Abstract
Aim
To investigate the effects of cryogenic treatment on nickel-titanium endodontic instruments. The null hypothesis was that cryogenic treatment would result in no changes in composition, microhardness or cutting efficiency of nickel-titanium instruments.
Methodology
Microhardness was measured on 30 nickel-titanium K-files (ISO size 25) using a Vicker’s indenter. Elemental composition was measured on two instruments using X-ray spectroscopy. A nickel-titanium bulk specimen was analysed for crystalline phase composition using X-ray diffraction. Half of the specimens to be used for each analysis were subjected to a cryogenic treatment in liquid nitrogen (−196 °C) for either 3 s (microhardness specimens) or 10 min (other specimens). Cutting efficiency was assessed by recording operator choice using 80 nickel-titanium rotary instruments (ProFile® 20, .06) half of which had been cryogenically treated and had been distributed amongst 14 clinicians. After conditioning by preparing four corresponding canals, each pair of instruments were evaluated for cutting efficiency by a clinician during preparation of one canal system in vitro. A Student’s t-test was used to analyse the microhardness data, and a binomial test was used to analyse the observer choice data. Composition data were analysed qualitatively.
Results
Cryogenically treated specimens had a significantly higher microhardness than the controls (P < 0.001; β > 0.999). Observers showed a preference for cryogenically treated instruments (61%), but this was not significant (P = 0.21). Both treated and control specimens were composed of 56% Ni, 44% Ti, 0% N (by weight) with a majority in the austenite phase.
Conclusions
Cryogenic treatment resulted in increased microhardness, but this increase was not detected clinically. There was no measurable change in elemental or crystalline phase composition.
Keywords: cryogenic, cutting efficiency, energy-dispersive X-ray spectroscopy, microhardness, nickel-titanium, X-ray diffraction
Introduction
In 1988 an orthodontic wire alloy, Nitinol (nickel-titanium or NiTi) was described in the endodontic literature (Walia et al. 1988). Nickel-titanium alloy exhibits the unique properties of shape memory and pseudoelasticity. Nickel-titanium instruments show considerably greater flexibility and resistance to torsional fracture when compared with stainless steel instruments (Walia et al. 1988). In addition, nickel-titanium alloy was shown to have a lower modulus of elasticity, a wider range of elastic deformation and a greater overall strength (Andreasen et al. 1985, Walia et al. 1988). However, due to the pseudoelastic property of NiTi alloy, NiTi instruments must be machined rather than twisted (Thompson 2000). This machining process may lead to surface defects within the cutting surfaces of the instrument, which have been implicated in the relatively low cutting efficiency of the nickel-titanium instrument (Thompson 2000). Nickel-titanium instruments also exhibit a lower microhardness (303–362 VHN) than stainless steel instruments (522–542 VHN) (Brockhurst & Denholm 1996, Brockhurst & Hsu 1998). Consequently, surface defects occur more readily resulting in wear. Therefore, this combination of surface wear and lower micro-hardness decreases cutting efficiency when compared with stainless steel instruments (Schafer 2002).
Cutting efficiency of endodontic instruments or reamers depends on the interaction of a number of factors, such as metallurgical properties, cross-sectional configuration of shaft, sharpness of flutes, flute design, tip design, lubrication during cutting, wear resistance, chip removal capability and mode of use (Felt et al. 1982). Currently, there is no standard in vitro method available to measure cutting efficiency of endodontic instruments, and various methods have been used in an attempt to evaluate cutting efficiency. Cutting efficiency has been measured during rotary (Oliet & Sorin 1973, Villalobos et al. 1980) and linear (push–pull) motions of instruments (Webber et al. 1980, Camps & Pertot 1995, Bramipour et al. 2001). Endodontic instrument cutting efficiency has been tested on a variety of materials including bovine bone (Oliet & Sorin 1973, Newman et al. 1983), human dentine (Kazemi et al. 1996), acrylic blocks (Tepel et al. 1995, Brau-Aguade et al. 1996) and Plexiglas (Stenman & Spangberg 1990, Haikel et al. 1996). Cutting efficiency has been measured a number of different ways including the effective volume cut out of a substrate removed per unit of cutting length under well-defined cutting conditions (Yguel-Henry & von Stebut 1994), the extracted volume per unit of expended energy (Felt et al. 1982), depth of cut or weight loss (Newman et al. 1983), time of specimen penetration (Oliet & Sorin 1973, Felt et al. 1982), and volume of material removed per unit of time (Machian et al. 1982, Haikel et al. 1996).
Recently, some studies have investigated improving the cutting efficiency of nickel-titanium instruments, specifically focusing on surface treatment techniques. The implantation of boron ions on the surface of nickel-titanium has been shown to increase surface hardness (Lee et al. 1996). Similarly, increased wear resistance and an increased cutting efficiency of nickel-titanium was demonstrated following a thermal nitridation process (Rapisarda et al. 2000) and physical vapour deposition of titanium nitride (Ti3N4) particles (Schafer 2002). All of these studies have yielded promising results, although further studies are needed to assess the impact of these surface treatments on the manufacture and use of nickel-titanium instruments.
Historically, the cold treatment of metals during manufacture had been advocated as a means of improving the surface hardness and thermal stability of the metal (Molinari et al. 2001). The optimum cold treatment temperature range lies between −60 and −80 °C for tool steels depending upon the material and on the quenching parameters involved (Molinari et al. 2001). For the past 30 years, researchers have reported substantial benefits from subjecting metals for industrial applications to a cryogenic process (Mohan Lal et al. 2001, Molinari et al. 2001, Huang et al. 2003). Cryogenic treatment involves submersing metal in a super-cooled bath containing liquid nitrogen (−196 °C/−320 °F) (Mohan Lal et al. 2001, Molinari et al. 2001) and then allowing the metal to slowly warm to room temperature. This cryogenic treatment is used to treat a wide range of metal components, including high-speed steel and hot work tool steel (Barron 1982, Huang et al. 2003). The cryogenic treatment was shown to have more beneficial effects than the traditional higher temperature cold treatment (Moore & Collins 1993). The benefits include increasing cutting efficiency as well as the overall strength of the metal (Molinari et al. 2001, Huang et al. 2003). Cryogenic treatment is an inexpensive treatment that affects the entire cross-section of the metal rather than just the surface in contrast to surface treatment techniques (Mohan Lal et al. 2001), such as ion implantation and vapour deposition. Currently, two mechanisms are believed to account for the change in the properties from cryogenic treatment for steel. The first is a more complete martensite transformation from the austenite phase following cryogenic treatment (Barron 1982). The second is the precipitation of finer carbide (eta) particles within the crystalline structure (Huang et al. 2003). Controversy exists as to which mechanism is responsible.
Two studies have been reported in the endodontic literature regarding cryogenic treatment of endodontic instruments. Both have investigated treatment on stainless steel instruments only. Bramipour et al. 2001 treated stainless steel endodontic instruments (Flex-R and Hedström) cryogenically and found no effect on cutting efficiency of either instrument type. Berls (2003) found no significant increase in wear resistance of the stainless steel hand instruments (S-type and K-type). In fact, cryogenic tempering produced a K-file that was inferior with respect to initial cutting efficiency and overall wear resistance. The difference between stainless steel alloys and NiTi alloys is in their martensite temperatures. Stainless steel alloys have a martensitic transformation temperature above room temperature and NiTi alloys have a martensitic transformation temperature below room temperature. As one of the theories proposed to explain the effects of cryogenic treatment is the completion of martensite formation within steel alloys, a question exists as to whether or not cryogenic treatment would improve the cutting efficiency of NiTi instruments in a similar manner.
The aim of this study was to investigate the effects of cryogenic treatment on nickel-titanium endodontic instruments. The effects were measured using a cutting efficiency test, X-ray diffraction (XRD) analysis, micro-hardness test and compositional analysis.
Materials and methods
Thirty size 25 nickel-titanium K-files (NTO2525; Dentsply-Tulsa Dental, Tulsa, OK, USA) were used for the microhardness and energy-dispersive X-ray spectroscopy (EDS) portions of the experiment. Eighty ProFile® size 20, .06 taper nickel-titanium rotary instruments (PIT062025; Dentsply-Tulsa Dental) were used for evaluation of cutting efficiency. A bulk specimen of nickel-titanium (Sportswire International, Tulsa, OK, USA) was used for the XRD analysis.
Cryogenic treatment
Both the test instruments and bulk NiTi specimen were cryogenically treated with liquid nitrogen at −196 °C according to the US Patent No. 5 259 200 (Kamody 1993). The patent specifies for the total immersion time to be ‘1 h per 1 in. of cross-section’ of the metal to be treated and the time period to reach ambient room temperature following the immersion to be ≤10 min plus 10 min per minimum cross-sectional dimension in inches. The immersion time for microhardness was 3 s according to the dimensional requirements specified by the patent, whilst the immersion time for the EDS, XRD and cutting efficiency was arbitrarily chosen as 10 min. After the specimens had been immersed in the bath, they were removed and allowed to return to room temperature by contact with ambient air for 10 min.
Microhardness
Thirty size 25 nickel-titanium K-files (NTO2525; Dentsply-Tulsa Dental) were embedded in epoxy (811-563-103 and 811-563-104; Leco, St Joseph, MI, USA) mixed according to the manufacturer’s instructions. Fifteen control instruments and fifteen cryogenically treated instruments were used. The ratio of resin to hardener was 75 : 10.5 mL. Each instrument was cut at the handle and then placed within a mounting ring (20-8161-010; Buehler, Lake Bluff, IL, USA). The mounting rings were brushed with releasing agent (20-8185-032; Buehler) and placed upon a flat surface. The resin and hardener were then mixed until clear in appearance and was then poured into each mounting ring. The epoxy was left to cure for 8 h. The resin blocks were then removed from the mounting rings and ground to reveal a cross-section of the instruments and polished flat using a grinder/polisher (Phoenix Beta; Buehler). Silicon carbide polishing papers (240, 320, 400, 600 and 1200) were used in succession followed by Al2O3 powder/H2O suspensions (1.0, 0.3 and 0.05 μm particle sizes) for final polishing. A Vicker’s indenter was used to make two indentations adjacent to the edge of the instrument cross-section (FM-7; Future Tech, Tokyo, Japan). A 9.8-N indentation load was applied for a 15-s dwell time. Both indentation diagonals were measured, and the Vicker’s microhardness, VHN, was calculated from the size of the indentation. According to the following equation:
| (1) |
where F is the indentation load (N) and d the average diagonal length of indentation (mm).
Energy-dispersive X-ray spectroscopy
Two size 25 nickel-titanium K-files (NTO2525; Dents-ply-Tulsa Dental), one cryogenically treated and one control instrument, were mounted in an electrically conductive moulding compound (Konductomet I #20-3375-016 and #20-3380-064; Buehler) to avoid the need for gold sputter coating. The instrument handles were removed, and the instruments were placed within a mounting ring (20-8161-010; Buehler) followed by the moulding compound. The mountings were created at a temperature of 150 °C, under 4200 psi pressure for 1 min using an automatic electrohydraulic mounting press (Simplemet 3; Buehler). The specimens were then polished flat using a grinder/polisher (Phoenix Beta; Buehler) according to the sequence previously discussed. The nickel-titanium surfaces were examined in a secondary electron image mode on a scanning electron microscope (SEM) (JSM-6300; JEOL, Peabody, MA, USA) with an EDS apparatus. A Si (Li) X-ray detector (Noran Instruments, Middleton, WI, USA) and an X-ray microanalysis and digital imaging system (5480; IXRF Systems, Houston, TX, USA) controlled by a workstation (EDS 2000; IXRF Systems) was used for the EDS analysis. Both cryogenically treated and control instruments were analysed with an EDS point composition analysis in the centre of cross-section followed by an EDS line profile analysis across the width of cross-section.
X-ray diffraction analysis
A nickel-titanium bulk specimen with a hexagonal cross-section was used. This specimen was purported by the manufacturer (Sportswire International) to have the same composition as the endodontic instruments. The specimen, for XRD, was sectioned into 1.5-cm lengths using a low-speed saw (Isomet; Buehler) under water irrigation. Four opposing sides of the hexagonal block were ground off to render a rectangular cross-section in the dimensions that were required for XRD, using a grinder/polisher (Phoenix Beta; Buehler) and 320-grit silicon carbide polishing paper. The two surfaces that were not polished served as the surfaces to be analysed and were oriented perpendicular to the incident radiation. The width of the block was 2 mm, and a total of eight blocks were placed next to one another for the analysis (Fig. 1). XRD analysis was performed at room temperature using Cu-Kα radiation (λ = 1.5418 Å) on an X-ray diffractometer (Miniflex CN2005; Rigaku, Tokyo, Japan) with a computer upgrade. The diffractometer was calibrated with a silicon standard (640b Silicon Powder XRD Spacing, Standard Reference Material; NIST, Gaithersburg, MD, USA). The experimental conditions were 2θ range 20–90° at 0.02°/step, with a 5-s photon counting time per step. The peaks on the XRD patterns were indexed to the X-ray polycrystalline powder diffraction files (ICDD 1998). Following the initial XRD analysis, the blocks were cryogenically treated for 10 min and analysed again at room temperature.
Figure 1.
Schematic representing bulk specimen for use in XRD and grinding sequence to render a rectangular cross-section.
Cutting efficiency
Eighty ProFile® 20, .06 nickel-titanium rotary instruments (PIT062025; Dentsply-Tulsa Dental) were used. Half of the instruments were treated cryogenically in a bath of liquid nitrogen (−196 °C) for 10 min. Extracted teeth were decoronated using a sectioning disc and high-speed saw (456, 275-02; Dremel Incorporated, Mount Prospect, IL, USA). The corresponding canals within the same root system were standardized to an ISO size 10. Then each instrument was conditioned by dulling it in either the buccal or lingual canals of the mesial roots of four separate lower molars for a total of 4 min. Pairs of instruments, one cryogenically treated and one non-treated, were then placed in each of 40 envelopes, and one instrument was marked. The proportion of marked and nonmarked instruments was controlled to ensure an equal distribution. Four graduate endodontic faculty and five second- and third-year graduate endodontic residents each compared three pairs of instruments for cutting efficiency. In addition, five first-year graduate endodontic residents each compared two pairs of instruments. All instruments were compared in additional decoronated extracted teeth.
Standardized corresponding canals were again used for the comparison. The instruments were used in an electric rotary handpiece (AEU-17BTT, AHP-88; Dents-ply-Tulsa Dental) at the manufacturer’s recommended speed of 350 rpm. The observers made a choice as to which instrument cut more efficiently.
Statistics
A Student’s t-test with α = 0.05 was used to test for a significant effect of cryogenic treatment on microhardness. A binomial test (α = 0.05) was used to determine if the proportion of cryogenically treated instruments, as chosen by observers, was significantly different from 50%. An a priori power analysis predicted that 30 pairs would be sufficient to detect a significant difference if the measured proportion was <30% or >70%.
Results
There was an increase in the microhardness following cryogenic treatment. Nontreated instruments had a mean VHN of 339.3 ± 23.0, and treated instruments had VHN of 346.7 ± 20.6 (Fig. 2). A Student’s t-test showed this to be a statistically significant difference (P < 0.001; β > 0.999).
Figure 2.
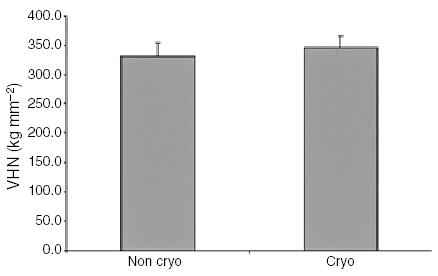
Results of microhardness.
The results of the clinical observer choice are found in Table 1. The proportion of treated instruments chosen was 61%. This was not significantly different than 50% (P = 0.21).
Table 1.
Results from the observer choice
| Category | n | Observer proportion | Test proportion | P-value |
|---|---|---|---|---|
| Cryogenic instrument | 25 | 0.61 | 0.50 | 0.212a |
| Noncryogenic instrument | 16 | 0.39 | ||
| Total | 41 | 1.00 |
Based on normal distribution.
Results from XRD analysis demonstrated a major NiTi austenite phase prior to cryogenic treatment. A minimum of three peaks were indexed to the austenite NiTi phase (powder diffraction file no. 18-0899). A minimum of three peaks of lower intensity were indexed to the martensite NiTi phase (powder diffraction file no. 35-1281). There were no changes detected in the diffraction pattern from cryogenic treatment when compared with the noncryogenically treated specimens (Fig. 3). Several low intensity peaks between 2θ (30–40°) were attributed to NiTiO3 (no. 33-960) and Ni3TiO5 (no. 30-865).
Figure 3.
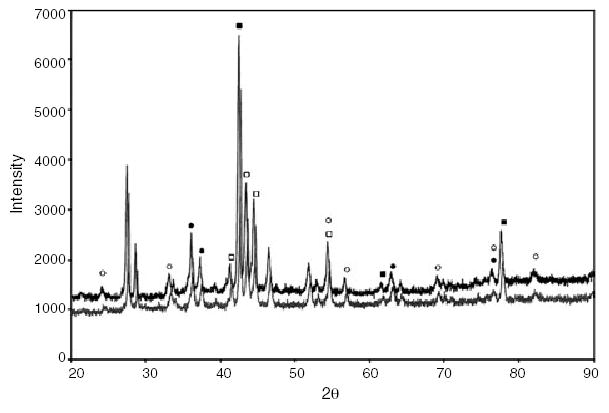
Superimposition of X-ray diffraction patterns of nontreated and cryogenically treated instruments (▪, Austenite NiTi; ▴, Martensite NiTi; •, NiTiO3; □, Ni3TiO3).
Results from the EDS are summarized in Table 2. EDS yielded a slight increase in the nitrogen Kα peak intensity following cryogenic treatment as compared with the control. However, after the ZAF correction, no measurable amount of nitrogen was detected on the control and cryogenically treated specimens.
Table 2.
Results of energy-dispersive X-ray spectroscopy
| Element | Line | Intensity (counts s−1) | Background intensity (count s−1) | Atomic % | Weight % |
|---|---|---|---|---|---|
| N | Kα | 1.17 | 1.12 | 0.00 | 0.00 |
| Ti | Kα | 206.34 | 5.86 | 49.15 | 44.08 |
| Ni | Kα | 108.95 | 2.57 | 50.85 | 55.92 |
| Total | 100.00 | 100.00 |
Discussion
X-ray diffraction
The XRD results were in agreement with other reports in the literature regarding XRD of NiTi alloy (Thayer et al. 1995, Iijima et al. 2002). Following cryogenic treatment, there were no changes detected when compared with the noncryogenically treated bulk specimens. Titanium nitride could not be identified following treatment. In fact, the peaks between the control specimens and treated specimens were identical except for the minor changes in intensity.
Energy-dispersive X-ray spectroscopy
Certain effects related to composition can affect the X-ray spectrum produced in EDS. These effects must be corrected in order for an accurate analysis to be performed. The corrections are called ZAF corrections, which are in reference to three confounding factors; atomic number (Z), absorption (A) and fluorescence (F). The intensity of X-rays is affected by the depth of electron penetration and the fraction of electrons within the specimen, which is a function of the atomic number (Z). The higher the atomic number of the element, the greater the X-ray intensity. The absorption (A) is the absorption of X-rays in the specimen that occurs as a function of composition and depth of electron penetration. X-rays are generated throughout the volume of material during EDS analysis. X-rays produced in the bulk must pass through a certain distance within the specimen and some are absorbed. The fluorescence (F) is caused by X-ray absorption of re-emission at a different wavelength.
After the ZAF correction, no measurable amount of nitrogen was detected on the control and cryogenic treated specimens. As nitrogen is a light element that possesses few electron energy levels, the X-ray energy may have been too low for the scintillation detector, which had an ultrathin carbon window, to accurately measure.
Microhardness
The microhardness results were consistent with those of Brockhurst & Hsu (1998) but not with those of Kuhn et al. 2001. The mean VHN was 339.3 ± 22.9 for controls and 346.7 ± 20.6 following cryogenic treatment. Brockhurst & Hsu (1998) demonstrated the microhardness of NiTi hand instruments to range from 296 to 349 VHN. Each instrument was cut at the handle, mounted in resin, ground to cross-section and tested for microhardness under a 300-g load and a 15-s dwell time. Six microhardness measurements were taken along each instrument, and they were averaged (Brockhurst & Hsu 1998). Kuhn et al. 2001 reported that both Hero 20 (.06 taper) and ProFile® 20 (.06 taper) instruments had a mean VHN >400 prior to a heat treatment. No mention was made of how the specimens were mounted or polished. In the present study, microhardness at the edge of the cross-section at the first cutting blade were investigated, where Kuhn et al. 2001 measured only at the ‘inactive’ part of the instrument that had not been machined.
Several mechanisms can be proposed to account for the increase in microhardness. These include: (i) A reaction between nitrogen and titanium atoms, resulting in titanium nitride formation on the surface (Rapisarda et al. 2000). (ii) Nitrogen atom deposition into the interstitial spaces within the atomic lattice of NiTi alloy causing lattice strain (Shackelford & Meier 2001). (iii) A more complete martensitic transformation of NiTi alloy (Barron 1982). (iv) Precipitation of finer carbide particles throughout the crystal lattice (Huang et al. 2003). The latter two mechanisms have been suggested to account for cryogenic changes in steel alloys (Barron 1982). As there is no carbon present within NiTi alloy, the fourth mechanism is ruled out immediately. The XRD results did not indicate any titanium nitride formation following cryogenic treatment. In addition, the increase in microhardness, although statistically significant, was only slightly higher as compared with our control. One study describing titanium nitride reported that the coating thickness ranges from 1 to 7 μm and that it is possible to obtain surface hardness of about 2200 VHN (Schafer 2002). Thus, one would expect a markedly greater increase in microhardness than what was found if titanium nitride formation was responsible. The slight increase in microhardness found in this study can be caused by strain within the atomic lattice due to the deposition of nitrogen within the interstitial spaces. Furthermore, EDS line profile analysis revealed that nitrogen was evenly distributed throughout the entire cross-section of the instrument following cryogenic treatment. This may have been due to the empty interstitial spaces within NiTi alloy that are large enough to be readily occupied by nitrogen atoms (Donachie 1988). One of the mechanisms implicated for the improvements to tool steels, by cryogenic treatment, has been a more complete martensitic transformation (Barron 1982). The difference between stainless steel and NiTi alloy is in their respective martensite transformation temperatures. Stainless steel alloy has a martensitic transformation temperature above room temperature, and NiTi alloy has a martensitic transformation temperature below room temperature. It is uncertain whether cryogenic treatment affected the stress-induced martensite transformation of NiTi alloy at room temperature.
Cutting efficiency
The observer choice involved determining whether the effect of cryogenic treatment was clinically detectable. The null hypothesis was that no difference could be detected following cryogenic treatment, and that 50% of the observers would choose the cryogenically treated instruments as cutting more efficiently. An a priori power analysis predicted that 30 instrument pairs would be sufficient to detect a significant difference approaching a proportion of either 30 or 70% (α = 0.05). The proportion of the observer choice for the cryogenically treated instruments over controls was 61%. This was not statistically different from 50% (P = 0.21).
The data indicated no effect from cryogenic treatment upon nickel-titanium endodontic instruments except for an increase in microhardness. An increased hardness corresponds to an increased wear resistance for most materials (Ashby & Jones 1980). An increased wear resistance would intuitively expect to correspond to an increased cutting efficiency. A statistically significant difference in microhardness was detected, but it did not result in a clinically detectable increase in cutting efficiency. This may be attributed to the statistical power in the microhardness test being >99.9%. The sample size was increased to n = 15 from n = 2 to promote a normal distribution of mean values. Due to the precision and number of measurements, a statistical but not clinically significant difference was detected. This was confirmed with the observer choice portion of the study. These results are similar to the other studies that looked at cryogenic treatment and wear resistance (Bramipour et al. 2001, Berls 2003). Both studies investigated the cryogenic treatment of stainless steel instruments and its effect on wear resistance. Bramipour et al. 2001 measured wear in terms of a decrease in the depth of groove cut into an acrylic wafer by the instruments at a specific number of cycles. The depth data were normalized by dividing the depth of the groove cut in the acrylic wafer after machining on dentine with the depth of the groove cut prior to machining dentine. Bramipour et al. 2001 concluded that cryogenic treatment did not increase the wear resistance of stainless steel instruments. Berls (2003) measured the depth of the groove cut in a Plexiglas block before and after machining bovine bone. The conclusion was that cryogenic treatment had no effect upon wear resistance of stainless steel instruments.
Conclusion
There was a slight increase in microhardness that was found to be statistically significant. However, the increase in microhardness was not clinically detectable in terms of cutting efficiency. Nitrogen concentration could not be measured following cryogenic treatment through EDS following the ZAF correction. There was no measurable change in crystalline phase composition.
Acknowledgments
This experiment would not have been possible without the donation of the necessary materials by Dr Ben Johnson of Dentsply-Tulsa Dental, Ms Marilyn Hale of Dentsply-Tulsa Dental and Mr Carl Behrendt of Sports-wire International.
Special thanks to Dr Eric Solomon for his assistance with the statistical analysis.
This study was funded by Baylor Research Funds, Baylor College of Dentistry, a component of the Texas A&M University System Health Science Center, and equipment was shared with NIH-NIDCR grant DE013358.
References
- Andreasen G, Wass K, Chan KC. A review of superelastic and thermodynamic nitinol wire. Quintessence International. 1985;16:623–6. [PubMed] [Google Scholar]
- Ashby MF, Jones DRH (1980) Engineering Materials 1: An Introduction to Their Properties and Applications Oxford: Pergamon Press.
- Barron R. Cryogenic treatment of metals to improve wear-resistance. Cryogenics. 1982;22:409–13. [Google Scholar]
- Berls RW. Effect of cryogenic tempering on the wear resistance of two types of stainless steel files [Abstract] Journal of Endodontics. 2003;29:300. [Google Scholar]
- Bramipour D, Svec TA, White KW, Powers JM. Wear resistance of cryogenically treated stainless steel files. Journal of Endodontics. 2001;27:212–3. doi: 10.1097/00004770-200103000-00019. [DOI] [PubMed] [Google Scholar]
- Brau-Aguade E, Canalda-Sahli C, Berastegui-Jimeno E. Cutting efficiency of K-files manufactured with different metallic alloys. Endodontics and Dental Traumatology. 1996;12:286–8. doi: 10.1111/j.1600-9657.1996.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Brockhurst PJ, Denholm I. Hardness and strength of endodontic files and reamers. Journal of Endodontics. 1996;22:68–70. doi: 10.1016/S0099-2399(96)80274-3. [DOI] [PubMed] [Google Scholar]
- Brockhurst P, Hsu E. Hardness and strength of endodontic instruments made from NiTi alloy. Australian Endodontic Journal. 1998;24:115–9. doi: 10.1111/j.1747-4477.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Camps JJ, Pertot WJ. Machining efficiency of nickeltitanium K-type files in a linear motion. International Endodontic Journal. 1995;28:279–84. doi: 10.1111/j.1365-2591.1995.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Donachie MJ Jr (1988) Titanium – A Technical Guide, 2nd edn. Materials Park: ASM International.
- Felt RA, Moser JB, Heuer MA. Flute design of endodontic instruments: its influence on cutting efficiency. Journal of Endodontics. 1982;8:253–9. doi: 10.1016/S0099-2399(82)80335-X. [DOI] [PubMed] [Google Scholar]
- Haikel Y, Serfaty R, Lwin TT, Allemann C. Measurement of the cutting efficiency of endodontic instruments: a new concept. Journal of Endodontics. 1996;22:651–6. doi: 10.1016/S0099-2399(96)80058-6. [DOI] [PubMed] [Google Scholar]
- Huang JY, Zhu YT, Liao XZ, Beyerlein IJ, Bourke MA, Mitchell TE. Microstructure of cryogenic treated M2 tool steel. Materials Science and Engineering. 2003;A339:241–4. [Google Scholar]
- ICDD (1998) Powder Diffraction File, Set 1–48. Swarthmore: ICPDS-International Center for Diffraction Data.
- Iijima M, Ohno H, Kawashima I, Endo K, Brantley WA, Mizoguchi I. Micro X-ray diffraction study of superelastic nickel-titanium orthodontic wires at different temperatures and stresses. Biomaterials. 2002;23:1769–74. doi: 10.1016/s0142-9612(01)00303-9. [DOI] [PubMed] [Google Scholar]
- Kamody DJ (1993) Process for the Cryogenic Treatment of Metal Containing Materials US Patent #5 259 200.
- Kazemi RB, Stenman E, Spangberg LS. Machining efficiency and wear resistance of nickel-titanium endodontic files. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 1996;81:596–602. doi: 10.1016/s1079-2104(96)80055-2. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Tavernier B, Jordon L. Influence of structure on nickel-titanium endodontic instruments failure. Journal of Endodontics. 2001;27:516–20. doi: 10.1097/00004770-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Lee DH, Park B, Saxena A, Serene TP. Enhanced surface hardness by boron implantation in Nitinol alloy. Journal of Endodontics. 1996;22:543–6. doi: 10.1016/S0099-2399(96)80015-X. [DOI] [PubMed] [Google Scholar]
- Machian GR, Peters DD, Lorton L. The comparative efficiency of four types of endodontic instruments. Journal of Endodontics. 1982;8:398–402. doi: 10.1016/S0099-2399(82)80093-9. [DOI] [PubMed] [Google Scholar]
- Mohan Lal D, Renganarayanan S, Kalanidhi A. Cryogenic treatment to augment wear resistance of tool and die steels. Cryogenics. 2001;41:149–55. [Google Scholar]
- Molinari A, Pellizzari M, Gialenella S, Straffelini G, Stiasny KH. Effect of deep cryogenic treatment on the mechanical properties of tool steels. Journal of Materials Processing Technology. 2001;118:350–5. [Google Scholar]
- Moore K, Collins DN. Cryogenic treatment of three heat treated tool steels. Key Engineering Materials. 1993;47:86–7. [Google Scholar]
- Newman JG, Brantley WA, Gerstein H. A study of the cutting efficiency of seven brands of endodontic files in linear motion. Journal of Endodontics. 1983;9:316–22. doi: 10.1016/S0099-2399(83)80145-9. [DOI] [PubMed] [Google Scholar]
- Oliet S, Sorin SM. Cutting efficiency of endodontic reamers. Oral Surgery, Oral Medicine, Oral Pathology. 1973;36:243–52. doi: 10.1016/0030-4220(73)90246-6. [DOI] [PubMed] [Google Scholar]
- Rapisarda E, Bonaccorso A, Tripi TR, Fragalk I, Condorelli GG. The effect of surface treatments of nickel-titaniumfiles on wear and cutting efficiency. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2000;89:363–8. doi: 10.1016/s1079-2104(00)70103-x. [DOI] [PubMed] [Google Scholar]
- Schafer E. Effect of physical vapor deposition on cutting efficiency of nickel-titanium files. Journal of Endodontics. 2002;28:800–2. doi: 10.1097/00004770-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Shackelford JF, Meier ML (2001) Introduction to Materials Science for Engineers, 5th edn. Upper Saddle River: Prentice Hall.
- Stenman E, Spangberg LS. Machining efficiency of endodontic K files and Hedstrom files. Journal of Endodontics. 1990;16:375–82. doi: 10.1016/s0099-2399(06)81909-6. [DOI] [PubMed] [Google Scholar]
- Tepel J, Schafer E, Hoppe W. Properties of endodontic hand instruments used in rotary motion. Part 1. Cutting efficiency. Journal of Endodontics. 1995;21:418–21. doi: 10.1016/S0099-2399(06)80828-9. [DOI] [PubMed] [Google Scholar]
- Thayer TA, Bagby MD, Moore RN, DeAngelis RJ. X-ray diffraction of nitinol orthodontic arch wires. American Journal of Orthodontics and Dentofacial Orthopedics. 1995;107:604–12. doi: 10.1016/s0889-5406(95)70103-6. [DOI] [PubMed] [Google Scholar]
- Thompson SA. An overview of nickel-titanium alloys used in dentistry. International Endodontic Journal. 2000;33:297–310. doi: 10.1046/j.1365-2591.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- Villalobos RL, Moser JB, Heuer MA. A method to determine the cutting efficiency of root canal instruments in rotary motion. Journal of Endodontics. 1980;6:667–71. doi: 10.1016/S0099-2399(80)80128-2. [DOI] [PubMed] [Google Scholar]
- Walia HM, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. Journal of Endodontics. 1988;14:346–51. doi: 10.1016/s0099-2399(88)80196-1. [DOI] [PubMed] [Google Scholar]
- Webber J, Moser JB, Heuer MA. A method to determine the cutting efficiency of root canal instruments in linear motion. Journal of Endodontics. 1980;6:829–34. doi: 10.1016/S0099-2399(80)80036-7. [DOI] [PubMed] [Google Scholar]
- Yguel-Henry S, von Stebut J. Cutting efficiency loss of root canal instruments due to bulk plastic deformation, surface damage, and wear. Journal of Endodontics. 1994;20:367–72. doi: 10.1016/S0099-2399(06)80292-X. [DOI] [PubMed] [Google Scholar]


