Abstract
Rationale
It has been suggested that the 5-HT1A receptor plays a significant modulatory role in the stimulus effects of the indoleamine hallucinogen lysergic acid diethylamide (LSD).
Objectives
The present study sought to characterize the effects of several compounds with known affinity for the 5-HT1A receptor on the discriminative stimulus effects of LSD.
Methods
12 Male F-344 rats were trained in a two-lever, fixed ratio10, food reinforced task with LSD (0.1 mg/kg; IP; 15 min pretreatment) as a discriminative stimulus. Combination and substitution tests with the 5-HT1A agonists, 8-OH-DPAT, buspirone, gepirone, and ipsapirone, with LSD-induced stimulus control were then performed. The effects of these 5-HT1A ligands were also tested in the presence of the selective 5-HT1A receptor antagonist, WAY-100,635 (0.3 mg/kg; SC; 30 min. pretreatment).
Results
In combination tests stimulus control by LSD was increased by all 5-HT1A receptor ligands with agonist properties. Similarly, in tests of antagonism, the increase in drug-appropriate responding caused by stimulation of the 5-HT1A receptor was abolished by administration of WAY-100,635.
Conclusions
These data, obtained using a drug discrimination model of the hallucinogenic effects of LSD, provide support for the hypothesis that the 5-HT1A receptor has a significant modulatory role in the stimulus effects of LSD.
Keywords: Lysergic acid diethylamide [LSD]; Drug discrimination; rat; 8-OH-DPAT; buspirone; gepirone; ipsapirone; WAY-100, 635
Introduction
Currently there are fourteen recognized 5-HT receptor subtypes which fall into 7 families, 5-HT1–7 (Raymond 2001). Although serotonergic systems are clearly relevant to the effects of LSD, questions still remain as to the contributions of specific serotonergic receptor subtypes (Winter et al. 1999). The blockade of the stimulus effects of LSD by administration of 5-HT2 receptor antagonists as well as a correlation between affinity of the 5-HT2 receptor and hallucinogenic potency in man led Glennon and colleagues (1984) to hypothesize that hallucinogens act as 5-HT2 agonists. In support of this idea Schreiber and colleagues (1994) found that the 5-HT2A receptor antagonist, MDL 100,907, but not the 5-HT2C receptor antagonist, SB 200,646, blocked the stimulus effects of the phenylalkylamine hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI). However, affinity at the 5-HT2A receptor could only account for 56% of the variability in the potency of an antagonist to block the stimulus effects of LSD in vivo (Fiorella et al. 1995a). Furthermore, compounds such as quipazine have high affinity for the 5-HT2A receptor yet are not hallucinogenic (Fiorella et al. 1995b; Egan et al. 1998). Thus, while 5-HT2A receptor stimulation is a necessary component of LSD-induced stimulus control, it is not the only mechanism by which the drug exerts its effects.
It has been suggested that functional interactions exist among the different populations of 5-HT receptors and that stimulation of one receptor subtype may influence the activity of another. 5-HT2A mediated behaviors have been shown to be influenced by the 5-HT1A receptor in a variety of experimental paradigms. For example, the head-twitch response, a behavior typically associated with 5-HT2A receptor stimulation (Green et al. 1983; Schreiber et al. 1995b), has been shown to be variably affected by 5-HT1A agonism. Prior research has found that quipazine induced head twitches are increased by the administration of the 5-HT1A agonist, gepirone (Eison et al. 1986; Yocca et al. 1991). However the effects of 5-HT1A agonism on this behavioral outcome are unclear as other investigations have shown that 8-hydroxy-2-(di-N-propylamino)tetralin (8-OH-DPAT) is able to significantly decrease DOI mediated head twitches (Darmani et al. 1989). 8-OH-DPAT is the prototypical 5-HT1A receptor agonist and has an affinity for the 5-HT1A receptor which is several hundred-fold greater than for the 5-HT2 receptor (Hamon 1984, 1986; Winter and Rabin 1987; Gozlan et al. 1988). Further complicating matters is a report showing that 8-OH-DPAT is able to increase 5-MeO DMT-induced, but not 5-hydroxytryptophan-induced, head-twitch response in rats (Darmani et al. 1989). In addition isobolographic analysis suggests that 5-HT1A and 5-HT2A receptors act antagonistically with regards to their locomotor suppressing effects (Krebs-Thompson and Geyer 1998). While complex, the relationship between 5-HT2 and 5-HT1A receptors seems to be of a reciprocal nature. This is evident from studies showing that 8-OH-DPAT-induced forepaw treading is increased 20 fold by administration of DOI (Arnt and Hyttel 1989).
The 5-HT1A receptor has been implicated in a variety of CNS responses and may play a role in depression and the formation of memory (Winter and Petti 1987; Sarnyai et al. 2000; Gingrich and Hen 2001; Hoyer at al. 2002). It has also been suggested that 5-HT1A receptors mediate the behavioral effects of the anxiolytics buspirone and ipsapirone (Cunningham et al. 1987a). The present study sought to characterize the effects of the 5-HT1A ligands 8-OH-DPAT, buspirone, gepirone, (Eison et al. 1986) and ipsapirone (Traber and Glaser 1987) on the discriminative stimulus effects of LSD.
Materials and Methods
Subjects
12 Male Fischer 344 rats were obtained at an age of approximately 6 weeks from Harlan Sprague-Dawley Inc. (Indianapolis, Ind., USA), housed in pairs under a 12-h light-dark cycle beginning at 6:00 a.m., and allowed free access to water in their home cages. All training and testing took place during the light cycle. Subjects were fed standard rat chow following experimental sessions. Caloric intake was controlled to maintain a mean body weight of 250 g. Caloric control has been shown to lengthen the life span and decrease the incidence of a variety of pathologies in Fischer 344 rats (Keenan et al.1994). Animals used in these studies were maintained in accordance with US Public Health Service Policy on Humane Care and Use of Laboratory Animals as amended August 2002. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Apparatus
Six small animal test chambers (Med Associates ENV-008) were used for all experiments. These were housed in larger light-proof, sound-insulated boxes which contained a house light and an exhaust fan. Chambers contained two levers mounted at opposite ends of one wall. Centered between the levers was a dipper that delivered 0.1 ml of sweetened condensed milk diluted 2:1 with tap water. Sessions were managed by a micro-computer using operant control software (MED-PC State Notation, Version IV).
Training procedures
After learning to drink from the dipper, rats were trained to press first one and then the other of the two levers. The number of responses for each reinforcement was gradually increased from 1 to 10. During this time, the reinforced lever was alternated on a random basis. All subsequent training and testing sessions used a fixed-ratio 10 (FR10) schedule of reinforcement and a 10 minute training session. Discrimination training was then begun. Subjects were trained to discriminate LSD [0.1 mg/kg, 15 min pretreatment time, intraperitoneal (IP) injection] (Hirschorn and Winter 1971; Fiorella et al. 1995b) from saline. Following the administration of drug, every tenth response on the drug-appropriate lever was reinforced. Similarly, responses on the saline-appropriate lever were reinforced on a FR10 schedule following the injection of saline. For half of the subjects, the left lever was designated as the drug-appropriate lever. During discrimination training, drug and saline were alternated on a daily basis. Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, 83% or more of all responses prior to the delivery of the first reinforcer were on the appropriate lever, i.e. no more than two incorrect responses prior to completion of the FR10 on the correct lever. After stimulus control was established tests were conducted once per week in each animal so long as performance did not fall below the criterion level of 83% correct responding in any one of the three previous training sessions.
Combination and substitution tests
After stimulus control with LSD was well established, combination and substitution tests were conducted once per week in each animal if the criterion for drug-induced stimulus control were met. Tests were balanced between subjects trained on the previous day with saline and drug, respectively. During test sessions, no responses were reinforced and the session was terminated after the emission of ten responses on either lever. The distribution of responses between the two levers was expressed as the percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated by dividing the total number of responses emitted prior to lever selection, that is, prior to the emission of ten responses on either lever, divided by elapsed time. Data for any subjects failing to emit ten responses within the constraints of the 10-min test session were not considered in the calculation of the percent drug-appropriate responding but were included in the analysis of response rates.
The effects of 5-HT1A agonists on LSD-induced stimulus control were assessed by co-administration of a 5-HT1A agonist [15 min. pretreatment, subcutaneous injection (SC)] and LSD (15 min before testing) as previously described (Winter et al. 2000). The interactions of 5-HT1A ligands and WAY-100,635 with stimulus control by LSD were assessed in experiments in which WAY-100,635 was administered 30 min, SC, before testing and the combination of LSD and a 5-HT1A agonist were administered 15 min before testing. For purposes of discussion an intermediate degree of antagonism is defined as less than 80% drug-appropriate responding and significantly different from both training conditions.
Drugs
Lysergic acid diethylamide [(+)-LSD (+)-tartrate (2:1)] was generously provided by the National Institute on Drug Abuse, Rockville, Md., USA. 8-hydroxy-2-(di-N-propylamino)tetralin, WAY-100,635, and buspirone were purchased from Tocris, USA. Gepirone and ipsapirone were gifts from Bristol-Meyers Squibb Company, Wallingford, CT and Miles Pharmaceuticals, West Haven, CT, respectively. Doses are expressed as mg/kg and refer to weights of the salts. LSD and the 5-HT1A ligands were dissolved in bacteriostatic water.
Statistical analysis
The statistical significance of combination tests with a 5-HT1A agonist and LSD were determined using two-way ANOVA with dose of LSD and treatment with the 5-HT1A agonist as factors. Two-way ANOVA was also used to determine the statistical significance of the antagonism of the effects of the 5-HT1A agonists on LSD induced stimulus control by WAY-100,635. In combination tests involving WAY-100,635, dose of LSD and treatment with the combination of 5-HT1A ligands were used as factors. For assessment of the statistical significance of antagonism of the stimulus effects of the training dose of LSD, by WAY-100,635 one-way ANOVA was used to compare the two training conditions (saline and 0.1 mg/kg LSD) with the combination of LSD and WAY-100,635. In all measures of analysis of variance subsequent multiple comparisons were made by the method of Student-Newman-Keuls. For analysis of individual points in substitution tests Student’s t-test was used. Differences were considered to be statistically significant if the probability of their having arisen by chance was <0.05. All analyses were conducted using SigmaStat 2.03 for Windows (Jandel Scientific Software, San Rafael, Calif., USA). Data for LSD and saline training sessions were repeated for each comparison and statistical analyses were applied using the appropriate training sessions. However, for purposes of clarity, mean values for training sessions are shown in all figures.
Results
Initial experiments determined the effects of 8-OH-DPAT when administered to LSD-trained animals. A maximum of 53.2% LSD-appropriate responding was achieved with the highest dose of 8-OH-DPAT tested (1.0 mg/kg) although significant impairment of test subjects resulted in only 9 of 12 animals completing the test session. Rate suppression precluded us from testing higher doses. One-way ANOVA revealed that the highest dose of 8-OH-DPAT yielded a level of drug-appropriate responding which was significantly different from both the training dose of LSD and saline (i.e., intermediate substitution) [F(2,30)=52.331; p=0.001]. The effects of the highest dose of 8-OH-DPAT on rate of responding and LSD substitution were blocked by the selective 5-HT1A receptor antagonist WAY-100,635 [Student’s t-test, p=.044 and p=.002, respectively] (Forster et al. 1995; Fletcher et al. 1996). However, when combined with the training dose of LSD, one-way ANOVA revealed that WAY-100,635 had no effect on drug-appropriate responding although there was a significant rate suppressant effect [F(2,34)=9.978; p<0.001]. A dose of 0.05 mg/kg 8-OH-DPAT was chosen for subsequent experiments as this dose yielded a degree of LSD-appropriate responding which did not differ significantly from that following the injection of saline. The rate of responding was not significantly decreased at this dose, and all subjects were able to complete the test session.
Figure 2 shows a dose-related increase in LSD-appropriate responding in rats trained and tested with LSD. When the same doses were tested in rats pretreated with a fixed dose of 8-OH-DPAT (0.05 mg/kg), LSD-appropriate responding increased for all doses of LSD less then the training dose (0.01 mg/kg and 0.03 mg/kg). Two-way ANOVA showed a significant increase in LSD-appropriate responding following the combination of LSD and 8-OH-DPAT compared with LSD alone [F(1,47)=9.057; p=0.004]. Neither the effect of dose nor the interaction term were significant. A significant decrease in the rate of responding was also seen with the combination of LSD and 8-OH-DPAT [F(1,47)=13.67; p<0.001] although the effects of dose and the interaction term did not reach significance. Although displaying a much higher affinity for the 5-HT1A receptor versus other subtypes, 8-OH-DPAT has been shown to be a partial agonist at the 5-HT7 receptor and have affinity for the α2-adrenoceptor (Winter and Rabin 1992; Ruat et al. 1993; Wood et al. 2000). To rule out the possibility of effects caused by administration of 8-OH-DPAT other then 5-HT1A receptor stimulation, WAY-100,635 (0.3 mg/kg) was used. Upon administration of the combination of 8-OH-DPAT, LSD, and WAY-100,635 drug-appropriate responding returned to levels which were not significantly different from LSD administered alone as measured by two-way ANOVA. However, a suppression of rate of responding was still seen with the combination of 8-OH-DPAT, LSD, and WAY-100,635 [F(1,47)=16.25; p<0.001]. In a manner similar to drug-appropriate responding, neither the effect of dose, nor the interaction term were significant.
Fig. 2.
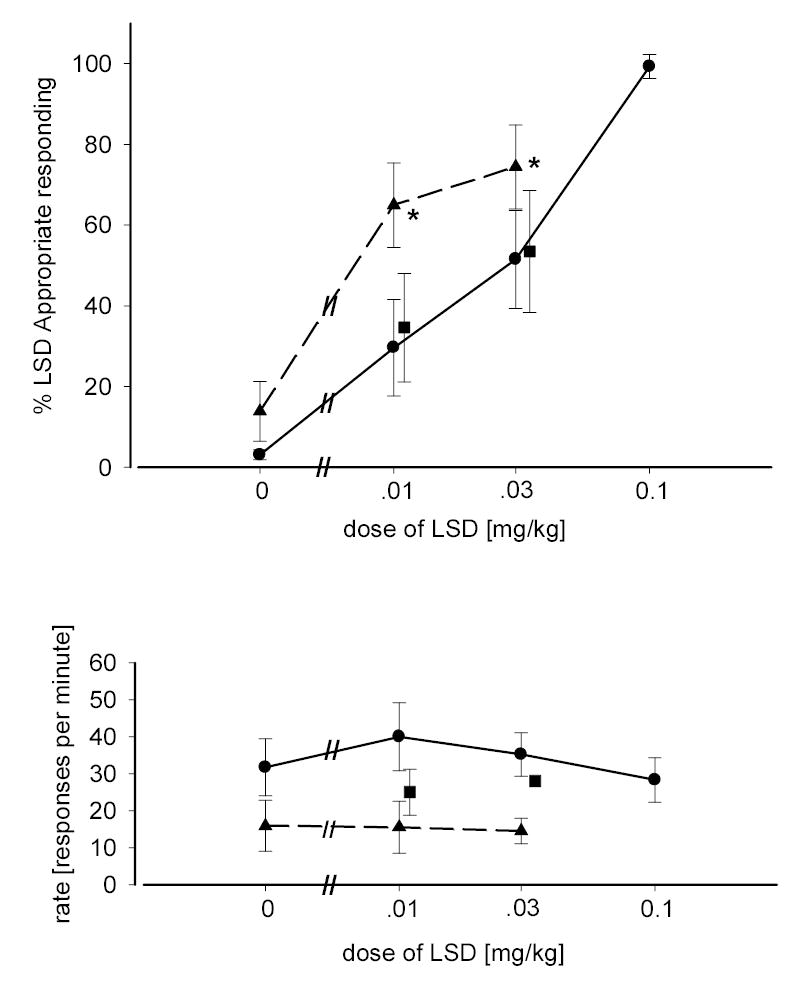
Dose-response relationship for LSD alone and in combination with 8-OH-DPAT. Circles represent the effects of LSD alone in rats trained with LSD as a discriminative stimulus (0.1 mg/kg). Triangles represent the effects of LSD in combination with 8-OH-DPAT (0.05 mg/kg). Squares represent the effects of LSD following treatment with 8-OH-DPAT and WAY-100,635 (0.3 mg/kg). LSD was administered IP 15 min. before testing. 8-OH-DPAT and WAY-100,635 were administered SC 15 min and 30 min respectively, before testing. Each point represents the mean of one determination in 12 rats. Standard errors of the means are indicated. * Significantly different from LSD given alone. Ordinate: upper panel: percent LSD-appropriate responding; lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
A similar potentiation of LSD-appropriate responding caused by administration of the clinically effective anxiolytic buspirone (Riblet et al. 1982; Goa and Ward 1986) is seen in figure 3. Buspirone has been shown to possess agonist activity and be relatively specific for receptors of the 5-HT1A receptor subtype (Riblet et al. 1982; Dourish et al. 1986). A dose of buspirone of 0.3 mg/kg was chosen for combination tests as this dose resulted in a level of LSD-appropriate responding which was not significantly different from that achieved with the injection of saline. When a dose-response curve was performed with doses of LSD less then the training dose (0.01 mg/kg and 0.03 mg/kg), the addition of buspirone (0.3 mg/kg) resulted in an increase in drug-appropriate responding. Two-way ANOVA revealed that the increase in LSD-appropriate responding was significant in comparison to LSD given alone [F(1,43)=23.46; p<0.001] while the effects of dose of LSD were significant [F(1,43)=5.865; p=.020] the interaction term was not. A significant decrease in rate of responding was also seen with the combination of LSD and buspirone [F(1,47)=16.28; p<.001] although neither the effect of dose, nor the interaction term were significant. However, when WAY-100,635 (0.3 mg/kg) was added to the combination of LSD and buspirone two-way ANOVA determined that drug-appropriate responding and rate of responding returned to levels which were not significantly different from LSD administered alone.
Fig. 3.
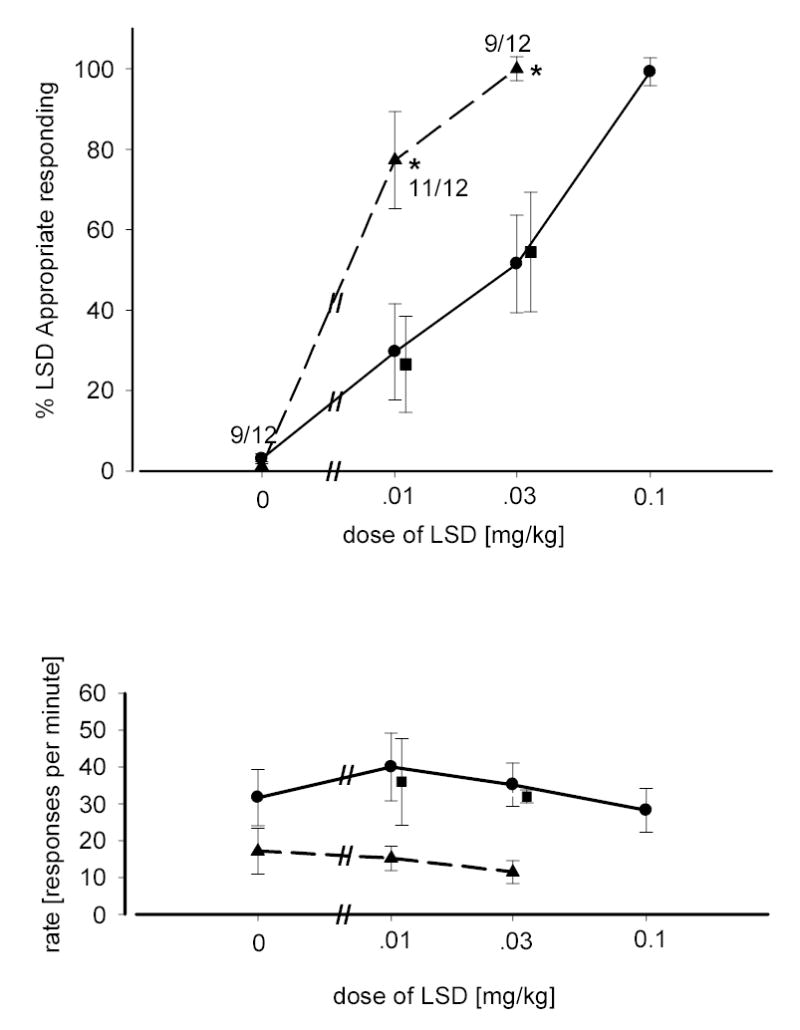
Dose-response relationship for LSD alone and in combination with buspirone. Circles represent the effects of LSD alone in rats trained with LSD as a discriminative stimulus (0.1 mg/kg). Triangles represent the effects of LSD in combination with buspirone (0.3 mg/kg). Squares represent the effects of LSD in combination with buspirone and WAY- 100,635. Number of subjects completing each session are indicated. Other details are as described in figure 2.
Figure 4 shows an orderly dose-related increase in LSD-appropriate responding in rats trained and tested with LSD. Substitution tests revealed that a 0.3 mg/kg dose of gepirone resulted in a level of LSD-appropriate responding which did not differ from that following the administration of saline. As was true for buspirone and 8-OH-DPAT, when doses of LSD less then the training dose of LSD were administered in the presence of a fixed dose of gepirone (0.3 mg/kg) an increase in drug-appropriate responding occurred. This increase was statistically significant as measured by two-way ANOVA [F(1,45)=4.227; p=0.046] the effects of dose were significant [F(1,45)=7.6; p=0.008] although the interaction term was not. A significant decrease in the rate of responding was also observed [F(1,47)=31.80; p<0.001] with a non-significant effect of dose, and interaction term. The effects of gepirone on LSD-appropriate responding were reversed by administration of WAY-100,635 (0.3 mg/kg), however gepirone’s effects on rate suppression remained unchanged and were significantly reduced in comparison to LSD given alone [F(1,47)=28.72; p<0.001].
Fig 4.
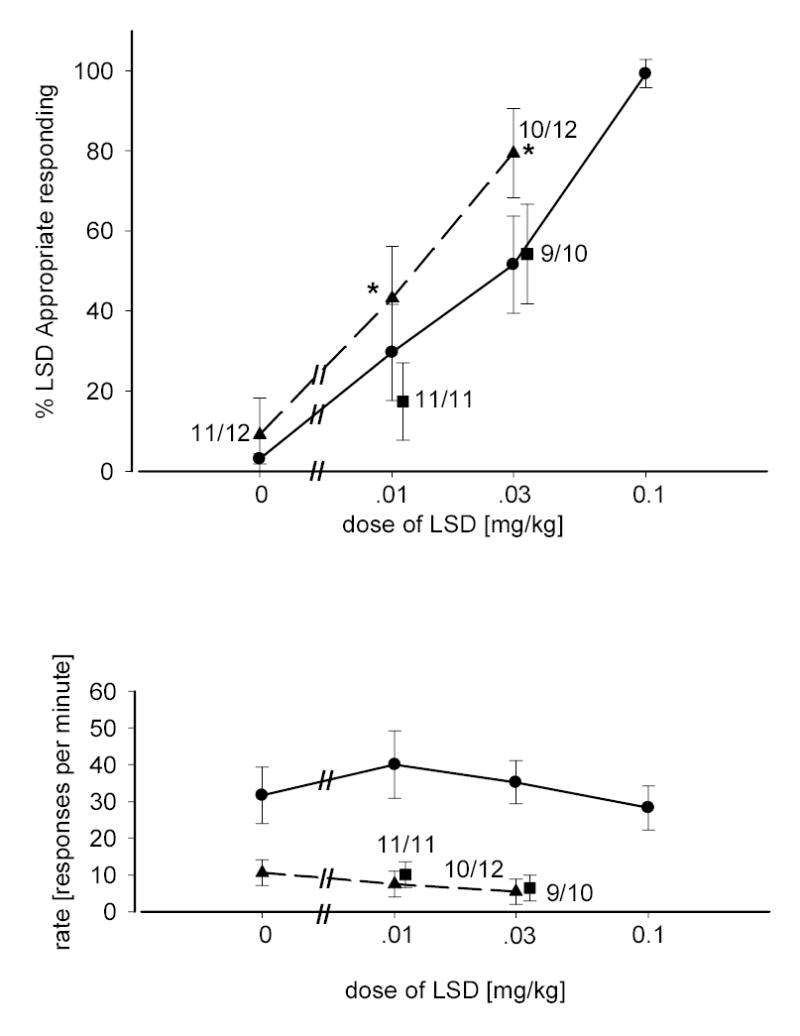
Dose-response relationship for LSD alone and in combination with gepirone. Circles represent the effects of LSD alone in rats trained with LSD as a discriminative stimulus (0.1 mg/kg). Triangles represent the effects of LSD given in combination with gepirone (0.3 mg/kg). Squares represent the effects of LSD in combination with gepirone and WAY-100,635. Other details are as described in figure 2.
Based upon its high affinity for the 5-HT1A receptor (Dompert et al. 1985), ipsapirone was also screened for potential interactions with the LSD stimulus cue. A dose of 0.3 mg/kg was chosen for combination tests as this dose resulted in a level of drug-appropriate responding which did not differ from that following the injection of saline. Results of the combination of ipsapirone (0.3 mg/kg), with a range of LSD doses are shown in figure 5. For LSD doses of 0.01 and 0.03 mg/kg, two-way ANOVA revealed a significant increase in LSD-appropriate responding following the combination of LSD and ipsapirone compared with LSD alone [F(1,38)=4.488; p=0.041] with a significant effect of dose [F(1,38)=4.25; p=0.047] and non-significant interaction term . An additional, significant, rate suppressing effect was also seen by administration of the combination of ipsapirone and LSD [F(1,43)=24.05; p<0.001] although neither dose, nor the interaction term were significant. Two-way ANOVA determined that the effects of ipsapirone on the LSD appropriate responding and rate suppression were eliminated by pretreatment with the 5-HT1A antagonist WAY-100,635 (0.3 mg/kg).
Fig 5.
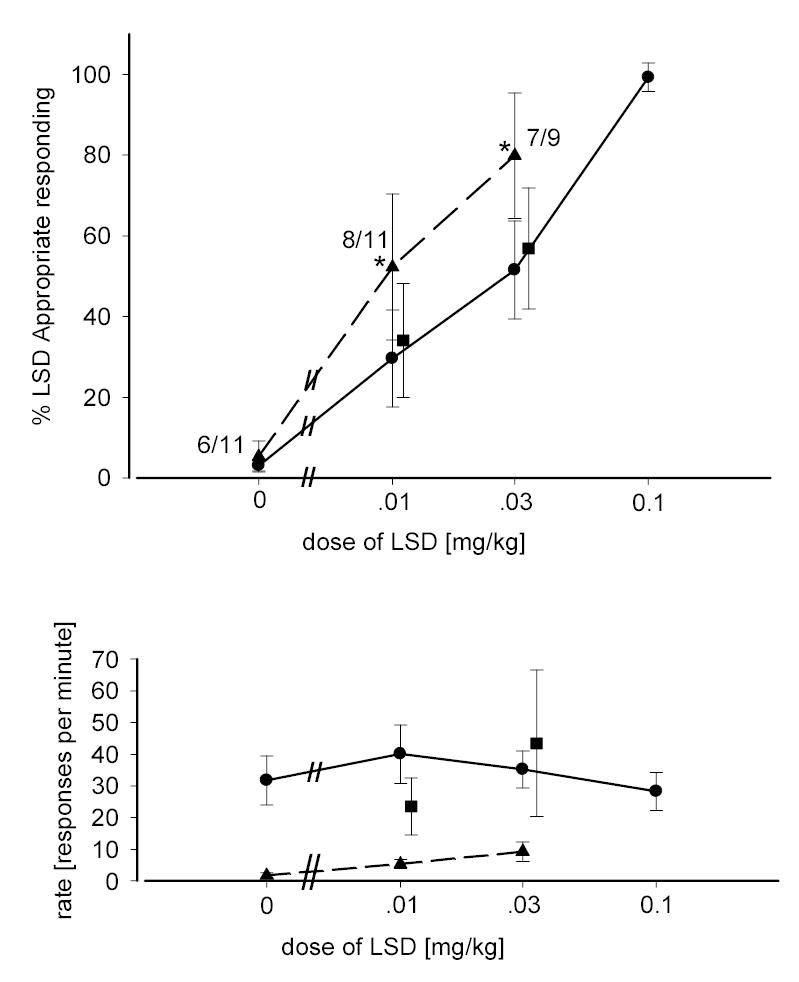
Dose-response relationship for LSD alone and in combination with ipsapirone. Circles represent the effects of LSD alone in rats trained with LSD as a discriminative stimulus (0.1mg/kg). Triangles represent the effects of LSD given in combination with ipsapirone (0.3 mg/kg). Squares represent the effects of LSD in combination with ipsapirone and WAY-100,635. Other details are as described in figure 2.
Discussion
The data of figure 1 are a confirmation of our previous findings showing the partial substitution of LSD to the 8-OH-DPAT stimulus cue (Winter and Rabin 1987). While Cunningham and Appel (1987b) failed to produce substitution between these two compounds, procedural differences may account for this discrepancy as the latter study utilized a different strain of rat, lower dose of the training agent (0.08 mg/kg LSD) and higher FR schedule (FR 20). The intermediate level of LSD substitution achieved with 8-OH-DPAT in the present investigation indicates that 8-OH-DPAT and LSD share a common stimulus component. However, 5-HT1A receptor stimulation seems to be a non-essential component of the LSD stimulus cue because the training dose of LSD was unaffected by WAY-100,635. Thus it would seem that LSD produces effects on 5-HT1A receptors which become apparent in drug discrimination studies when drugs which are active at 5-HT1A receptors are tested. Although the salient characteristics of LSD induced stimulus control are mediated via agonist actions at 5-HT2A receptors (Fiorella et al. 1995a), the data shown in Fig. 1 suggest that an additional, albeit smaller role is played by the 5-HT1A receptor.
Fig. 1.
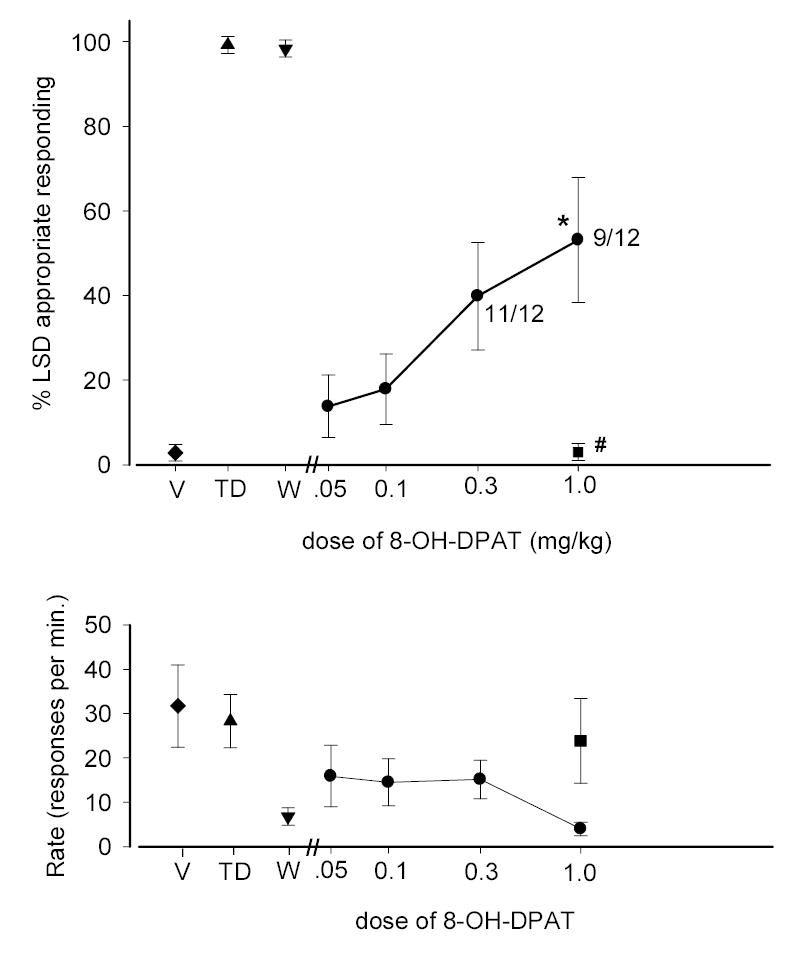
Effects of 8-OH-DPAT, the selective 5-HT1A antagonist WAY-100,635, and their combination in rats trained to discriminate LSD (0.1 mg/kg) from saline. The diamond represents the effects of water administered IP 15 min before testing. Circles represent the effects of 8-OH-DPAT administered IP 15 min. before testing. The Square represents the effects of 8-OH-DPAT in the presence of WAY-100,635 (0.3 mg/kg, SC, 30 min pretreatment). The Triangle represents the training dose of LSD. The Inverted Triangle represents the effects of the training dose of LSD given in the presence of WAY-100,635. Each point represents the mean of one determination in each of 12 rats. Standard errors of the mean are shown. * Significantly different from both training conditions. # Significantly different from 8-OH-DPAT (1.0 mg/kg). Ordinate: upper panel: percent LSD-appropriate responding; lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
The 5-HT1A receptor is found throughout the brain, with high concentrations in dorsal raphe nucleus (DRN), medial raphe nucleus (MRN), hippocampus, lateral septum, entorhinal cortex, and central amygadala. The raphe nuclei are the major source of serotonergic cell bodies in the brain and send projections to cortical and limbic areas (Aghajanian et al. 1968). In general, projections from DRN and MRN overlap one another with the former sending projections to the frontal cortex (Molliver 1987) an area containing a high density of 5-HT2A receptors (Pazos and Palacios 1985) and thought to play a significant role in hallucinogenesis and psychosis (Arvanov et al. 1999; Gewirtz and Marek 2000). Studies in our laboratory support a role of the medial raphe nucleus in hallucinogenesis as systemically administered (-) 2,5-dimethoxy-4-methylamphetmine (DOM) generalized completely to DOM infused into this area (Doat et al. 2003). Indeed, LSD is known to produce a complete inhibition of neuronal activation within the raphe nucleus (Aghajanian and Haigler 1975) which would likely affect the activity of downstream cortical neurons and contribute to the drug’s effects. Although suppression of neurons within the raphe nucleus does not seem to be a tenable hypothesis for the primary mechanism of hallucinogenesis, it may be important for the overall psychopharmacology of psychotropic compounds (Nichols 2004). A similar conclusion was reached by Penington and Fox (1994) who suggested that inhibition of 5-HT release resulting from 5-HT1A receptor activation may play a role in the hallucinogenic actions of LSD.
A previous investigation has found a potentiation of the phenethylamine hallucinogen DOM by pretreatment with 8-OH-DPAT (Glennon 1991). Prior research examining the head twitch response and its interaction with 5-HT1A agonists has found that quipazine induced head twitches were increased by the administration of gepirone (Darmani et al. 1989; Yocca et al. 1991). DOI induced ear scratch stereotypy (another behavior thought to be mediated via 5-HT2A receptor stimulation) was also increased by administration of 8-OH-DPAT (Darmani et al. 1990). These data suggest a potentiation of 5-HT2A function caused by 5-HT1A agonism and is fully in keeping with the results in fig. 2–5. The precise mechanism by which this potentiation occurs however remains obscure.
Several other investigations have been made into the complex mechanism of action of LSD. Considering its relatively non-selective binding profile it is not surprising that numerous pharmacological stimuli are able to affect the stimulus properties of LSD. Studies in our laboratory have demonstrated that the stimulus effects of LSD are modulated by 5-HT2C receptors, (Fiorella et al. 1995a) significantly reduced by the antipsychotic clozapine (Palumbo and Winter 1994), and potentiated by selective serotonin reuptake inhibitors (SSRI’s) (Fiorella et al. 1996). The last observation is interesting in light of data suggesting that the efficacy of antidepressant therapies and the azapirone anxiolytics may be due to region specific changes in 5-HT1A receptor function (Hensler 2002) and that the decrease in the subjective effects of LSD following chronic treatment with serotonergic antidepressants may involve changes in 5-HT1A receptor sensitivity (Bonson et al. 1996). These findings suggest that manipulation of serotonergic neurotransmission can affect the behavioral outcome of LSD administration. To our knowledge, this is the first report to show the potentiation of the stimulus properties of LSD with multiple compounds having agonist actions at the 5-HT1A receptor.
Schreiber and colleagues (1995) have correlated the ability of a drug to substitute for the 8-OH-DPAT stimulus cue with its affinity for the 5-HT1A receptor. 8-OH-DPAT has previously been shown to generalize to ipsapirone, buspirone, and gepirone (Winter 1988; Winter and Rabin 1989; Rabin and Winter 1993) all of which have appreciable selectivity for the 5-HT1A receptor with pKD values ranging from 8.90-7.49 (Eison et al. 1986; Traber and Glaser 1987; Rabin and Winter 1993). These data suggest that the primary stimulus component of these compounds is mediated via 5-HT1A stimulation and that all have similar stimulus properties. The fact that the observed potentiation of the LSD stimulus cue by 5-HT1A agonists was completely reversed by WAY-100,635 further supports this hypothesis.
In vitro studies have also demonstrated similarities among the 5-HT1A ligands tested. Electrophysiological experiments have shown that application of 8-OH-DPAT, buspirone, or gepirone all result in the suppression of firing of neurons within the DRN through activation of Gi/o proteins (Innis and Aghajanian 1987; Blier et al. 1993). Suppression of raphe firing and second messenger systems may be a potential mechanism by which the ligands studied are able to potentiate the stimulus effects of LSD. Microdialysis studies have shown that buspirone and ipsapirone mimic one another in their ability to enhance dopamine outflow within the prefrontal cortex of the rat (Wedzony et al. 1996). Although it appears buspirone produced the largest amount of potentiation of the LSD stimulus cue, the sum of these data indicate that the agonists used in this study have similar pharmacological properties. Subtle differences in the mechanisms of action of these compounds may account for the slight differences in the levels of potentiation observed with each compound.
In summary, it has been demonstrated that 5-HT1A receptor agonists are able to increase the stimulus effects of LSD. This supports the idea that the 5-HT1A receptor plays a modulatory role in the stimulus effects of LSD. The exact mechanism of this increase is unknown, although it likely involves modulation of serotonergic neurotransmission and 5-HT2A receptor function. Further study of this receptor subtype may offer a greater understanding of its functional role with respect to hallucinogens and the etiology of numerous psychiatric disorders. A suggestive role for the 5-HT1A receptor for increasing the efficacy of current antipsychotic medications has been proposed (Meltzer 1999). Indeed, it has been suggested that the atypical profile of the antipsychotic aripiprazole may be derived in part, from its 5-HT1A agonist effect (Marona-Lewicka and Nichols 2004). Further investigation is needed to determine the precise role of this receptor in the mechanism of action of LSD and other psychotomimetic substances.
Acknowledgments
This study was supported, in part by U.S. Public Health Service Grant DA 03385 and by National Research Service Awards DA 13920-01 (J.R.E.) and DA 16457-01 (C.J.R).
References
- Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide:sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–708. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Haigler HJ. Hallucinogenic indoleamines: preferential action upon presynaptic serotonin. Psychopharmacol Commun. 1975;1:619–629. [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OH-DPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Russo A, Wang RY. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci. 1999;11:3064–3072. doi: 10.1046/j.1460-9568.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- Blier P, Lista A, deMontigny C. Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors: II. Effect of pertussis and cholera toxins. J Pharmacol and Exp Ther. 1993;265:6–23. [PubMed] [Google Scholar]
- Bonson KR, Buckholtz JW, Murphy DL. Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology. 1996;14:425–436. doi: 10.1016/0893-133X(95)00145-4. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM, Appel JB. Discriminative stimulus properties of 8-hydroxy-2-(din-propylamino tetralin (8-OHDPAT): implications for understanding the actions of novel anxiolytics. Eur J Pharmacol. 1987a;138:29–36. doi: 10.1016/0014-2999(87)90333-5. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Appel JB. Neuropharmacological reassessment of the discriminative stimulus properties of d-lysergic acid diethylamide (LSD) Psychopharmacology. 1987b;91:67–73. doi: 10.1007/BF00690929. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors. Pharmacol Biochem Behav. 1989;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Pharmacological characterization of ear-scratch response in mice as a behavioral model for selective 5-HT2 receptor agonists and evidence for 5-HT1B and 5-HT2 receptor interactions. Pharmacol Biochem Behav. 1990;37:95–99. doi: 10.1016/0091-3057(90)90047-l. [DOI] [PubMed] [Google Scholar]
- Doat MM, Rabin RA, Winter JC. Characterization of the discriminative stimulus properties of centrally administered (−)-DOM and LSD. Pharmacol Biochem Behav. 2003;74(3):713–21. doi: 10.1016/s0091-3057(02)01074-2. [DOI] [PubMed] [Google Scholar]
- Dompert WU, Glaser T, Traber J. [3H]TVX Q 7821: Identification of 5-HT1 binding sites as a target for a novel putative anxiolytic. Naunyn Schmiedebergs Arch Pharmacol. 1985;328:467–470. doi: 10.1007/BF00692918. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Hutson PH, Curzon G. Putative anxiolytics 8-OH-DPAT, buspirone and TVXQ7821 are agonists at 5-HT1A autoreceptors in raphe nuclei. Trends Pharmacol Sci. 1986;7:212–214. [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5-HT2A and 5-HT2C receptors. Psychopharmacology. 1998;136(4):409–14. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Stanley M, Riblet LA. Serotonergic mechanisms in the behavioural effects of buspirone and gepirone. Pharmacol Biochem Behav. 1986;24:701–701. doi: 10.1016/0091-3057(86)90577-0. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995a;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: reassessment of LSD false positives. Psychopharmacology. 1995b;(3):357–63. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Rabin RA, Winter JC. Potentiation of LSD-induced stimulus control by fluoxetine in the rat. Life Sci. 1996;59(18):283–287. doi: 10.1016/0024-3205(96)00490-0. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe EA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critcheley DJP, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte A-M, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal, and behavioural studies with WAY-100,635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist WAY100635. Eur J Pharmacol. 1985;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacol. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Gingrich J, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology. 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Glennon RA (1991) Discriminative Stimulus Properties of Hallucinogens and Related Designer Drugs. Glennon, Jarbe, Frankenheim eds. In: Drug discrimination: Applications to Drug Abuse Research. USGPO, Washington, D.C. pp 25–31 [PubMed]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic events. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Ponchant M, Daval G, Menard F, Beaucourt JP, Hamon M. 125I-Bolton-Hunter-8methoxy-2-[N-propyl-N-propylamino]tetralin as a new selective radioligand on 5-HT1A sites in the rat brain. In-vitro binding and autoradiographic studies. J Pharmacol and Exp Ther. 1988;244:751. [PubMed] [Google Scholar]
- Goa KL, Ward A. Buspirone, A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114–129. doi: 10.2165/00003495-198632020-00002. [DOI] [PubMed] [Google Scholar]
- Green AR, O’Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2 antagonist pirenperone. Neuropharmacology. 1983;(5):573–8. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Hamon M, Bourgoin S, Gozlan H, Hall MD, Goetz C, Artaud F, Horn AS. Biochemical evidence for the 5-HT agonist properties of 8-hydroxy-8-OH-DPAT in the rat brain. Eur J Pharmacol. 1984;100:263–276. doi: 10.1016/0014-2999(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Hamon M, Cossery JM, Spampinato U, Gozlan H. Are there selective ligands for 5-HT1A and 5-HT1B receptor binding sites in the brain? Trends Pharmacol Sci. 1986;9:336–338. [Google Scholar]
- Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Hirschorn ID, Winter JC. Mescaline and lysergic diethylamide (LSD) as discriminative stimuli. Psychopharmacology. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon J, Martin G. Molecular pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin block 5-HT1A and GABA receptor mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Smith PF, Hertzog P, Soper K, Ballman GC, Clark RL. The effects of overfeeding and dietary restriction on Sprague-Dawley rat survival and early pathology biomarkers of aging. Toxicol Pathol. 1994;22:300–315. doi: 10.1177/019262339402200308. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in rats. Psychopharmacology. 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols D. Aripiprazole (OPC-14597) fully substitutes for the 5-HT1A receptor agonist, LY293284 in the drug discrimination assay in rats. Psychopharmacology. 2004;172:415–421. doi: 10.1007/s00213-003-1677-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7(6 Suppl):3S–23S. [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Palumbo PA, Winter JC. Interactions of Clozapine with the stimulus effects of DOM and LSD. Pharmacol Biochem Behav. 1994;49(1):115–120. doi: 10.1016/0091-3057(94)90464-2. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Fox AP. Effects of LSD on Ca++ currents in central 5-HT-containing neurons: 5-HT1A receptors may play a role in hallucinogenesis. J Pharmacol and Exp Ther. 1994;269(3):1160–1165. [PubMed] [Google Scholar]
- Rabin RA, Winter JC. Studies of the biochemical basis for the discriminative properties of 8-hydroxy-2-(di-n-propylamino)tetralin. Eur J Pharmacol. 1993;235:237–243. doi: 10.1016/0014-2999(93)90142-5. [DOI] [PubMed] [Google Scholar]
- Raymond J, Mukhin Y, Gelasco A, Turner J, Collinsworth G, Gettys T, Grewal J, Garnovskaya M. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Riblet LA, Taylor DP, Eison MS, Stanton HC. Pharmacology and neurochemistry of buspirone. J Clin Psychiatry. 1982;12:11–16. [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. 1993;90:8547. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Sibelle EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin1A receptors. Proc Natl Acad Sci USA. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan M. Blockade of the discriminative stimulus effects of DOI by MDL100,907 and the ‘atypical’ antipsychotics clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Lefebvre De Ladonchamps B, Monneyron S, Millan M. A drug discrimination analysis of the actions of novel serotonin1A receptor ligands in the rat using the 5-HT1A agonist, 8-hydroxy-2-(di-n-propylamino)tetralin. J Pharmacol and Exp Ther. 1995;275:822–831. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol and Exp Ther. 1995b;273(1):101–12. [PubMed] [Google Scholar]
- Traber J, Glaser T. 5-HT1A receptor-related anxiolytics. Trends Pharmacolo Sci. 1987;8:432–437. [Google Scholar]
- Wedzony K, Mackowiak M, Fijal K, Golembiowska K. Ipsapirone enhances the dopamine outflow via 5-HT1A receptors in the rat prefrontal cortex. Eur J Pharmacol. 1996;305(1–3):73–8. doi: 10.1016/0014-2999(96)00150-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between Serotonergic Agonists and Antagonists in Rats Trained with LSD as a discriminative stimulus. Pharmacol, Biochem Behav. 1987;30:617–624. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Petti DT. The effects of 8-OH-DPAT and other serotonergic agonists on performance in radial maze: A possible role for 5-HT1A receptors in memory. Pharmacol Biochem Behav. 1987;27:625–628. doi: 10.1016/0091-3057(87)90184-5. [DOI] [PubMed] [Google Scholar]
- Winter JC. Generalization of the discriminative stimulus properties of 8-OH-DPAT and ipsapirone to yohimbine. Pharmacol Biochem Behav. 1988;29:193–195. doi: 10.1016/0091-3057(88)90295-x. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine and serotonergic agonists: Stimulus properties and receptor binding. Drug Dev Res. 1989;16:327–333. [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a Serotonergic Agent: Evidence from Receptor Binding and Drug Discrimination. J Pharmacol and Exp Ther. 1992;263(2):682–689. [PubMed] [Google Scholar]
- Winter JC, Fiorella DJ, Timineri DM, Filipink RA, Helsley SE, Rabin RA. Serotonergic receptor subtypes and hallucinogen-induced stimulus control. Pharmacol Biochem Behav. 1999;64(2):283–93. doi: 10.1016/s0091-3057(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Winter JC, Fiorella DJ, Helsley S, Rabin RA. Partial generalization of (-)DOM to fluvoxamine in the rat: implications for SSRI-induced mania and psychosis. Int J Neuropsychopharmacol. 1999;2:165–172. doi: 10.1017/S1461145799001510. [DOI] [PubMed] [Google Scholar]
- Winter J, Doat M, Rabin R. Potentiation of DOM-induced stimulus control by non-competitive NMDA antagonists. A link between the glutamatergic and serotonergic hypotheses of schizophrenia. Life Sci. 2000;68:337–344. doi: 10.1016/s0024-3205(00)00934-6. [DOI] [PubMed] [Google Scholar]
- Wood M, Chaubey M, Atkinson P, Thomas DR. Antagonist activity of meta-chlorophenylpiperazine and partial agonist activity of 8-OH-DPAT at the 5-HT(7) receptor. Eur J Pharmacol. 2000;396(1):1–8. doi: 10.1016/s0014-2999(00)00213-2. [DOI] [PubMed] [Google Scholar]
- Yocca FD, Eison AS, Hyslop DK, Ryan E, Taylor DP, Giantusos G. Unique modulation of central 5-HT2 receptor binding sites and 5-HT2 receptor-mediated behavior by continuous gepirone treatment. Life Sci. 1991;49:1777–1785. doi: 10.1016/0024-3205(91)90478-t. [DOI] [PubMed] [Google Scholar]


