Abstract
Background
Upper respiratory tract infections are a major source of morbidity throughout the world. Extracts of the root of North American ginseng (Panax quinquefolium) have been found to have the potential to modulate both natural and acquired immune responses. We sought to examine the efficacy of an extract of North American ginseng root in preventing colds.
Methods
We conducted a randomized, double-blind, placebo-controlled study at the onset of the influenza season. A total of 323 subjects 18–65 years of age with a history of at least 2 colds in the previous year were recruited from the general population in Edmonton, Alberta. The participants were instructed to take 2 capsules per day of either the North American ginseng extract or a placebo for a period of 4 months. The primary outcome measure was the number of Jackson-verified colds. Secondary variables measured included symptom severity, total number of days of symptoms and duration of all colds. Cold symptoms were scored by subjects using a 4-point scale.
Results
Subjects who did not start treatment were excluded from the analysis (23 in the ginseng group and 21 in the placebo group), leaving 130 in the ginseng group and 149 in the placebo group. The mean number of colds per person was lower in the ginseng group than in the placebo group (0.68 [standard deviation (SD) 0.82] v. 0.93 [SD 0.91], difference 0.25%, 95% confidence interval [CI] 0.04–0.45). The proportion of subjects with 2 or more Jackson-verified colds during the 4-month period (10.0% v. 22.8%, 12.8% difference, 95% CI 4.3–21.3) was significantly lower in the ginseng group than in the placebo group, as were the total symptom score (77.5 [SD 84.6] v. 112.3 [SD 102.5], difference 1.5%, 95% CI 1.2–2.0) and the total number of days cold symptoms were reported (10.8 [SD 9.7] v. 16.5 [SD 13.8] days, difference 1.6%, 95% CI 1.3–2.0) for all colds.
Interpretation
Ingestion of a poly-furanosyl-pyranosyl-saccharide–rich extract of the roots of North American ginseng in a moderate dose over 4 months reduced the mean number of colds per person, the proportion of subjects who experienced 2 or more colds, the severity of symptoms and the number of days cold symptoms were reported.
Upper respiratory tract infections are a major source of morbidity throughout the world; in the United States alone at least 1 billion colds per year have been reported, with a frequency of 2–6 colds per person.1 Finding effective ways to reduce the frequency of these infections is therefore an important issue. Agents such as analgesics, antihistamines and decongestants have been found to be ineffective because of their limited efficacy against specific symptoms,2 whereas safety concerns for some antiviral drugs limit their use.3,4 Natural health products with properties that stimulate the immune system have been used to combat the common cold, but the results are often inconsistent.5
Extracts of North American ginseng (Panax quinquefolium) containing polysaccharides and oligosaccharides have been shown to have immunomodulatory effects.6,7,8,9,10,11,12 These extracts have been shown to enhance immune responses such as immunoglobulin production by lymphocytes and natural immune responses by peritoneal exudate macrophages.6 They have also been found to enhance anticomplementary and reticuloendothelial system activities,7 enhance macrophage Fc receptor expression,8increase the phagocytosis index along with phagocytosis fraction,9 and induce messenger RNA expression of interleukin-2 (IL-2), interferon-gamma (IFN-γ), interleukin-1α and granulocyte-macrophage colony-stimulating factor as well as lymphokine-activated killer cells and CD8+ cells.10 In addition, these extracts appear to stimulate cell-mediated immune response and natural killer cell cytotoxicity11 as well as to have cytotoxic effects on a wide range of tumour cell lines without major histocompatibility complex restriction.12
Recently, a patented poly-furanosyl-pyranosyl-saccharide–rich extract of North American ginseng (COLD-fX, CV Technologies Inc., Edmonton) was also shown to be capable of enhancing lymphocyte function and initiating acquired immune responses.13 In a recent study on human peripheral blood mononuclear cells cultured with live influenza virus, the extract was shown to be effective in enhancing the production of IL-2 and IFN-γ (unpublished data). IL-2 and IFN-γare major T-cell and natural killer cell cytokine responses associated with virus-elicited adaptive immunity, and IFN-γplays a role in skewing virus-specific antibody responses to the IgG2a isotype.14 An increase in natural killer cell activity is also thought to decrease susceptibility to frequent colds.15 These results from in vitro studies parallel those of a clinical trial involving elderly people living in institutions, where ginseng extract was found to effectively prevent acute respiratory illness due to influenza and respiratory syncytial virus by 89%.16
We sought to determine the efficacy of this North American ginseng extract in reducing the number of upper respiratory tract infections during 4 months of a cold and influenza season and to test whether prophylactic treatment with the extract would decrease the severity and duration of symptoms related to upper respiratory tract infections.
Methods
The study was conducted as a randomized, double-blind, placebo-controlled trial from September 2003 to April 2004 at the University of Alberta, Edmonton.
Subjects were recruited through media advertisements from Edmonton and the surrounding areas. Volunteers who responded to the advertisements were initially screened by telephone for inclusion and exclusion criteria. They were required to be in good general health, between 18 and 65 years of age and to have contracted at least 2 colds in the past year. To avoid the confounding effects of vaccination, subjects were excluded if they had been vaccinated against influenza in the previous 6 months. Subjects with medical conditions such as multiple sclerosis, tuberculosis, diabetes, cancer, lupus, HIV/AIDS, cardiovascular disease, hypertension, neurologic or psychiatric disease, and renal, pulmonary and hepatic abnormalities were excluded. Subjects taking medications such as immunosuppressive drugs, corticosteroids, warfarin, phenalzine, pentobarbital, haloperidol or cyclosporine were also excluded, as were pregnant or lactating women and heavy smokers. Because the potential impact of the North American ginseng extract in patients with these conditions has not been specifically examined in clinical studies, these exclusion factors were chosen on the basis of the results of a systematic review of the safety of Asian ginseng.17
Volunteers who qualified were asked to report to the University of Alberta for an information session. At these sessions the subjects completed a questionnaire pertaining to the history of past cold infections. The study protocol was also explained, consent forms were signed and a 2-month supply of either the ginseng extract or placebo was distributed along with the information sheet and daily cold assessment questionnaires. The standardized extract, for which batch-to-batch consistency is ensured in the production process, contains 80% poly-furanosyl-pyranosyl-saccharides and 10% protein formulated from the roots of North American ginseng (P. quinquefolium). The freeze-dried extract was encapsulated to contain 200 mg/capsule. The placebo was rice powder, which was encapsulated identically to the active treatment.
Subjects were randomly assigned to receive either the ginseng extract or placebo using an unrestricted randomization scheme generated using Excel 2003. EGA Biosciences (Edmonton, Alberta) administered the randomization scheme, providing investigators with numbered, opaque, sealed envelopes containing the treatment codes. Separate envelopes were to be opened only in case of medical emergency. The randomization codes were not broken until all of the data were analyzed.
Subjects were instructed to take 2 capsules per day for a period of 4 months following the onset of influenza season (November 2003). The study physician, G.P., notified the investigators when to start the treatment. Those with respiratory infections at the start of treatment were asked to delay beginning until absolute recovery.
Subjects were asked to take the capsules daily, in the mornings, after breakfast with a glass of water. They were instructed not to take any other cold medication unless advised by their family physicians. Subjects were contacted by e-mail or telephone each month to assure adherence to the study protocol. Compliance was also verified by weighing the returned bottles.
Subjects were asked to complete a daily log at about the same time every evening, to document the severity of their cold-related symptoms (sore throat, runny nose, sneeze, nasal congestion, malaise, fever, headache, hoarseness, earaches and cough) on a 4-point scale (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). The total symptom score3,4,18 was calculated by summing the daily scores for all symptoms. Subjects were told to contact the investigators and the study physician at the onset of a cold. A 2-day total symptom score greater than 14 (modified Jackson criteria)19 was considered to indicate a Jackson-verified cold; these were used in the analysis of number of colds. A daily total symptom score exceeding 4 was used in the analysis of symptom severity, number of days cold symptoms were reported, and duration of colds. This assessment was further verified by telephone or e-mail contact. The study physician also interviewed subjects, enquiring about the presence of symptoms that suggested secondary complications such as sinusitis, bronchitis, otitis media or pneumonia, and recommended family physician follow-up if necessary. To assess the tolerability of the treatments, subjects were asked to record any adverse side effects.
Subjects returned to the university at 2 months to receive additional medication and again at 4 months. At these visits they returned their daily assessment forms and unused medication. To ensure the integrity of randomization, identically numbered bottles (4 per subject), each containing 70 capsules of either the ginseng extract or placebo, were supplied by EGA Biosciences. These were distributed in numerical order. The numbers on the bottles were also used as the subjects' identification number. During the second visit the investigators as well the study subjects verified that the bottles returned were replaced with identically numbered bottles. As well, after completion of the intervention, subjects were asked whether they thought they had received the ginseng extract or placebo (“What do you think you received — ginseng extract or placebo?”).
All analyses were performed by a statistician under blinded conditions. A sample of about 123 in each group was found to be adequate. This was determined assuming the mean number of colds in the control group to be 2.5 per subject (primary efficacy variable), a treatment efficacy of 30%, a standard deviation of 2.0, a significance level of 5% and a power of 80%.
Our primary efficacy end point was the number of colds reported and Jackson-verified per subject. The secondary efficacy variables included severity of symptoms (total symptom score), number of days symptoms were experienced and duration of all colds during the 4-month intervention period.
The data were analyzed for all of the subjects who had been randomly assigned to a group, excluding those who provided only baseline information. An intention-to-treat analysis was performed. For those who discontinued the study, the last value available was carried forward. Those with incomplete daily logs were called and the scores obtained.
The 2 groups were compared with respect to baseline characteristics. The primary analysis was performed using an unpaired t test on the log-transformed data, with a Satterthwaite adjustment made to the degrees of freedom to allow for unequal variance. We used χ2 tests to compare the percentages of subjects contracting colds and receiving additional medication, and Fisher's exact 2-tailed probability test to compare the percentage of subjects reporting adverse events. Unpaired t tests were used to compare the 2 groups in terms of the number of colds, symptom scores and duration of symptoms.
The study was approved by the Human Ethics Committee of the University of Alberta.
Results
After the screening process, a total of 323 volunteers were enrolled and randomly assigned to the placebo or ginseng group (Fig. 1). Of these, 153 were assigned to receive the ginseng extract and 170 to receive placebo. The treatment assignment imbalance was due to the lack of a blocking factor in the randomization process. Of subjects in the ginseng group, 23 (15.0%) did not start their treatment, and in the placebo group, 21 (12.4%) failed to start. A number of reasons for withdrawal were given, including changing employment, compulsory immunization and holiday commitments. Some withdrew without giving a reason. A total of 130 in the ginseng group and 149 in the placebo group began the treatments and were included in data analysis. Twelve (9.2%) in the ginseng group and 11 (7.4%) in the placebo group discontinued for various reasons (Fig. 1).
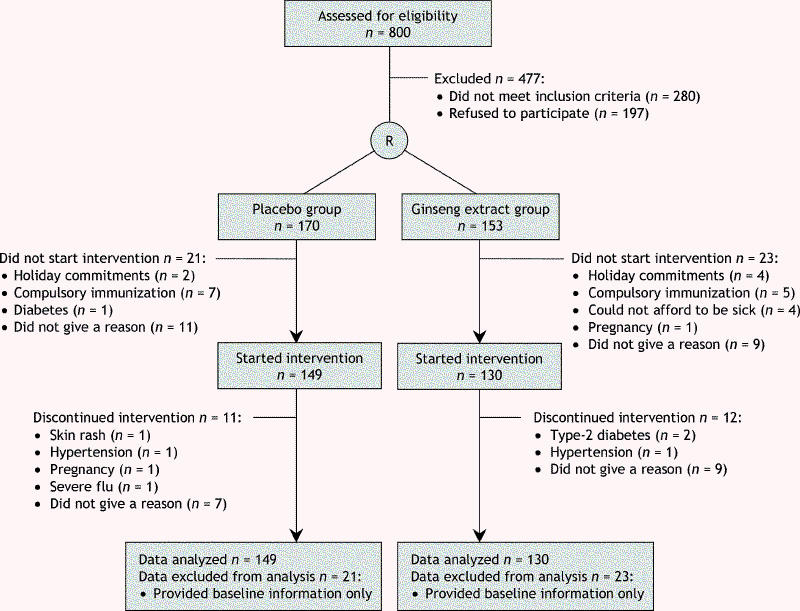
Fig. 1: Flow of participants through the study.
There were no significant differences in baseline characteristics between the subjects included and those excluded from data analysis (Table 1). The 2 study groups were similar with respect to age, sex and history of colds.
Table 1
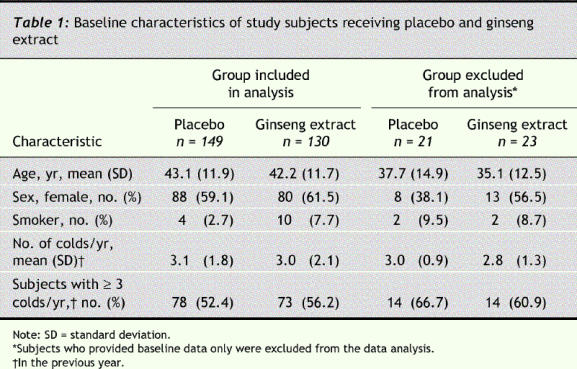
Compliance was high in both groups: 90% in the ginseng group and 92% in the placebo group reported taking more than 75% of the medication. Blinding was also maintained adequately during the treatment period. On completion of the study, 69.8% of those taking ginseng and 77.3% of those taking placebo thought they had been given the ginseng extract.
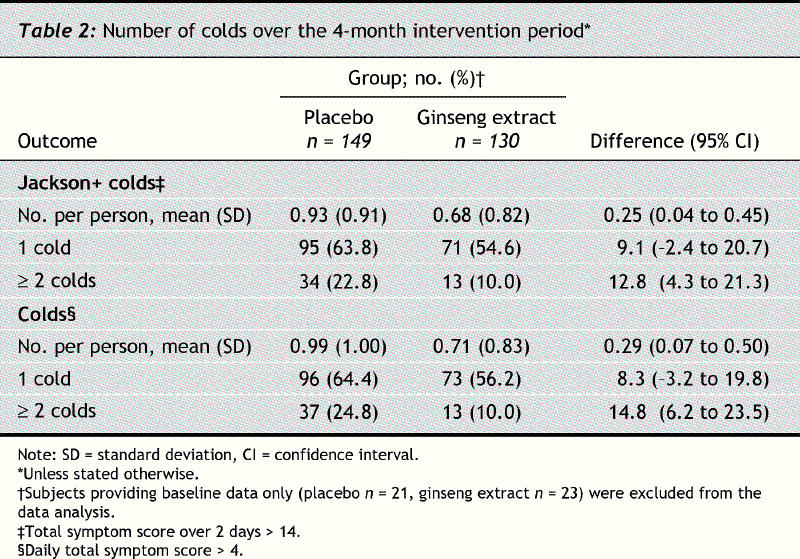
The mean number of Jackson-verified colds per person was less in the ginseng group than in the placebo group (0.68 v. 0.93, difference 0.25%, 95% confidence interval [CI] 0.04 to 0.45, p = 0.017) (Table 2). Fewer subjects in the ginseng group reported contracting at least 1 cold during the 4-month intervention period than in the placebo group (54.6% [71/130] v. 63.8% [95/149], difference 9.1%, 95% CI –2.4 to 20.7), but the difference was nonsignificant. However, the difference in recurrent colds was significant: 10.0% in the ginseng group reported having more than one cold compared with 22.8% in the placebo group (difference 12.8%, 95% CI 4.3 to 21.3, p = 0.004). Similar results were observed for all colds reported.
Table 2
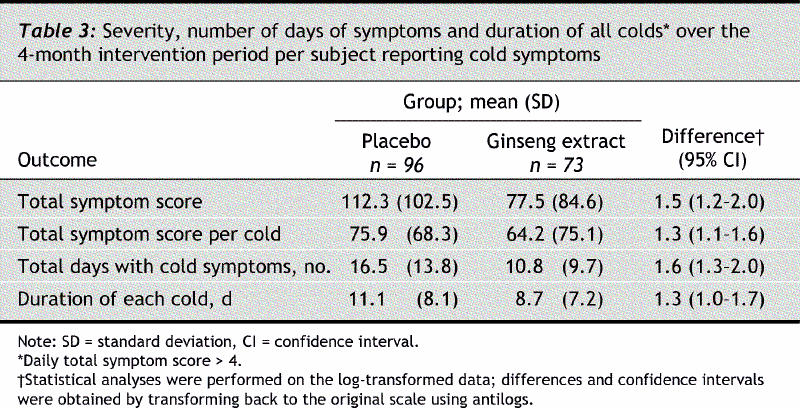
The total symptom score of all colds during the 4-month intervention period in the ginseng and placebo groups was 77.5 and 112.3 respectively (difference 1.5%, 95% CI 1.2–2.0, p = 0.002) (Table 3). A similar result was obtained when the total symptom scores per cold were compared. The total number of days that cold symptoms were experienced was significantly fewer in the ginseng group (10.8 v. 16.5 days, difference 1.6%, 95% CI 1.3–2.0, p < 0.001).
Table 3
The interventions were generally well tolerated by the subjects (Table 4). The frequency of adverse events was similar in the 2 groups. Type 2 diabetes mellitus was diagnosed in 2 subjects in the ginseng group, and they were subsequently withdrawn from the study.
Table 4
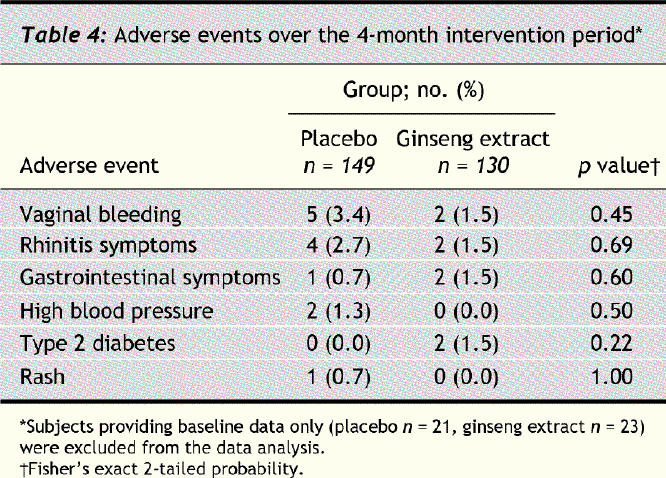
Supplementary medications, particularly NSAIDs, were used by 26.2% (34/130) and 29.5% (44/149) of the subjects in the ginseng and placebo groups respectively. Four (3.1%) in the ginseng group and 5 (3.4%) in placebo reported taking antibiotics for their colds and flu. The differences in the incidence of NSAIDs and antibiotic use between the 2 groups were not found to be statistically significant.
Interpretation
A moderate dose of a poly-furanosyl-pyranosyl-saccharide–rich extract of the root of North American ginseng (P. quinquefolium) was associated with an absolute risk reduction of recurrent colds meeting the Jackson criteria of 12.8% as well with as a reduction in the mean number of colds per person. A similar effect was also observed for all colds reported.
The ginseng extract was also found to be effective in reducing the severity of symptoms and the number of days symptoms related to all colds were reported. The total symptom score was 31.0% lower and the total number of days symptoms were reported 34.5% less in the ginseng group than in the placebo group over the 4-month intervention period. A similar effect was observed when the severity of symptoms was analyzed for individual colds: the prophylactic, low-dose treatment with the extract reduced the total symptom score of individual colds by 15.4%.
One of the limitations of the study is that subjects who provided only baseline data were excluded from analysis. However, since the demographic characteristics of the 2 study groups were virtually identical, it is unlikely that this exclusion created an imbalance of unknown prognostic factors.
Our results can be compared with those for many of the common antiviral drugs such as rimantadine, amantadine, zanamivir or oseltamivir20,21,22,23 for the prevention or treatment of influenza. Although our study was not designed to distinguish influenza infection from that of a common cold, an earlier study involving elderly people indicated that the extract reduced the relative risk of laboratory-confirmed influenza infections by 89%.16 These results are similar to those reported for zanamivir and oseltamivir therapy.24 These antiviral agents have been reported to reduce the severity and duration of illness by 1.5–2.5 days.25 In comparison, the ginseng extract treatment was found to reduce the duration of a cold by 2.4 days. Another limitation of these agents is their viral specificity; recent evidence also suggests that they may cause viral mutations.26 This North American ginseng extract has a broader range of activity and hence is potentially more effective in combating different strains of virus.
The standardized extract of North American ginseng was effective in reducing the absolute risk of recurrent colds and the mean number of colds per person. The safety of this formulation was also evident. It therefore appears to be an attractive natural prophylactic treatment for upper respiratory tract infections. However, further studies are required to assess its efficacy and safety for children and immunocompromised populations.
@ See related article page 1051
Acknowledgments
We thank CV Technologies Inc. for their financial support for this study. They had no role in planning the design of the study or in the collection or analysis of the data, nor did they have any role in the decisions related to preparing the manuscript for publication.
Footnotes
Editor's take
· Many herbal remedies are promoted to prevent colds or reduce symptoms, but few are subjected to randomized trials.
· In this randomized controlled trial a proprietary product containing North American ginseng reduced the mean number of colds per person from 0.9 in the placebo group to 0.7 in the ginseng group. Cold symptoms were less severe and were reported for fewer days in the experimental group.
Implications for practice: Ginseng products may be useful in preventing some viral upper respiratory infections (see the accompanying Commentary on page 1051).
This article has been peer reviewed.
Contributors: Tapan Basu was responsible for the conception and design of the study. Gerald Predy contributed to the design and execution of the clinical aspects of the study. Vinti Goel and Ray Lovlin were responsible for the recruitment and regular monitoring of the study subjects and the acquisition of data. Allan Donner, Larry Stitt, Vinti Goel and Ray Lovlin analyzed the data. Gerald Predy, Allan Donner and Larry Stitt interpreted the data. Vinti Goel, Ray Lovlin and Tapan Basu drafted the manuscript. Allan Donner and Larry Stitt were responsible for the statistical design of the study. All of the authors revised the manuscript for critical content and approved the final version to be published.
Competing interests: None declared for Allan Donner and Larry Stitt. Gerald Predy has received funding for research from CV Technologies Inc. but no specific honorarium. Vinti Goel is working for CV Technologies Inc. as a scientist for the research and development of nutraceuticals for cardiovascular health through a program funded by the Alberta Ingenuity Fund. Ray Lovlin is currently employed by CV Technologies Inc. for a project unrelated to this study. Tapan Basu has received a consultancy fee unrelated to this study and travel expenses from CV Technologies Inc.
Correspondence to: Dr. Tapan K. Basu, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton AB T6G 2P5; fax 780 492-4265; tapan.basu@ualberta.ca
REFERENCES
- 1.Gwaltney JM Jr. Clinical significance and pathogenesis of viral respiratory infections. Am J Med 2002;112:13S-18S. [DOI] [PubMed]
- 2.Turner RB. Epidemiology, pathogenesis and treatment of the common cold. Am J Allergy Asthma Immunol 1997;78:531-40. [DOI] [PMC free article] [PubMed]
- 3.Management of Influenza in the Southern Hemisphere Trialists Study Group. Randomized trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet 1998;352:1877-81. [PubMed]
- 4.Nicholson KG, Aoki FY, Osterhaus ADM, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000;355:1845-50. [DOI] [PubMed]
- 5.Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: A randomized, double-blind, placebo-controlled trial. Arch Intern Med 2004;164:1237-41. [DOI] [PubMed]
- 6.Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, et al. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J Pharm Pharmacol 2001;53:1515-23. [DOI] [PubMed]
- 7.Tomoda M, Hirabayashi K, Shimizu N, Gonda R, Ohara N, Takada K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull 1993;16:1087-90. [DOI] [PubMed]
- 8.Shin KS, Kiyohara H, Matsumo T, Yamada H. Rhamnogalactouronan II from the leaves of Panax ginseng C.A. Meyer as macophage Fc-receptor expression enhancing polysaccharide. Carbohydr Res 1997;300:239-49. [DOI] [PubMed]
- 9.Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res 1990;16:537-42. [PubMed]
- 10.Kim JY, Germolec DR, Luster MI. Panax ginseng as a potential immunomodulator: Studies in mice. Immunopharmacol Immunotoxicol 1990;12:257-76. [DOI] [PubMed]
- 11.Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, et al. Acidic polysaccharide from Panax ginseng, ginsan induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Plant Med 1998;64:110-5. [DOI] [PubMed]
- 12.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginseng isolated from Panax ginseng. Anticancer Res 1997;17:323-31. [PubMed]
- 13.Hu S. A contribution to our knowledge of ginseng. Am J Clin Med 1977;5:1-23. [DOI] [PubMed]
- 14.Almed R, Biron CA. Immunity to viruses. In: Paul WE. Fundamental immunology. Philadelphia, PA: Raven Publishers;1999. pp. 1303-19.
- 15.Cohen S, Hamrick N, Rodriguez MS, Feldman DJ, Rabin BS, Manuck SB. Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosom Med 2002;64:302-10. [DOI] [PubMed]
- 16.McElhaney JE, Gravenstein S, Cole S, Davidson E, O'Neill D, Petigean S, et al. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc 2004;52:13-9. [DOI] [PubMed]
- 17.Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-44. [DOI] [PubMed]
- 18.Prasad AS, Fitzgerald JT, Bao B, Beck FWJ, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. Ann Intern Med 2000;133:245-52. [DOI] [PubMed]
- 19.Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. 1. The common cold as a clinical entity. Arch Intern Med 1958;101:267-78. [DOI] [PubMed]
- 20.Claussen DW. Flumadine (rimantadine hydrochloride). Gastroenterol Nurs 1996;19:72-3. [DOI] [PubMed]
- 21.Marra F, Marra CA, Stiver HG. A case for rimantadine to be marketed in Canada for prophylaxis of influenza A virus infections. Can Respir J 2003;10:381-8. [DOI] [PubMed]
- 22.Garman E, Laver G. Controlling influenza by inhibiting the virus's neraminidase. Curr Drug Targets 2004;5:119-36. [DOI] [PubMed]
- 23.Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor Oseltamivir in treating acute influenza. A randomized controlled trial. JAMA 2000;283:1016-24. [DOI] [PubMed]
- 24.Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modeling for the prevention and treatment of influenza A and B. Health Technol Assess 2003;7:1-170. [DOI] [PubMed]
- 25.Stiver G. The treatment of influenza with antiviral drugs. CMAJ 2003;168(1):49-57. [PMC free article] [PubMed]
- 26.Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir. Lancet 2004;364:759-65. [DOI] [PubMed]


