Abstract
There is accumulating evidence that universally vaccinating school children would reduce the transmission of influenza. The authors sought to identify target age groups, within the pediatric population, which develop influenza the earliest and which are most strongly linked with mortality in the population. Patient visits for respiratory illness were monitored, with real time syndromic surveillance systems, at six Massachusetts healthcare settings, including ambulatory care sites and emergency departments at tertiary care and community hospitals. Visits from January 1, 2000 to September 30th, 2004 were segmented into age group subpopulations. Timeliness and prediction of each subpopulation was measured against pneumonia and influenza mortality in New England with time series analyses and regression models. Patient age significantly influences timeliness (p=0.026) with pediatric age groups arriving first (p<0.001). Three to four year olds are consistently the earliest (p=0.0058). Age also influences the degree of prediction of mortality (p =0.036) with children under five years old most strongly associated with mortality than all other patients (p <0.001). Our findings add to a growing of body support for a strategy to vaccinate children older than the currently targeted 6–23 month olds, and specifically suggest that there may be value in vaccinating preschool aged children.
Medical Subject Headings: Disease Transmission, Fourier Analysis, Influenza, Influenza Vaccines, Mass Immunization, Population Surveillance, Sentinel Surveillance, Vaccination
Abbreviations: ACIP: Advisory Committee on Immunization Practices, AC: Ambulatory Care, ANOVA: Analysis of Variance, ED: Emergency Department, DF: Degrees of Freedom, ICD: International Classification of Disease, ILI: Influenza-Like Illness, P&I: Pneumonia and Influenza, SE: Standard Error, SD: Standard Deviation
Each year in the United States, an influenza epidemic causes hundreds of thousands of hospitalizations (1–4), tens of thousands of deaths (5, 6) and has enormous economic impact (7, 8). Vaccination against influenza is the mainstay of prevention efforts and was initially targeted at older individuals and those with high risk of complications (3). In 2003, the Advisory Committee on Immunization Practices (ACIP) recommended universal vaccination of infants and children 6 to 23 months (9, 10). ACIP continues to only recommend influenza vaccination of children aged ≥23 months who have high-risk medical conditions. Given the evidence that the vaccination of schoolchildren significantly reduces influenza transmission (11–13), expanding the recommended target population to include healthy children has been suggested (14).
We take a novel approach to identifying high value populations for influenza vaccination. We leverage a real time population health monitoring system that acquires and processes clinical data collected in the routine process of internal medicine, pediatric and emergency care (15–19). Specifically, we identify within a regional healthcare-based population of patients with respiratory illness, the age cohorts which present the earliest and which have patterns of illness most strongly associated with adverse outcomes from influenza.
MATERIALS AND METHODS
Patient Populations
We performed time series analysis of five healthcare populations, identified retrospectively, with respiratory illness syndromes. Four populations consisted of patients presenting to emergency departments (ED) which share overlapping catchment areas in Eastern Massachusetts but differ in the age distribution of the patients. The first is a pediatric ED at a large children’s hospital and has an average patient age of 6.8 (SD, 6.3) years. The second is an adult ED with an average patient age of 52.2 (SD, 22.7) years. The populations from these departments included encounters from January 1st, 2000 to August 1st, 2004. The third is a general ED that sees both children and adults with an average patient age of 44.8 (SD, 27.1) and includes patients seen from October 1st, 2002 to September 30th, 2004. The final healthcare population is a group of community EDs which comprises patients seen at three affiliated community-based emergency departments and includes both children and adults with an average patient age of 37.8 (SD, 21.1) seen from July 1st, 2001 to June 30th, 2004. ED presenting complaints were used for classification of patients with respiratory illness as previously described (20, 21).
We also studied daily counts of respiratory illness from ambulatory care (AC) encounters at a large group practice among insurees of a Health Maintenance Organization in eastern Massachusetts. Approximately 175,000 members are included. These cases were identified from physician-assigned International Classification of Disease (ICD) encoding of telephone contacts, regular visits, and urgent-care encounters, but not ED visits. The grouping of patients with respiratory illness was based on merging ICD-9 diagnoses codes assigned by the clinician at the time of consultation using a modification of a provisional classification scheme produced by the Department of Defense ESSENCE project (22). The AC population included patients seen between January 1st, 2000 and December 31st, 2003. All of these data were obtained from two real-time population health monitoring systems, the AEGIS (Automated Epidemiologic Geotemoporal Integrated Surveillance) system (23) and the National Bioterrorism Syndromic Surveillance Demonstration Project (24).
The effect of patient age was evaluated by considering separately the following age groups: 0–2 (infant and toddler), 3–4 (preschool age), 5–11 (school age), 12–17 (adolescent), 18–39 (youngest adults), 40–64 (older adults), and over 64 (elderly adults).
For comparison with an extant surveillance system, we obtained data from the CDC U.S. Influenza Sentinel Providers Surveillance Network. For this system, influenza morbidity data is collected from sentinel health-care providers who report the number of patients they have seen with influenza-like illness (ILI) symptoms. These symptoms include fever (temperature above 100°F) plus either a cough or a sore throat. Weekly ILI counts from September 30th, 2001 to October 2nd, 2004 were obtained for Massachusetts.
We evaluated earliness of presentation and association with adverse outcomes for the healthcare populations and the sentinel surveillance by comparing them with pneumonia and influenza (P&I) mortality data. Deaths due to P&I in New England for all ages combined were obtained from the CDC 122 Cities Mortality Reporting System published weekly in Table III of the Morbidity and Mortality Weekly Report (25).
Analysis of timeliness
The temporal relationships between the healthcare encounter and mortality datasets were characterized using time series analysis. We first removed linear trends in the data and standardized the residuals from this analysis. Given that we already expect that each of the data sets will display strong yearly components, we used a finite Fourier transform to remove random noise from the yearly signals and produce a smoothed picture of seasonal change.(26) We then performed cross-spectral analysis to find the estimated lead time (i.e., phase shift) between the underlying yearly components of each of the monitored patient populations and the pneumonia and influenza mortality time series. The lead time is the lag between two time series of interest. All analyses were carried out in SAS v. 9 for Windows (The SAS Institute Inc., Cary, NC).
Cross-spectral analysis was initially applied to data streams: pediatric ED, adult ED, general ED, community EDs, AC, ILI and P&I. The sine and cosine coefficients were obtained for the yearly frequency of approximately 52 weeks. The lead time was calculated from all monitored patient populations and ILI to P&I mortality. The yearly signals of respiratory illness from the monitored patient population partitioned into age subgroups were also obtained and compared to that of overall population mortality due to P&I mortality.
Differences in estimated mean phase shift by age group and site of care were evaluated by Analysis of Variance (ANOVA). We used randomized complete block design ANOVA where the blocks are the sites of care and the treatments are the age groups, with the estimated phase-shift from each site, by age group, as the outcome (27). The adult ED and pediatric ED data blocks were treated as one block to account for missing age groups at each of these sites. The hypothesis that specific pediatric age groups as well as aggregated pediatric age ranges (0–4 and 0–18) were timelier than other age groups was tested.
Analysis of predictive value for mortality
We assessed the relative predictive value of the time-lagged healthcare population data streams by fitting generalized linear models to P&I mortality counts (28). A Poisson distribution for the P&I was assumed as it is usually appropriate for modeling counts. We ran separate models for each population where the predictor was the respiratory counts from the prior week suggested by the cross-spectral analysis. The same method was used for each age group of each population and ILI data. Overall model fit for each of the Poisson regression models was calculated by comparing deviance statistics with their asymptotic chi-square (29). The value of each population’s respiratory counts in predicting mortality was determined by calculating the proportion of the deviance explained, similar to the R2.
Differences in mean predictive value by age group and site of care were evaluated by ANOVA. We once again used randomized complete block design ANOVA where the blocks are the sites of care and the treatments are the age groups, with the percent deviance explained in each site by age group as the outcome. The hypothesis that specific pediatric age groups as well as aggregated pediatric age ranges (0–4 and 0–18) were more predictive than other age groups was tested.
RESULTS
Timeliness by patient population
The populations are described in Table 1. The EDs have approximately the same visit rate ranging from 99.6 (SD, 24.0) to 174.7 (SD, 43.0) visits per week. The AC has approximately ten-fold more volume with 1585.9 (SD, 556.3) visits per week. The sentinel ILI surveillance system reports cases at an average rate of 71.1 (SD, 47.9) per week. Each population displays a highly seasonal cycle where peaks of illness occurred from the beginning of December to the end of February. Figure 1 shows the results of the cross-spectral analysis. The P&I mortality peak is last, at the end of February.
Table 1.
Summary of five retrospective patient populations with respiratory illness syndromes presenting to healthcare sites and the CDC sentinel ILI surveillance system in Eastern Massachusetts, USA, 2000–2004.
| Data Source* | Number of weeks in study | Population size | Mean visits per week (SD) |
|---|---|---|---|
| Ambulatory | 208 | 329 876 | 1 585.9 (556.3) |
| Pediatric ED | 208 | 29 372 | 141.2 (44.8) |
| Adult ED | 208 | 20 715 | 99.6 (34.0) |
| Community ED | 156 | 27 260 | 174.7 (29.3) |
| General ED | 105 | 13 185 | 125.6 (43.0) |
| Sentinel ILI | 157 | 7 495 | 71.1 (47.9) |
ED, Emergency Department
ILI, Influenza-Like Illness
Figure 1. Predicted yearly seasonality of respiratory illness from five retrospective patient populations with respiratory illness syndromes presenting to healthcare sites and the CDC sentinel influenza-like illness surveillance system, Eastern Massachusetts, USA, 2000–2004.
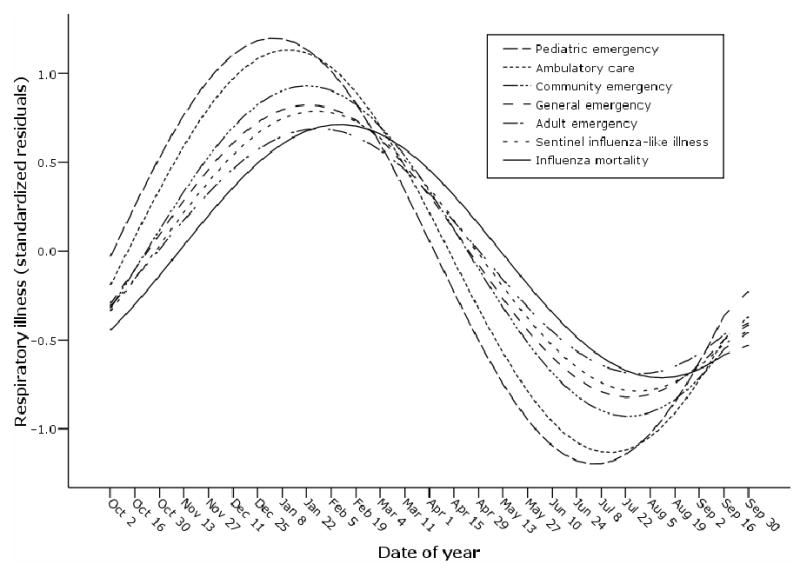
Yearly cycles were obtained by cross-spectral analysis performed on each data stream after linear detrending and standardization (standardized regression residuals). The phase shift of each data stream with the CDC influenza mortality surveillance represents the timeliness of the data stream.
Timeliness was calculated as the lead time from each of the respiratory illness datasets to P&I mortality. The ambulatory care population, with both pediatric and adult patients, has a mean lead time of about four weeks (29 days), peaking in mid to late January. The pediatric ED population displays the earliest peak of respiratory illness, occurring on average five weeks (38 days) prior to the peak in mortality, during the first week of January. Sentinel ILI data peaks on average 20 days prior to influenza mortality, well after both the pediatric ED and ambulatory care populations. The adult ED, general ED and community populations are the least timely for warning about influenza mortality with a mean lead time of about two weeks (12,10,14 days, respectively), peaking during the first week of February.
Timeliness by age
The lead times varied by age (Table 2). Separate cross-spectral analysis of the age groups revealed that among patients presenting to the different healthcare settings, children constitute the earliest signal of P&I mortality irrespective of site of care. Among children, three to four year olds are seen first with a mean lead time of 34 days (95% confidence interval (CI): 14.5, 53.5). This age group presents to the pediatric ED with the longest lead time (50 days). Pediatric patients seek care prior to all adults age groups,(18–39, 40–64 and over 65) in the AC and ED settings with a mean lead times of compared to 12.0 (95% CI: −4, 28), 10.5 (95% CI: −19.8, 40.8) and 14.5 days (95% CI: −6.8, 35.8) days, respectively.
Table 2.
Timeliness of patient populations with respiratory illness for signaling P&I mortality by site of care and patient age in Eastern Massachusetts, USA, 2000–2003.
| Timeliness (days) † | |||||||
|---|---|---|---|---|---|---|---|
| Data Source* | <3 | 3–4 | 5–10 | 11–17 | 18–39 | 40–64 | >64 |
| Ambulatory | 35 | 37 | 25 | 25 | 26 | 30 | 33 |
| Pediatric ED | 35 | 50 | 40 | 28 | - | - | - |
| Adult ED | - | - | - | - | 7 | 4 | 11 |
| Community ED | 21 | 26 | 12 | 25 | 3 | 21 | 13 |
| General ED | 19 | 23 | 15 | 32 | 12 | −13 | 1 |
| Mean (95% CI ) | 27.5 (13.6, 41.3) | 34.0 (14.5, 53.5) | 23.0 (2.9, 43) | 27.5 (22.2, 32.8) | 12.0 (−4.0, 28;0) | 10.5 (−19.8.40.8) | 14. 5 (−.6.8. 35.8) |
ED, Emergency Department
CI, Confidence Interval
Lead time to pneumonia and influenza mortality calculated by cross-spectral analysis
Randomized complete block design ANOVA confirmed a significant effect of both age group (F= 3.19; DF= 6; p =0.021) and site of care (F=4.14; DF=3; p =0.026) on timeliness. Posthoc mean contrast revealed that children three to four had significantly greater mean lead time than older age groups (p =0.00142). Overall, pediatric patients (age ≤18) were timelier than adults (p <0.001) and the youngest children, under four years, arrived before all other groups (p=0.0058).
Mortality prediction by patient population
Using the lead times defined by the cross-spectral analysis, each healthcare population was found to be a statistically significant predictor of mortality (p < 0.0001). A comparison of the predictive abilities of these populations shows the AC, general ED and pediatric ED (30–31%) to explain more of the variation than the adult and community ED’s (24–25%) and the sentinel ILI data (25%).
Mortality prediction by age
Prediction of influenza mortality varied by age (Table 3). Among age groups presenting to the different healthcare settings, the pattern of illness among children is most predictive of P&I mortality across sites of care. Children under three years old provided the best prediction of mortality explaining on average 40.8 percent (SE, 4.4) of the deviance. This was followed by children three to four years old, who explain 36.8 percent (SE, 6.1) of the deviance. Figure 2 plots mortality prediction versus timeliness for age group of each healthcare population and reveals that, in general, pediatric age groups have the best combination of the two indicators.
Table 3.
Predictive ability of patient populations with respiratory illness for signaling P&I mortality by site of care and patient age in Eastern Massachusetts, USA, 2000–2003.
| Mortality Prediction (percent deviance explained)† | |||||||
|---|---|---|---|---|---|---|---|
| Data Source | <3 | 3–4 | 5–10 | 11–17 | 18–39 | 40–64 | >64 |
| Ambulatory | 28.3 | 31.1 | 28.5 | 27.8 | 26.1 | 32.0 | 27.6 |
| Pediatric ED | 34.4 | 23.1 | 17.7 | 14.7 | - | - | - |
| Adult ED | - | - | - | - | 19.8 | 17.3 | 17.2 |
| Community ED | 42.2 | 41.9 | 40.0 | 41.6 | 40.3 | 41.5 | 39.9 |
| General ED | 58.3 | 50.9 | 44.2 | 45.4 | 49.5 | 41.2 | 44.6 |
| Mean (95% CI) | 40.8 (20.1, 61.5) | 36.8 (17.4,56.1) | 32.6 (13.6, 51.6) | 32.4 (10.1, 54.7) | 33.9 (12.5, 55.4) | 33.0 (15.9,51.1) | 32.3 (12.6, 52.0) |
ED, Emergency Department
CI, Confidence Interval
Percent deviance explained calculated by Poisson regression analyses
Figure 2. Prediction and timeliness of patients with respiratory illness syndromes for signaling pneumonia and influenza mortality by age group and site of care, Eastern Massachusetts, USA, 2000–2003.
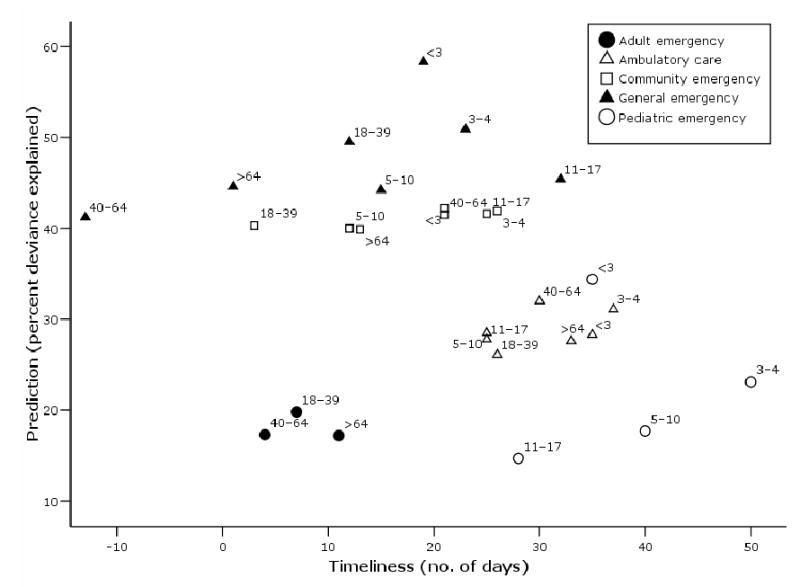
Timeliness is the lead time to influenza mortality, obtained by cross-spectral analysis. Mortality prediction is proportion of variance explained by each healthcare population calculated by Poisson regression. The plot reveals the pediatric age groups to have the best combination of timeliness and prediction.
Randomized complete block design ANOVA revealed a significant effect of both age group (F=2.92; DF=6; p =0.036) and site of care (F=74.79; DF=3; p <0.0001) on predictive ability. Children under three had significantly greater predictive ability than all other age groups (p =0.0019). When grouped together, pediatric patients, age 0–18, did not explain significantly more of the deviance than adults (p =0.0906). However, the youngest children, under five years, clearly provided the best prediction of all age groups (p =0.0012). When plotted against timeliness, these two youngest age groups have the maximum prediction while also providing the earliest signal (Figure 3).
Figure 3. Prediction and timeliness of patients with respiratory illness syndromes for signaling pneumonia and influenza mortality summarized by age group, Eastern Massachusetts, USA, 2000–2003.
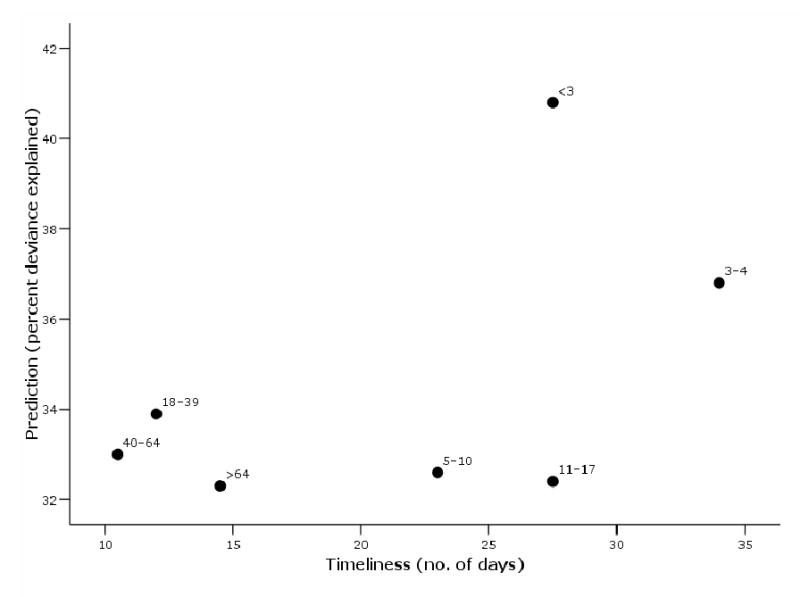
Timeliness is the average lead time to influenza mortality for each age group across all sites of care obtained by cross-spectral analysis. Mortality prediction is the average proportion of variance explained by each age group across healthcare sites calculated by Poisson regression. The plot reveals the two youngest age groups to have the best combination of timeliness and prediction.
DISCUSSION
Patient age is a key determinant in the timing of visits for respiratory illness; pediatric patients, and specifically preschool aged children, three to four years old, seek ambulatory and emergency care earliest. Further, respiratory illness among children less than five years of age is significantly associated with mortality from P&I with a four to five week lead time. Pediatric populations are sentinels of infection and they signal the consequent burden of illness. Though this does not necessarily prove that preschool age children are driving the yearly influenza epidemics, these findings intriguingly suggest that preschool age children are the initial group infected and may be important in the subsequent spread.
There is ample prior evidence that children play a primary role in influenza transmission. Given their increased tendency to acquire and shed influenza, children have been identified as predominant vectors in the household spread of influenza (30–32). Our findings support the notion that specifically targeting the preschool children may reduce transmission. Children under five years of age have higher infection rates than older children (33–35). In addition, vaccination of this age group has been shown to significantly reduce morbidity among their household contacts (36). For this reason, concentrating immunization efforts on preschool children may eliminate the primary pathway of infection.
Other studies have shown that older children (5–18 years old) are the most important targets and that their routine vaccination would reduce disease burden across the community level (11–13, 37–40). Our results suggest that younger children may initiate spread to these older children and therefore may be of value as targets of vaccination out of proportion to their lesser numbers.
While our study suggests that young children are infected first, there are other possible explanations for their early presentation to the health care system. It may be not just the inherent vulnerability of children, but also health care seeking behaviors that make them timely sentinels of influenza (41). Family members may have a lower threshold for bringing in febrile young children because of morbidity concerns specific to the pediatric population and will thus been seen by physicians at the earlier stages of viral illnesses (42, 43). However, we find that the pediatric ED populations arrive prior to the pediatric ambulatory populations. Because the ED populations are naturally more acutely ill (24), the reason for the early presentation of children is likely at least partly rooted in genuine morbidity, and not just parental behavior. In addition, if the early arrival children were could be explained primarily by the behavior of worried parents and pediatricians, we would instead expect to see the youngest, most fragile children, infants, arriving before the preschoolers; in preschoolers, simple febrile illnesses simply do not pose the same risks or require as much testing (44).
A limitation of our study is that we are measuring respiratory illness, but not virologically confirmed influenza infection. Our findings are confounded by co-circulation with other viruses, for which there are no vaccinations currently available, including respiratory syncytial virus and parainfluenza virus. Another limitation is that our data are from the Greater Boston Area and may not be entirely generalizable to other regions. However, the patients are seen at seven diverse institutions and are likely to be highly representative of the region; also, a priori, it is not clear why there would be regional differences.
This study has other implications as well. Since the data are available in a real time population health monitoring system, understanding the temporal dynamics of respiratory illness through different age groups can be used to inform medical practice and enable improved prevention and control efforts by individual clinicians. Monitoring respiratory illness in the ambulatory care and pediatric ED populations using syndromic surveillance systems was shown to provide even earlier detection and better prediction of influenza activity then the current CDC sentinel surveillance system. Supplying physicians with a mechanism to identify the earliest and most sensitive warning of respiratory mortality can help them implement prevention strategies that will protect their general patient population.
We demonstrate clearly, across a region, that preschool age children are the first to seek healthcare for respiratory infections and further that there is a strong association between their temporal patterns of illness and subsequent mortality in the general population from influenza. While our findings do not definitively indict preschool age children as those initially infected and primarily responsible for spread to other age groups, this age group does appear to have an important role in influenza transmission. These results bolster arguments for a recommendation currently under consideration by the ACIP to begin to universally vaccinate preschool aged children.
Acknowledgments
This work was supported by grant R01 LM007677-01 from the National Library of Medicine (National Institutes of Health), contract 290-00-0020 from the Agency for Healthcare Quality and Research, and by contract 52253337HAR from the Massachusetts Department of Public Health. We gratefully acknowledge the thoughtful input of Drs. Tracy Lieu, Ben Reis, Cecily Wolfe and Karen Olson. We thank Andrew Ellingson and Drs. John Halamka and Tom Stair for their participation in the AEGIS surveillance system.
References
- 1.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2003;52:1–34. [PubMed] [Google Scholar]
- 4.Glezen WP, Couch RB. Estimating deaths due to influenza and respiratory syncytial virus. Jama. 2003;289:2500. doi: 10.1001/jama.289.19.2500-a. author reply 2500–2. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181:831–7. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 7.Schoenbaum SC. Economic impact of influenza. The individual’s perspective. Am J Med. 1987;82:26–30. doi: 10.1016/0002-9343(87)90557-2. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis. 1999;5:659–71. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reduction of the influenza burden in children. Pediatrics. 2002;110:1246–52. doi: 10.1542/peds.110.6.1246. [DOI] [PubMed] [Google Scholar]
- 10.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53:1–40. [PubMed] [Google Scholar]
- 11.Monto AS, Davenport FM, Napier JA, Francis T., Jr Effect of vaccination of a school-age population upon the course of an A2-Hong Kong influenza epidemic. Bull World Health Organ. 1969;41:537–42. [PMC free article] [PubMed] [Google Scholar]
- 12.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344:889–96. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 14.Longini IM, Jr, Halloran ME. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol. 2005;161:303–6. doi: 10.1093/aje/kwi053. [DOI] [PubMed] [Google Scholar]
- 15.Reis BY, Pagano M, Mandl KD. Using temporal context to improve biosurveillance. Proc Natl Acad Sci U S A. 2003;100:1961–1965. doi: 10.1073/pnas.0335026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner MM, Robinson JM, Tsui FC, Espino JU, Hogan WR. Design of a national retail data monitor for public health surveillance. J Am Med Inform Assoc. 2003;10:409–18. doi: 10.1197/jamia.M1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinman K, Lazarus R, Platt R. A generalized linear mixed models approach for detecting incident clusters of disease in small areas, with an application to biological terrorism. Am J Epidemiol. 2004;159:217–24. doi: 10.1093/aje/kwh029. [DOI] [PubMed] [Google Scholar]
- 18.Lombardo J, Burkom H, Elbert E, et al. A Systems Overview of the Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE II) J Urban Health. 2003;80:I32–I42. doi: 10.1007/PL00022313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller B, Kassenborg H, Dusmuir W, et al. Syndromic surveillance for influenzalike illness in an ambulatory care network. Emerging Infectious Diseases. 2004;10:1806–1810. doi: 10.3201/eid1010.030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beitel AJ, Olson KL, Reis BY, Mandl KD. Use of Emergency Department Chief Complaint and Diagnostic Codes for Identifying Respiratory Illness in a Pediatric Population. Pediatr Emerg Care. 2004;20:355–360. doi: 10.1097/01.pec.0000133608.96957.b9. [DOI] [PubMed] [Google Scholar]
- 21.Tsui F-C, Espino JU, Dato VM, Gesteland PH, Hutman J, Wagner MM. Technical description of RODS: a real-time public health surveillance system. J Am Med Inform Assoc. 2003;10:399–408. doi: 10.1197/jamia.M1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarus R, Kleinman K, Dashevsky I, DeMaria A, Platt RM. Using automated medical records for rapid identification of illness syndromes (syndromic surveillance): the example of lower respiratory infection. BMC Public Health. 2001;1:9. doi: 10.1186/1471-2458-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandl KD, Overhage JM, Wagner MM, et al. Implementing syndromic surveillance: a practical guide informed by the early experience. J Am Med Inform Assoc. 2004;11:141–150. doi: 10.1197/jamia.M1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus R, Kleinman K, Dashevsky I, et al. Use of automated ambulatory-care encounter records for detection of acute illness clusters, including potential bioterrorism events. Emerging Infectious Diseases. 2002;8:753–60. doi: 10.3201/eid0808.020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brammer TL, Murray EL, Fukuda K, Hall HE, Klimov A, Cox NJ. Surveillance for influenza--United States, 1997–98, 1998–99, and 1999–00 seasons. MMWR Surveill Summ. 2002;51:1–10. [PubMed] [Google Scholar]
- 26.Priestly MB. Spectral Analysis and Time Series. New York: Academic Press, Inc., 1981.
- 27.Crowder MJ, Hand DJ. Analysis of Repeated Measures. London: Chapman and Hall, 1990.
- 28.Nelder JA, Wedderburn RW. Generalized linear models. Journal of the Royal Statistical Society, Series A. 1972;175:370–384. [Google Scholar]
- 29.Williams DA. Generalized linear model diagnostics using the deviance and single case deletions. Applied Statistics. 1987;36:181–191. [Google Scholar]
- 30.Longini IM, Jr, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115:736–51. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 31.Fox JP, Hall CE, Cooney MK, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–27. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 32.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121:811–22. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 33.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 34.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–9. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 35.Viboud C, Boelle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. Jama. 2000;284:1677–82. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 37.Elveback LR, Fox JP, Ackerman E, Langworthy A, Boyd M, Gatewood L. An influenza simulation model for immunization studies. Am J Epidemiol. 1976;103:152–65. doi: 10.1093/oxfordjournals.aje.a112213. [DOI] [PubMed] [Google Scholar]
- 38.Halloran ME, Longini IM, Jr, Gaglani MJ, et al. Estimating efficacy of trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against influenza A (H1N1) and B using surveillance cultures. Am J Epidemiol. 2003;158:305–11. doi: 10.1093/aje/kwg163. [DOI] [PubMed] [Google Scholar]
- 39.White T, Lavoie S, Nettleman MD. Potential cost savings attributable to influenza vaccination of school-aged children. Pediatrics. 1999;103:e73. doi: 10.1542/peds.103.6.e73. [DOI] [PubMed] [Google Scholar]
- 40.Longini IM, Halloran ME, Nizam A, et al. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: implications for influenza epidemic control. Vaccine. 2000;18:1902–9. doi: 10.1016/s0264-410x(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 41.Long CE, Hall CB, Cunningham CK, et al. Influenza surveillance in community-dwelling elderly compared with children. Arch Fam Med. 1997;6:459–65. doi: 10.1001/archfami.6.5.459. [DOI] [PubMed] [Google Scholar]
- 42.Glezen WP. Influenza vaccination for healthy children. Curr Opin Infect Dis. 2002;15:283–7. doi: 10.1097/00001432-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113:585–93. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 44.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602–14. doi: 10.1067/mem.2000.110820. [DOI] [PubMed] [Google Scholar]


