus (FasL, χ 2 =77.9, df =1; Fas, χ 2 =51.9, df =1) (Fig. 1).
Fig. 1. Expression of FasL or Fas in anterior pituitary cells from rats killed at selected stages of the estrous cycle.
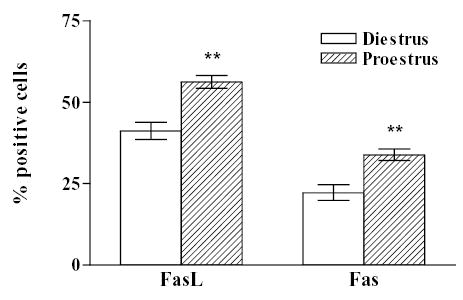
The expression of FasL and Fas was detected by immunocytochemistry in cultures of anterior pituitary cells from rats killed at diestrus or proestrus. Each column represents the percentage ± confidence limits (CL) of immunoreactive cells for FasL or Fas of the total number of cells in each condition (n = 1000–2500 cells/group from at least 2 separate experiments). ** p<0.01 vs respective controls at diestrus. (χ 2 test).
Considering that the expression of the Fas/FasL system could vary in the anterior pituitary according to the steroid hormone profile during the estrous cycle we determined the expression of FasL and Fas in anterior pituitary cells from OVX rats incubated with or without 17β-estradiol (10−9 M). No remarkable difference in the intensity of FasL and Fas immunoreactivity was observed between cells from OVX rats incubated in the presence or absence of 17β-estradiol (Fig. 2). Nevertheless, the presence of 17β-estradiol significantly increased the percentage of anterior pituitary cells from OVX rats expressing either FasL ( χ 2 =43.6, df =1) or Fas (χ 2 =146.8, df =1) (Fig. 2). The expression of FasL and Fas was localized in lactotropes, somatotropes, corticotropes and gonadotropes (Fig. 3 upper panel). FasL immunoreactivity was distributed among lactotropes (25 %), somatotropes (35 %) and other cell types (Fig 3 lower panel A). About 60 % of Fas immunoreactive cells were lactotropes and 26% were somatotropes (Fig. 3 lower panel B). Approximately 20 % of lactotropes expressed FasL and a similar percentage of this cell subpopulation exhibit Fas immunoreactivity (Fig. 4 A and B). 17β-estradiol significantly increased the percentage of lactotropes with positive staining for either FasL ( χ 2 =29.8, df =1) or Fas (χ 2 =11.45, df =1) (Fig. 4 A and B). Almost all somatotropes expressed FasL whereas about half of this cell subpopulation showed Fas immunoreactivity. 17β-estradiol significantly increased the percentage of somatotropes immunoreactive for Fas (χ 2 = 4.4, df =1), but did not significantly modify the number of somatotropes showing FasL staining (Fig. 4 A and B).
Fig. 2. Effect of 17β-estradiol on the expression of FasL and Fas in anterior pituitary cells from OVX rats.
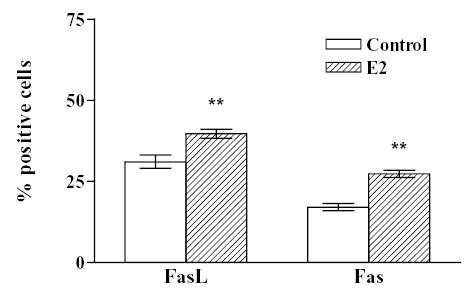
Cells from OVX rats were cultured with vehicle (ethanol 1 μl/l, Control) or 17β-estradiol (10−9 M, E2). The expression of FasL and Fas was detected by immunocytochemistry. Each column represents the percentage ± CL of immunoreactive cells for FasL or Fas of the total number of cells in each condition. (n = 2000–6000 cells/group from at least 2 separate experiments). ** p<0.01 vs respective controls without 17β-estradiol.( χ 2 test).
Fig. 3. Expression of FasL and Fas in different anterior pituitary cell subpopulations.
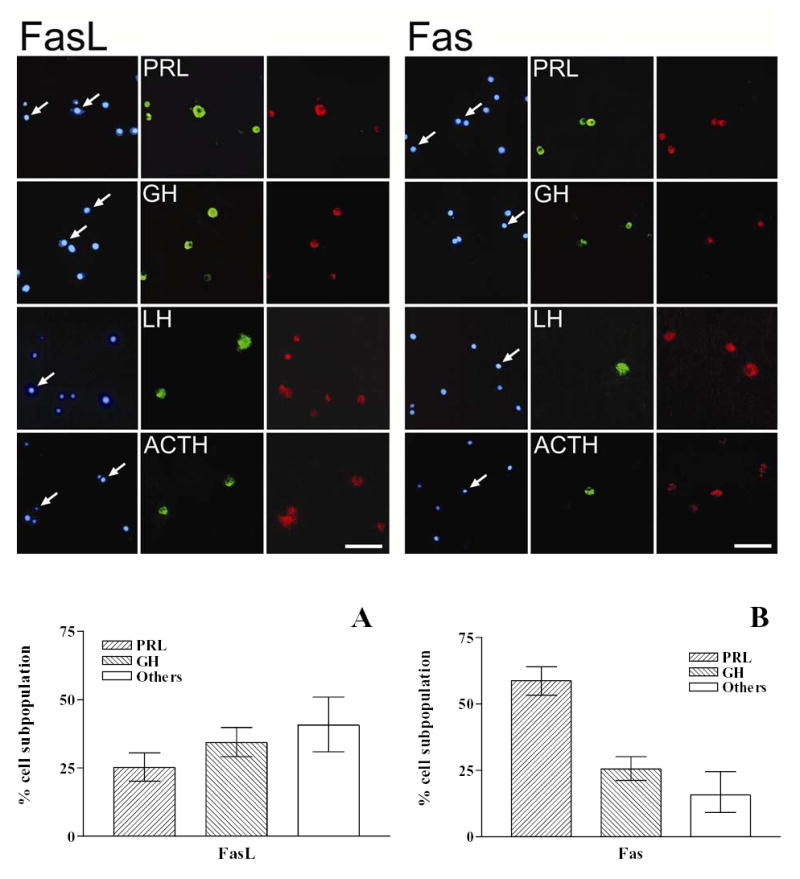
Upper panel: Cultured anterior pituitary cells from OVX rats were processed for identification of FasL or Fas and pituitary hormones by double immunofluorescence. Left panels: nuclear morphology by staining with DAPI, middle panels: immunocytochemistry for each cell subpopulation (lactotropes: PRL, somatotropes: GH, gonadotropes: LH, corticotropes: ACTH), and right panels: immunocytochemistry for FasL or Fas. Arrows indicate co-localization. Scale bar= 50 μm. Lower panel: Distribution of FasL (A) and Fas (B) immunoreactivity in different anterior pituitary cell subpopulations. Each column represents the percentage ± CL of each cell type within the immunoreactive population for FasL and Fas. (n = 300–1000 cells/group from at least 2 separate experiments).
Fig. 4. Effect of 17β-estradiol on the expression of FasL (A) and Fas (B) in lactotropes or somatotropes.
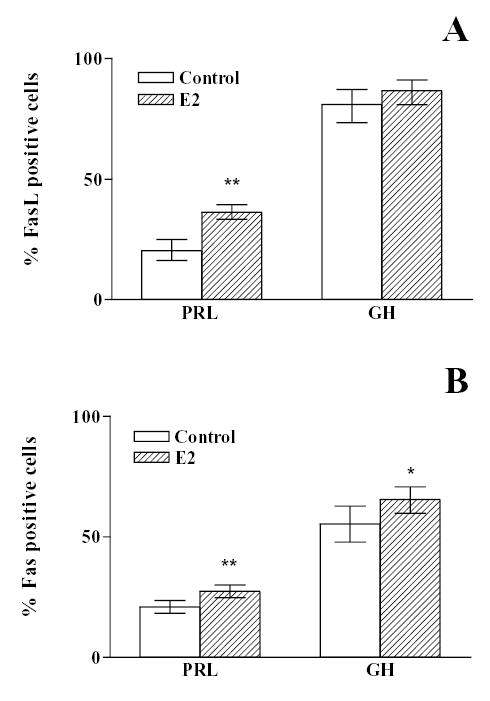
Anterior pituitary cells from OVX rats were cultured with vehicle (ethanol 1 μl/l, control) or 17β-estradiol (10−9 M, E2). The expression of FasL (A), Fas (B) prolactin (PRL) and GH was detected by double immunocytochemistry. Each column represents the percentage ± CL of immunoreactive cells for FasL or Fas of the total number of lactotropes or somatotropes in each condition. (n = 150–1000 cells/group from at least 2 separate experiments). * p<0.05; ** p<0.01 vs respective control without 17β-estradiol. (χ 2 test).
Fas-mediated apoptosis in anterior pituitary cells
To test the hypothesis that Fas activation induces apoptosis of anterior pituitary cells, we studied the effect of an anti-Fas monoclonal antibody (Mab-Fas), capable of activating the Fas receptor. We evaluated the effect of Mab-Fas (0.1-10 μg/ml) on the metabolic activity of viable anterior pituitary cells from OVX rats cultured in the presence of 17β-estradiol (10−9 M). The viability of these cells decreased in a dose-dependent manner in the presence of Mab-Fas (F3,44 = 5.51) (Fig. 5 A). Also, recombinant FasL (10 ng/ml) significantly decreased the cell viability of anterior pituitary cells from OVX rats only when cells were incubated with 17β-estradiol (F1,44 = 4.94) (Fig. 5 B). We also assessed the apoptotic effect of recombinant FasL (5–20 ng/ml) by flow cytometry. FasL at concentrations equal or above 10 ng/ml significantly increased the percentage of hypodiploid anterior pituitary cells (F3,7 =19.4) (Fig. 6 A). To determine the influence of 17β-estradiol on the apoptosis triggered by Fas activation, we explored the percentage of TUNEL positive anterior pituitary cells incubated with Mab-Fas in the presence of this gonadal steroid. The microscopic evaluation of nuclear morphology of TUNEL-positive cells showed that Fas induces cell death with apoptotic features. Mab-Fas (1 μg/ml) significantly increased the percentage of apoptotic anterior pituitary cells from OVX rats only when cells were cultured in the presence of 17β-estradiol (χ 2 =61.7, df =3) (Fig. 7 A). Considering that lactotropes are the secretory cells with the highest turnover in the anterior pituitary during the estrous cycle (17–19), we determined the effect of Mab-Fas on the percentage of TUNEL positive lactotropes from OVX rats incubated with 17β-estradiol. Mab-Fas significantly induced apoptosis of lactotropes only when cells were cultured in the presence of 17β-estradiol (χ 2 =61, df=3)(Fig. 7B and C).
Fig. 5. Effect of Fas activation on the metabolic activity of anterior pituitary cells.
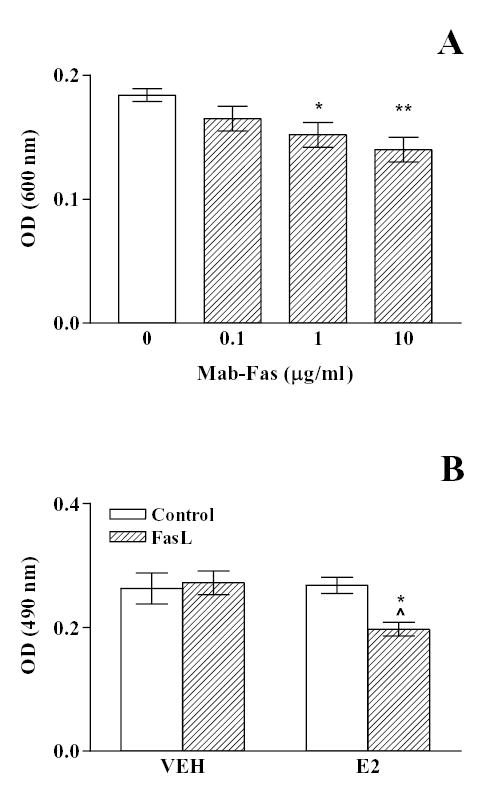
A, Anterior pituitary cells from OVX rats cultured with 17β-estradiol (10−9 M) were incubated with an agonistic anti-Fas antibody, (Mab-Fas, 0.1–10 μg/ml) for 6 h. Cell viability was assessed by the MTT assay. Each column represents the mean ± SE of 10 wells. * p < 0.05; ** p < 0.01 vs control. (One-way ANOVA followed by Dunnet multiple comparison test). B, Anterior pituitary cells from OVX rats cultured with vehicle (ethanol 1 μl/l, VEH) or 17β-estradiol (10−9 M, E2) were incubated in the presence of recombinant FasL (10 ng/ml, FasL) for 16 h and cell viability was assessed by the MTS assay. Each column represents the mean ± SE of 10 wells. * p < 0.05 vs respective control without FasL; ^ p < 0.05 vs respective control without 17β-estradiol. (Two-way ANOVA followed by Tukey test).
Fig. 6. Effect of Fas activation on percentage of hypodiploid anterior pituitary cells.
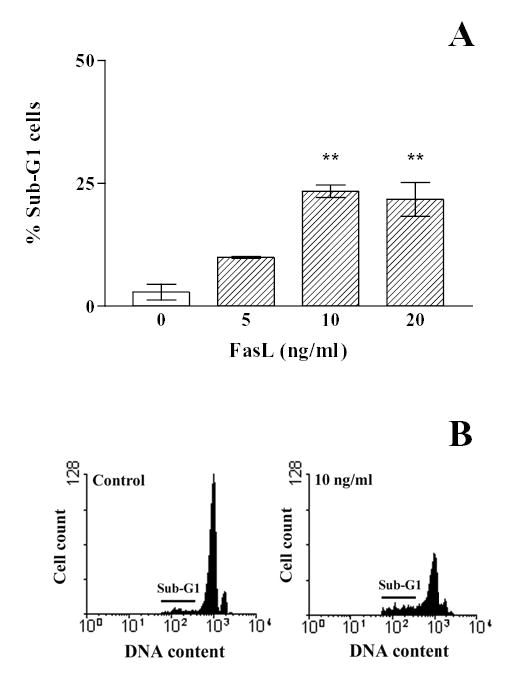
A, Anterior pituitary cells from OVX rats cultured with 17β-estradiol (10−9 M) were incubated with recombinant FasL (5–20 ng/ml, FasL) for 16 h. The percentage of hypodiploid cells was determined by FACS. Each column represents the mean ± SE of the percentage of sub-G1 cells of 3 wells. ** p<0.01 vs control. (One-way ANOVA followed by Dunnet multiple comparison test). B, Representative DNA histograms of anterior pituitary cells incubated without (control) or with FasL (10 ng/ml) and stained with propidium iodide.
Fig. 7. Fas-mediated apoptosis in anterior pituitary cells (A) and lactotropes (B, C) from OVX rats cultured in the presence of 17β-estradiol.
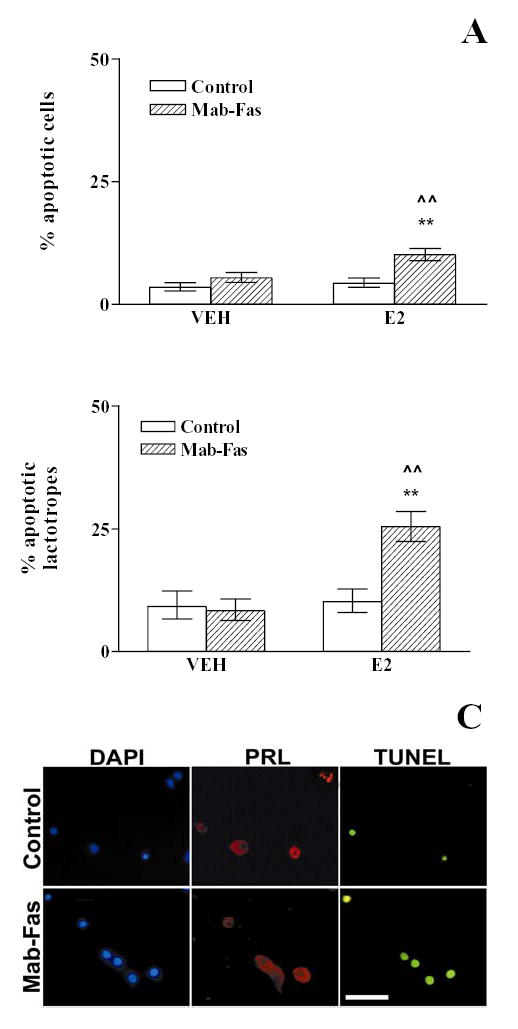
Anterior pituitary cells from OVX rats cultured with vehicle (ethanol 1 μl/l, VEH) or 17β-estradiol (10−9 M, E2) were incubated in the presence of anti-Fas antibody (1 μg/ml, Mab-Fas) for 24 h. Each column represents the percentage ± CL of TUNEL-positive cells. A, Anterior pituitary cells. (n>2000 cells/group from at least 5 separate experiments). ** p<0.01 vs respective controls without Mab-Fas, ^^ p<0.01 vs respective controls without 17β-estradiol; B, Lactotropes (n>450 cells/group from at least 3 separate experiments). ** p<0.01 vs respective controls without Mab-Fas; ^^ p<0.01 vs respective controls without 17β-estradiol. (χ 2 test). C, Representative TUNEL-positive lactotropes from OVX rats cultured in the presence of 17β-estradiol without (control) or with 1 μg/ml Mab-Fas. Prolactin (PRL)-bearing cells were identified by indirect immunofluorescence. Scale bar= 50 μm
To explore whether the apoptosis induced by Fas activation varies during the estrous cycle we determined the effect of Mab-Fas on anterior pituitary cells from cycling rats killed at different stages of the estrous cycle. Mab-Fas (1 μg/ml) significantly decreased the viability (t =3.9, df =10) and increased the percentage of TUNEL positive (χ 2 =148.5, df =1) anterior pituitary cells from rats killed at proestrus but not at diestrus (Fig. 8A and B).
Fig. 8. Effect of Fas activation on the metabolic activity (A) and percentage of apoptosis (B) in anterior pituitary cells from rats killed at selected stages of the estrous cycle.
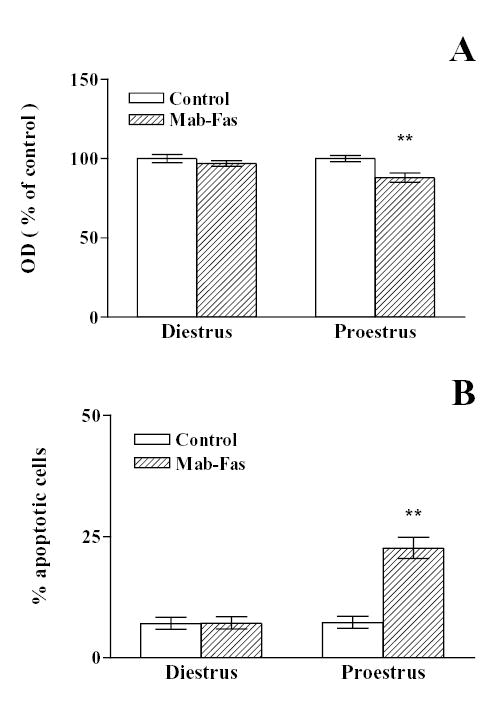
Anterior pituitary cells from rats killed at diestrus or proestrus were incubated in the presence of anti-Fas antibody (1 μg/ml, Mab-Fas) for 24 h. A, Cell viability was assessed by the MTT assay. Each column represents the mean ± SE of 6 wells. ** p< 0.01 vs. respective control. (Student t test). B, Each column represents the percentage ± CL of TUNEL-positive cells of the total number of cells in each condition. (n = 1300–1800 cells/group from at least 3 separate experiments). ** p<0.01 vs respective control without Mab-Fas. (χ 2 test).
DISCUSSION
In tissues where both Fas and FasL are expressed, the Fas/FasL apoptotic pathway has been suggested to participate in their physiological cell renewal (1, 16, 29, 30). In the present study, we report the expression of both Fas and FasL in anterior pituitary cells. Although we could detect Fas and FasL in gonadotropes and corticotropes, the expression of these proteins was mainly distributed among lactotropes and somatotropes. The fact that the expression of Fas and FasL is present in lactotropes and somatotropes suggests the participation of the Fas/FasL system in the apoptosis of these subpopulations of anterior pituitary cells. Indeed, our results indicate that Fas receptor activation induces apoptosis of lactotropes. Since we did not study the induction of apoptosis in other pituitary subpopulations we cannot discard that FasL can induce apoptosis in other cell types where Fas is also expressed.
The Fas/FasL system is involved in the cell renewal of many hormone-dependent tissues where its expression is modulated by cyclic changes in circulating gonadal steroids (1, 14, 15). The expression of Fas and FasL in the mammary gland seems to respond to circulating levels of gonadal steroids. Thus, during pregnancy, when serum levels of estrogen and progesterone are high, FasL protein levels increase but Fas expression is inhibited, resulting in a blockade of apoptosis (16). On the contrary, after the end of lactation, a simultaneous rise in Fas and FasL synthesis is induced, a process accompanied by apoptosis of epithelial cells, suggesting that gonadal steroids may affect mammary gland cell renewal through modulation of Fas/FasL expression (16). In the endometrium, where Fas activation induces apoptosis of epithelial cells, the expression of FasL fluctuates during the menstrual cycle (9, 15). The expression of Fas and FasL in the ovary varies depending on cell type and throughout the menstrual/estrous cycle. In this gland, estrogen upregulates FasL expression in epithelial cells (14). Also, the Fas pathway mediates apoptosis of luteal cells during the regression of the corpus luteum whose cells show a marked increase in Fas expression (1). During the estrous cycle, cell renewal also occurs in the anterior pituitary gland where proliferation takes place at estrus and apoptosis during proestrus (17–19). In the present report, we show evidence suggesting that the expression of Fas and FasL in anterior pituitary cells is sensitive to the hormonal environment. The expression of FasL and Fas is higher in anterior pituitary cells of rats killed at proestrus than at diestrus. Considering that it has been reported that cultured anterior pituitary cells can express a phenotype according to their previous in vivo condition (31, 32), the differential expression of FasL and Fas according to the stage in which the animals were sacrificed suggests cyclic changes in the expression of the Fas/FasL system. Tallying with the upregulation of FasL and Fas expression at proestrus, the activation of Fas triggers apoptosis of anterior pituitary cells from rats at this stage of the estrous cycle, suggesting that estrogen stimulates the Fas/FasL system in the anterior pituitary gland. Indeed, Fas activation did not induce apoptosis of anterior pituitary cells from rats killed in diestrus, when circulating levels of estrogen are low.
Lactotropes are the anterior pituitary cell subpopulation with the highest turnover during the estrous cycle, proliferating during estrus and showing high sensitivity to proapoptotic stimuli at proestrus, when circulating levels of estrogens are the highest (18, 20). Our results show that 17β-estradiol stimulates the expression of Fas and FasL in lactotropes. Moreover, Fas-induced apoptosis of lactotropes is estrogen-dependent, suggesting that the Fas/FasL system participates in the renewal of lactotropes at proestrus. We previously showed that TNF-α exerts a proapoptotic effect on lactotropes in an estrogen-dependent manner (20). Since proinflammatory cytokines can induce apoptosis by increasing Fas and FasL expression (33), it is possible that both proapoptotic factors, TNF-α and FasL, may interact in the induction of apoptosis in lactotropes at proestrus. The release of TNF-α from anterior pituitary cells is higher at proestrus and stimulated by estrogens (34) suggesting that the Fas/FasL system could be regulated by TNF-α during the estrous cycle. In fact, it has been reported that TNF-α enhances Fas-mediated apoptosis by up-regulating Fas expression in an NF-κB-dependent pathway (35).
It has been suggested that the peak in circulating levels of estrogens at proestrus may sensitize anterior pituitary cells to proapoptotic signals (20–22). On the contrary, high progesterone levels may impair apoptosis of anterior pituitary cells at other stages of the estrous cycle (21). Considering that the expression of the Fas/FasL system in anterior pituitary cells varies according to the characteristic gonadal hormone profile of the estrous cycle and that Fas-induced apoptosis of lactotropes was strongly enhanced by 17β-estradiol, it can be suggested that estrogen modulates lactotrope apoptosis both by increasing Fas expression and by sensitizing cells to Fas activation. It has been proposed that estradiol may function either as a protective agent or as an inducer of apoptosis, probably depending on the estrogen receptor subtype present in the cell (36–38). A growing body of evidence indicates that estrogens not only sensitize anterior pituitary cells to mitogenic stimuli, but also to proapoptotic factors (39). In fact, estradiol increases the expression of the proapoptotic proteins p53 and Bad in the anterior pituitary gland (40, 41).
In conclusion, the present study shows that the Fas/FasL system is expressed in several anterior pituitary cell types, mainly in lactotropes and somatotropes, and that the activation of Fas induces apoptosis in lactotropes. The expression of Fas/FasL and Fas-mediated apoptosis of anterior pituitary cells are higher in cells from rats at proestrus and modulated by estradiol. Our results suggest that the Fas/FasL system may participate in the process of cell renewal in the anterior pituitary gland during the estrous cycle. The complete elucidation of Fas-mediated apoptosis and its role in the physiology or pathology of the anterior pituitary gland will contribute to a better understanding of not only the life and death of anterior pituitary cells, but also of the basic mechanisms that could be involved in the pathogenesis of pituitary tumors.
Acknowledgments
We acknowlege the funding received from Agencia Nacional de Investigaciones Científicas y Tecnológicas, CONICET and Universidad de Buenos Aires, Argentina and from NIH-1RO1-NS44556 (MGC) and NIH-1RO3-TW006273-01A1 (MGC and AS).
Footnotes
Grant support: This work was supported by grants from Agencia Nacional de Investigaciones Científicas y Tecnológicas, CONICET and Universidad de Buenos Aires, Argentina and by NIH grants 1RO1-NS44556 (MGC) and 1RO3-TW006273-01A1 (MGC and AS).
References
- 1.Mor G, Straszewski S, Kamsteeg M. Role of the Fas/Fas Ligand system in female reproductive organs: survival and apoptosis. Biochem Pharmacol. 2002;64:1305–1315. doi: 10.1016/s0006-2952(02)01267-4. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Fergunson T. Biology of FasL. Cytokine Growth Factor Rev. 2003;14:325–335. doi: 10.1016/s1359-6101(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 4.Paasch U, Grunewald S, Agarwal A, Glandera H. Activation pattern of caspases in human spermatozoa. Fertil Steril. 2004;81:802–809. doi: 10.1016/j.fertnstert.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golstein P. Controlling cell death. Science. 1997;275:1081–1082. doi: 10.1126/science.275.5303.1081. [DOI] [PubMed] [Google Scholar]
- 7.Choi C, Benveniste E. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Golstein P. The Fas Death Factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Rutherford T, Naftolin F, Brown S, Mor G. Hormonal regulation of apoptosis and the Fas and Fas ligand system in human endometrial cells. Mol Hum Reprod. 2002;8:447–455. doi: 10.1093/molehr/8.5.447. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessio A, Riccioli A, Lauretti P, Padula F, Muciaccia B, De Cesaris P, Filippini A, Nagata S, Ziparo E. Testicular FasL is expressed by sperm cells. Proc Natl Acad Sci. 2001;13:3316–3321. doi: 10.1073/pnas.051566098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Cancer Biology. 2002;12:309–315. doi: 10.1016/s1044-579x(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 12.Thompson EB. Apoptosis and steroid hormones. Mol Endocrinol. 1994;8:665–673. doi: 10.1210/mend.8.6.7935482. [DOI] [PubMed] [Google Scholar]
- 13.Marti A, Jaggi R, Vallan C, Ritter PM, Baltzer A, Srinivasan A, Dharmarajan AM, Friis RR. Physiological apoptosis in hormone-dependent tissues: involvement of caspases. Cell Death Differ. 1999;6:1190–1200. doi: 10.1038/sj.cdd.4400610. [DOI] [PubMed] [Google Scholar]
- 14.Sapi E, Brown WD, Aschkenazi S, Lim C, Munoz A, Kacinski BM, Rutherford T, Mor G. Regulation of Fas ligand expression by estrogen in normal ovary. J Soc Gynecol Investig. 2002;4:243–250. [PubMed] [Google Scholar]
- 15.Selam B, Kayisli UA, Mulayim N, Arici A. Regulation of Fas ligand expression by estradiol and progesterone in human endometrium. Biol Reprod. 2001;4:979–985. doi: 10.1095/biolreprod65.4.979. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Sapi E, Brown W, Nilsen J, Tartaro K, Kacinski BM, Craft J, Naftolin F, Mor G. Roles of Fas and Fas ligand during mammary gland remodeling. J Clin Invest. 2000;106:1209–1220. doi: 10.1172/JCI10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi Y, Okuda M, Takahashi H, Fujii T, Morii S. Cellular proliferation in the anterior pituitary gland of normal adult rats: influences of sex, estrous cycle, and circadian change. Anat. Rec. 1993;235:111–120. doi: 10.1002/ar.1092350111. [DOI] [PubMed] [Google Scholar]
- 18.Hashi A, Mazawa S, Kato J, Arita J. Pentobarbital anesthesia during the proestrous afternoon blocks lactotroph proliferation occurring on estrus in female rats. Endocrinology. 1995;136:4665–4471. doi: 10.1210/endo.136.10.7664687. [DOI] [PubMed] [Google Scholar]
- 19.Yin P, Arita J. Proestrous surge of gonadotropin-releasing hormone secretion inhibits apoptosis of anterior pituitary cells in cycling female rats. Neuroendocrinology. 2002;76:272–282. doi: 10.1159/000066626. [DOI] [PubMed] [Google Scholar]
- 20.Candolfi M, Zaldivar V, De Laurentiis A, Jaita G, Pisera D, Seilicovich A. TNF-alpha induces apoptosis of lactotropes from female rats. Endocrinology. 2002;143:3611–3617. doi: 10.1210/en.2002-220377. [DOI] [PubMed] [Google Scholar]
- 21.Candolfi M, Jaita G, Zaldivar V, Zarate S, Ferrari L, Pisera D, Castro MG, Seilicovich A. Progesterone antagonizes the permissive action of estradiol on tumor necrosis factor-alpha-induced apoptosis of anterior pituitary cells. Endocrinology. 2005;146:736–743. doi: 10.1210/en.2004-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisera D, Candolfi M, Navarra S, Ferraris J, Zaldivar V, Jaita G, Castro MG, Seilicovich A. Estrogens sensitize anterior pituitary gland to apoptosis. Am J Physiol Endocrinol Metab. 2004;287:E767–771. doi: 10.1152/ajpendo.00052.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Shin MS, Park WS, Kim SY, Dong SM, Lee HK, Park JY, Oh RR, Jang JJ, Lee JY, Yoo NJ. Immunohistochemical analysis of Fas ligand expression in normal human tissues. APMIS. 1999;107:1013–1019. doi: 10.1111/j.1699-0463.1999.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang P, Tofighi R, Emgard M, Ceccatelli S. Cell death induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) in AtT-20 pituitary cells. Toxicology. 2005;207:391–399. doi: 10.1016/j.tox.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Wosik K, Becher B, Ezman A, Nalbantoglu J, Antel JP. Caspase 8 expression and signaling in Fas injury-resistant human fetal astrocytes. Glia. 2001;33:217–224. doi: 10.1002/1098-1136(200103)33:3<217::aid-glia1020>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Southgate TD, Windeatt S, Smith-Arica J, Gerdes CA, Perone MJ, Morris I, Davis JR, Klatzmann D, Lowenstein PR, Castro MG. Transcriptional targeting to anterior pituitary lactotrophic cells using recombinant adenovirus vectors in vitro and in vivo in normal and estrogen/sulpiride-induced hyperplastic anterior pituitaries. Endocrinology. 2000;141:3493–505. doi: 10.1210/endo.141.9.7639. [DOI] [PubMed] [Google Scholar]
- 27.Windeatt S, Southgate TD, Dewey RA, Bolognani F, Perone MJ, Larregina AT, Maleniak TC, Morris ID, Goya RG, Klatzmann D, Lowenstein PR, Castro MG. Adenovirus-mediated herpes simplex virus type-1 thymidine kinase gene therapy suppresses oestrogen-induced pituitary prolactinomas. J Clin Endocrinol Metab. 2000;85:1296–1305. doi: 10.1210/jcem.85.3.6482. [DOI] [PubMed] [Google Scholar]
- 28.Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C, Brambilla E. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Yano T, Matsumi H, Osuga Y, Yano N, Xu J, Wada O, Koga K, Fujiwara T, Kugu K, Taketani Y. Cross-Talk between Fas/Fas ligand system and nitric oxide in the pathway subserving granulosa cell apoptosis: a possible regulatory mechanism for ovarian follicle atresia. Endocrinology. 2005;146:808–815. doi: 10.1210/en.2004-0579. [DOI] [PubMed] [Google Scholar]
- 30.Aschkenazi S, Straszewski S, Verwer KM, Foellmer H, Rutherford T, Mor G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66:1853–1861. doi: 10.1095/biolreprod66.6.1853. [DOI] [PubMed] [Google Scholar]
- 31.Nagy GM, Frawley LS. Suckling increases the proportions of mammotropes responsive to various prolactin-releasing stimuli. Endocrinology. 1990;127:2079–2084. doi: 10.1210/endo-127-5-2079. [DOI] [PubMed] [Google Scholar]
- 32.Castaño JP, Kineman RD, Frawley LS. Dynamic fluctuations in the secretory activity of individual lactotropes as demonstrated by a modified sequential plaque assay. Endocrinology. 1994;135:1747–1752. doi: 10.1210/endo.135.5.7956898. [DOI] [PubMed] [Google Scholar]
- 33.Jo SK, Cha DR, Cho WY, Kim HK, Chang KH, Yun SY, Won NH. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415. doi: 10.1159/000064280. [DOI] [PubMed] [Google Scholar]
- 34.Theas S, Pisera D, Duvilanski B, De Laurentiis A, Pampillo M, Lasaga M, Seilicovich A. Estrogens modulate the inhibitory effect of tumor necrosis factor-alpha on anterior pituitary cell proliferation and prolactin release. Endocrine. 2000;12:249–255. doi: 10.1385/ENDO:12:3:249. [DOI] [PubMed] [Google Scholar]
- 35.Starace D, Riccioli A, D'Alessio A, Giampietri C, Petrungaro S, Galli R, Filippini A, Ziparo E, De Cesaris P. Characterization of signaling pathways leading to Fas expression induced by TNF-alpha: pivotal role of NF-kappaB. FASEB J. 2005;19:473–475. doi: 10.1096/fj.04-2726fje. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen J, Mor G, Naftolin F. Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 38.Osipo C, Liu H, Meeke K, Jordan VC. The consequences of exhaustive antiestrogen therapy in breast cancer: estrogen-induced tumor cell death. Exp Biol Med. 2004;229:722–731. doi: 10.1177/153537020422900804. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S. Intrapituitary regulatory system of proliferation of mammotrophs in the pituitary gland. Zoolog Sci. 2004;21:601–611. doi: 10.2108/zsj.21.601. [DOI] [PubMed] [Google Scholar]
- 40.Ying C. Potential involvement of tumor suppressor gene expression in the formation of estrogen-inducible pituitary tumors in rats. Endocr J. 2000;47:1–5. doi: 10.1507/endocrj.47.1. [DOI] [PubMed] [Google Scholar]
- 41.Kulig E, Camper SA, Kuecker S, Jin L, Lloyd RV. Remodeling of hyperplastic pituitaries in hypothyroid αus-subunit knockout mice after thyroxine and 17β-estradiol treatment: role of Apoptosis. Endocr Pathol. 1998;9:261–274. doi: 10.1007/BF02739967. [DOI] [PubMed] [Google Scholar]


