Abstract
The capsaicin-sensitive vanilloid receptor (VR1) was recently shown to play an important role in inflammatory pain (hyperalgesia), but the underlying mechanism is unknown. We hypothesized that pain-producing inflammatory mediators activate capsaicin receptors by inducing the production of fatty acid agonists of VR1. This study demonstrates that bradykinin, acting at B2 bradykinin receptors, excites sensory nerve endings by activating capsaicin receptors via production of 12-lipoxygenase metabolites of arachidonic acid. This finding identifies a mechanism that might be targeted in the development of new therapeutic strategies for the treatment of inflammatory pain.
VR1, a cloned capsaicin receptor, is a nonspecific cation channel expressed preferentially in small sensory neurons and activated by the vanilloids, capsaicin and resiniferatoxin (1). Because VR1 is also activated by heat and acid (1, 2), it is now considered to be a molecular sensor that detects a variety of painful stimuli. Indeed, recent experiments performed in mice that lack VR1 demonstrated that the receptor is essential for inflammation-induced heat hyperalgesia (3, 4). Therefore, understanding the cellular mechanisms by which capsaicin receptors are activated by inflammatory mediators may be a key to identifying novel therapeutic targets for pain treatment. Because of the presence of VR1 in sensory neurons and an apparent role in inflammatory hyperalgesia, endogenous activators of VR1 have been suspected. We recently demonstrated that products of the lipoxygenase pathway of arachidonic acid (AA) metabolism can activate capsaicin receptors (5). Among the eicosanoids tested, the 12-lipoxygenase product, 12-hydroperoxyeicosatetraenoic acid (12-HPETE), structurally similar to capsaicin, was the most potent VR1 agonist. Thus, metabolic products of lipoxygenases become candidates for the endogenous capsaicin-like substances. However, the upstream signals that stimulate lipoxygenase and activate VR1 are elusive.
Bradykinin (BK) is a potent inflammatory mediator that causes pain and hyperalgesia. BK is known to activate as well as sensitize sensory neurons to other stimuli. Various signaling pathways have been suggested to mediate the sensitizing effect of BK on sensory neurons (6, 7). However, activation mechanism by BK is not known. BK is now known to stimulate the production of AA in sensory neurons (8), a key substrate of lipoxygenases. Therefore, on the basis of previous observations that products of lipoxygenase activate VR1 (5), we hypothesized that BK excites sensory neurons by opening the capsaicin receptor via production of 12-lipoxygenase products of AA metabolism.
Materials and Methods
Cell Culture.
Experiments were carried out according to the Ethical Guidelines of the International Association for the Study of Pain and approved by the research ethics committee for the use of animals of the Seoul National University and the University of California, San Francisco. Thoracic and lumbar dorsal root ganglia (DRGs) were dissected from 1- to 2 day-old neonatal rats anesthetized by hypothermia. The culture medium, a mixture of Dulbecco's modified Eagle's MEM and Ham's F-12 solution (50:50), contained 10% FBS, 1 mM sodium pyruvate, 50–100 ng/ml of nerve growth factor (GIBCO), and 100 units/ml of penicillin/streptomycin. Ganglia were incubated for 30 min in 1 mg/ml of collagenase (37°C; Worthington) followed by 30 min in 2.5 mg/ml of trypsin (37°C; Boehringer Mannheim). Dissociated cells were plated on poly-l-lysine-treated glass cover slips and incubated at 37°C in 95% air/5% CO2.
Whole-Cell and Single-Channel Current Recording.
Whole-cell and single-channel currents were recorded in cultured DRG neurons as described previously (5, 9). For whole-cell recordings, pipette solution contained (in mM) 4 ATP, 0.1 GTP, 130 KCl, 2 MgCl2, 5 EGTA, 0.5 CaCl2, and 10 KOH/Hepes (pH 7.2) and bath solution contained 130 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, and 10 NaOH/Hepes (pH 7.2). For single-channel recordings, pipette and bath solutions contained (in mM) 130 NaCl, 2 MgCl2, 5 EGTA, and 10 NaOH/Hepes (pH 7.2). Experiments were performed at room temperature.
In Vitro Skin-Nerve Preparation.
The saphenous nerve innervating the thigh-to-hind-paw region with an attached overlying skin flap was excised from a pentobarbital-anesthetized (120 mg/kg, i.p.) rat (300- to 500-g male Sprague–Dawley) (10). The skin was placed in an organ bath chamber with the volar surface exposed to the superfused solution (10 ml/min). Single-unit action potentials were recorded from a nerve filament via platinum wire electrodes. The superfusion solution (pH 7.4) contained (in mM): 107 NaCl, 26.2 NaHCO3, 9.6 sodium gluconate, 5.5 glucose, 7.6 sucrose, 3.5 KCl, 1.7 NaH2PO4, 1.5 CaCl2, 0.7 MgSO4 saturated with 95% O2 and 5% CO2, at 32°C. Receptive fields were identified by probing with a blunt glass rod, and conduction velocity was measured by electrical stimulation of the nerve trunk. BK and other chemicals were applied to the skin for 200 s in a cylinder placed onto the volar receptive field. In each experiment, BK was applied to the receptive field three times with a 15-min washout period between each application. Inhibitors were applied with the second application of BK.
Measurement of Intracellular Ca2+ Concentration ([Ca2+]i).
[Ca2+]i was measured with a confocal laser scanning microscope. Cells were loaded with 50 μM Fluo-3/AM (Molecular Probes) and incubated for 30 min at 37°C. Fluo-3/AM was excited at 488 nm and emitted fluorescence was collected at 515 nm. [Ca2+]i changes were expressed as the ratio F/F0, where F0 was the initial fluorescence intensity.
Single-Cell Reverse Transcriptase–PCR (RT-PCR).
Adult rat DRGs were harvested, and cells were cultured as described previously (11). Action potentials were recorded in whole-cell current-clamp mode between 24 and 72 hr after plating. The bath medium contained (in mM) 137 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 0.4 Na2HPO4, 10 Hepes, pH 7.4. After recording the BK response, cell content was harvested by suction into the recording pipette that contained 10 μl of intracellular solution. RT-PCR was performed by using 0.5 μl of reverse transcriptase (Superscript II, Life Technologies, Grand Island, NY). Two successive amplifications (initial Taq activation step at 95°C for 15 min, then 94°C for 45 sec, 58°C for 1 min, 72°C for 1 min, and 72°C for 4 min) were performed for 30 and 38 cycles, respectively, by using Hotstar Taq (Quiagen, Chatsworth, CA). The following multiplexed upstream and downstream primers, respectively, were used for VR1: 5′-TGAGTCCCCACCCCAAGAGAAC-3′ and 5′-CCAGCCCGCCTTCCTCATAAG-3′ (nucleotides 116–291); for 12-lipoxygenase: 5′-CACAGCACTCTTCCGTCCATCTTG-3′ and 5′-CCCAGGAGCCAAACGACATTTATC-3′ (nucleotides 1635–1812); for BK B2 receptor: 5′-TGCCTCTGCTTCTTCTGCTGTAATG-3′ and 5′-GTGTCTCCGTGTGATGTGTGTCTTG-3′ (nucleotides 2177–2418).
[3H]-Resiniferatoxin (RTX)-Binding Assay.
[3H]-RTX-binding assays were carried out by using 96-well filtration plates as described previously (12). Briefly, VR1-transfected human embryonic kidney (HEK) cells were incubated with various concentrations of [3H]-RTX in 150 μl of assay buffer at 37°C. After 1 h of incubation, ice-cold α1-acid glycoprotein (1 mg/well) was added to reduce nonspecific binding (13). The solution in the microplates was then immediately aspirated. Filter membranes of the microplates were then washed with the assay buffer containing α1-acid glycoprotein, dried completely, and collected for liquid scintillation counting. Parameters for ligand binding were determined by fitting the Hill equation (12, 13). For the competition assay, VR1 transfected HEK cells were incubated with 100 pM [3H]-RTX and various concentrations of 12-HPETE (0.1–20 μM) or capsaicin (0.1–100 μM). Inhibition constants (Ki) were calculated, Ki = IC50/(1 + L/Kd), where IC50 is the concentration of competing ligand that inhibited [3H]-RTX binding by 50%, and L is the concentration of [3H]-RTX (13).
Measurement of 12-Lipoxygenase Metabolites.
Cells isolated from DRGs were incubated with 0.1 μCi/ml of 14C-AA (48 mCi/mmol, Amersham Pharmacia) and 10 nM cold AA for 16 h. After washing with PBS five times, the cells were incubated in the serum-free medium for 2 h. Incubation continued for an additional 10 min after applying 1 μM BK. The reaction was stopped with addition of 99% methanol at pH 5.0. Radio-labeled lipids in the reaction medium were extracted by centrifugation (100 × g) after addition of ethyl acetate (99.5%). The organic layer was evaporated with argon, and the residue was dissolved in ethanol and stored at −70°C.
Samples were injected into a C18 column (Merck) connected to a HPLC (Waters) with a flow rate of 0.5 ml/min. The initial concentration (vol/vol) of acetonitrile was 50% and gradually increased to 95% at pH 3.0. The effluent was monitored at 235 nm and by a radioisotope detector (IN/US, Fairfield, NJ). The effluent of the radio-labeled peak was collected and stored at −70°C for a mass spectral analysis.
Mass spectroscopic analyses for the samples were performed with a mass electroionization spectrometer (Model 1100, Agilent, Waldbronn, Germany). Nitrogen was used as the nebulizing gas maintained at 325°C for drying. The samples were introduced to the ion source at a flow rate of 4 μl/min in the positive scan mode from m/z 100 to 1,500.
12-(S)-Hydroxyeicosatetraenoic Acid [12(S)-HETE] Immunoassay.
The level of 12(S)-HETE in DRG primary cultures was determined as described (14) by using a 12(S)-HETE enzyme immunoassay kit (Assay Designs, Ann Arbor, MI). The 12(S)-HETE antibody showed low crossreactivity (<1%) with 5- or 15-HETE, or prostaglandins, and with 12(R)-HETE (7.2%). Briefly, standards and samples were loaded in 96-well plates coated with goat anti-rabbit IgG. 12(S)-HETE enzyme immunoassay conjugate and antibody were added to each well and incubated for 2 h. Colorimetric density of the reaction was read at 405 nm immediately after 3-h incubation with p-nitrophenyl phosphate.
Behavioral Testing.
Thermal nociception was quantified by using radiant heat focused on the dorsal surface of the hind paw of lightly restrained 250- to 300-g male Sprague–Dawley rats (Bantin & Kingman, Fremont, CA). For each measurement of paw withdrawal latency, the value reported was the mean of three trials performed 10 min apart. BK was injected intradermally at the site of heat stimulation in a volume of 2.5 μl. Baicalein was injected 5 min before BK.
In addition to direct sensitization of sensory nerve endings, BK can also induce hyperalgesia via mechanisms mediated by sympathetic nerves (15). To analyze the functional significance of 12-lipoxygenase activity within sensory nerves, the indirect sympathetic-mediated mechanism was eliminated by surgical sympathectomy (16).
Statistics.
Data were presented as mean ± SEM. Student's t test was used to compare two means.
Results
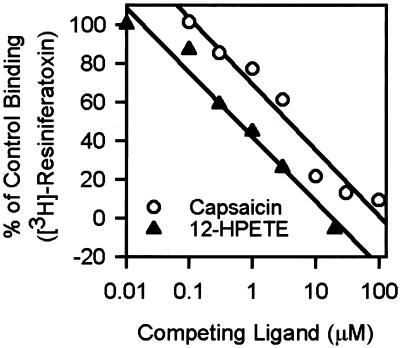
We sought to determine whether 12-HPETE, an immediate metabolic product of 12-lipoxygenase that shows the strongest potency in activating VR1 among the lipid products (5), actually binds to VR1 with saturability, by testing whether it competes for binding with a known ligand of VR1. VR1-transfected HEK cells were incubated with VR1 with 100 pM [3H]-RTX, a potent agonist of the capsaicin receptor (13). In the incubation medium, various concentrations of 12-HPETE were added to compete with [3H]-RTX. Capsaicin was used for a positive control. Comparable to the results observed by others (13), [3H]-RTX displayed saturable binding to HEK-VR1 cells with apparent Kd and Bmax of 102.8 and 206.0 pM, respectively (triplicate preparations). The nonspecific binding was about 6.1% at Kd.
As shown in Fig. 1, 12-HPETE and capsaicin inhibited [3H]-RTX binding to HEK-VR1 cells. The apparent inhibition constant (Ki) for 12-HPETE was 0.35 μM (quadruplicate preparations), and the apparent Ki for capsaicin (2.5 μM) was comparable to that reported previously (13). These results clearly suggest that 12-HPETE is indeed capable of binding to VR1, with a somewhat greater potency than capsaicin.
Figure 1.
12-HPETE inhibits the specific binding of [3H]-RTX to VR1-HEK cells. Cells were incubated with various concentrations of 12-HPETE and 100 pM [3H]-RTX. Capsaicin was used as a control. Apparent Ki values for 12-HPETE and capsaicin were 0.35 and 2.5 μM, respectively (quadruplicate preparations).
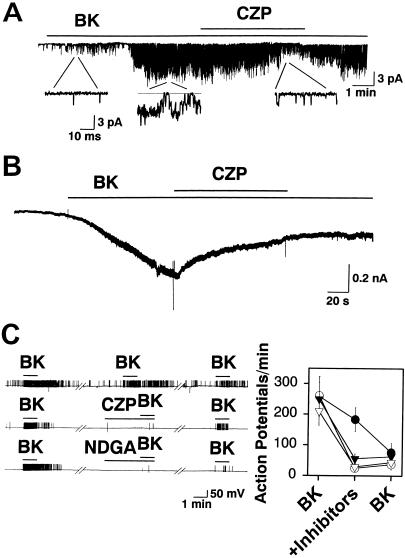
Single-channel current recordings were performed to test whether BK can cause capsaicin receptors to be activated in cultured rat DRG neurons. In 13 of 101 cell-attached patches, bath application of BK (1 μM) activated single-channel currents (Fig. 2A). The delay for activation of the currents (153 ± 31 sec, n = 13) is consistent with a requirement for generation of intracellular second messengers. When BK (0.5 μM) was applied via the pipette to the extracellular surface of the patch, channels were activated in 30 of 86 patches, and these currents were all reduced by bath application of 5 μM capsazepine (CZP) in 7 patches so tested (73.0 ± 12.7% reduction in channel activity, P < 0.01).
Figure 2.
BK excites cultured sensory neurons via the PLA2/lipoxygenase/VR1 pathway. (A) Bath application of 10 μM CZP antagonizes single-channel currents activated by bath application of 1 μM BK in a cell-attached patch. (B) CZP (10 μM) antagonizes whole-cell currents induced by 1 μM BK. (C) Action potential firing induced by 0.1 μM BK is antagonized by 5 μM CZP (open circles, n = 9), a lipoxygenase inhibitor, 5 μM NDGA (open triangles, n = 9), and a PLA2 inhibitor, 10 μM QN (filled triangles, n = 6). For control experiments (filled circles, n = 10), application of BK was repeated three times. Gaps represent 5-min intervals.
In whole-cell voltage-clamp recordings, BK (1 μM) induced small inward currents (<500 pA) in 26 of 201 neurons (Fig. 2B). In 12 BK-responsive neurons tested, 10 μM CZP significantly reduced the BK-evoked whole-cell current by 71.6 ± 6.6% (P < 0.01). Current-clamp recordings were performed to determine whether the BK-induced activation of capsaicin receptors is sufficient to evoke action potential firing in the cultured sensory neurons. BK (0.1 μM) was able to induce firing of action potentials, which, in some cells, continued after washout of BK from the bath (Fig. 2C). The response could be evoked repeatedly from a single cell, although tachyphylaxis occurred. The response to BK was greatly attenuated by 5 μM CZP (86.1 ± 6.1%, n = 9), suggesting that capsaicin receptors mediate this effect. Consistent with the idea that BK activates capsaicin receptors by stimulating production of AA metabolites via a phospholipase A2 (PLA2)/lipoxygenase pathway, BK-induced action potential generation was attenuated by 10 μM quinacrine (QN; an inhibitor of PLA2) and by 5 μM nordihydroguaiaretic acid (NDGA), a lipoxygenase inhibitor (Fig. 2C). Application of NDGA (5 μM, n = 5) or QN (10 μM, n = 7) alone failed to inhibit capsaicin-evoked single-channel currents or activate capsaicin receptors, indicating that these inhibitors have no direct effect on the capsaicin receptor.
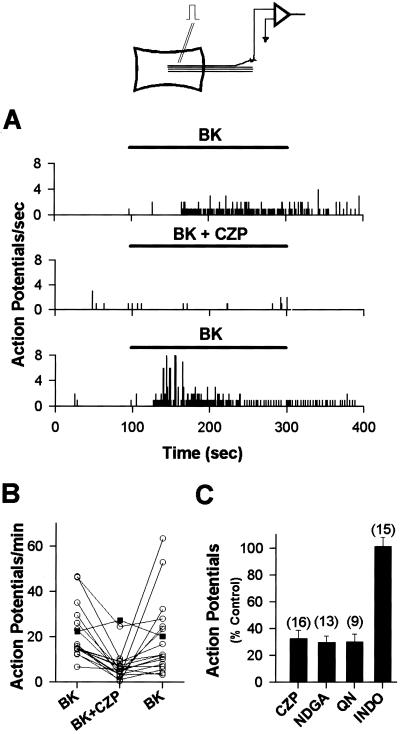
The foregoing in vitro electrophysiological observations, performed in a perfused recording chamber, suggest that BK can act directly on sensory neurons to stimulate AA metabolism within those cells, leading to activation of CZP-sensitive currents. To verify whether BK can stimulate AA metabolism leading to activation of capsaicin currents in situ, action potentials from rat cutaneous sensory nerve fibers attached to skin, isolated in vitro (10, 17, 18), were recorded. Superfusion of BK (1 μM) over the volar surface of the skin excited 67 of 122 fibers identified as unmyelinated C-fiber nociceptors (velocities <1 m/s) (Fig. 3A). Coapplication of CZP (10 μM) and BK significantly reduced the neural responses to BK (67.2 ± 6.0%, P < 0.01, n = 16) (Fig. 3 A–C), whereas application of the CZP vehicle with BK did not significantly change the BK response (25.2 ± 7.6% increase, P < 0.12, n = 7). The neural response to BK was also reduced by the PLA2 inhibitor, QN (10 μM) (69.1 ± 5.1%, n = 9) and by the lipoxygenase inhibitor, NDGA (20 μM) (70.1 ± 4.6%, n = 13). Finally, application of 2 μM indomethacin (INDO), to inhibit the lipoxygenase-independent metabolism of AA via cyclooxygenase, did not significantly reduce the response to BK (1.3 ± 7.0%, n = 15).
Figure 3.
BK (1 μM)-induced excitation of cutaneous nerve fibers in the in vitro skin-nerve preparation is antagonized by 10 μM CZP, 20 μM NDGA, and 10 μM QN, but not by a cyclooxygenase inhibitor, 2 μM INDO. (A) Peristimulus-time histograms of BK-induced activity, which is reversibly inhibited by 10 μM CZP. Bin width = 100 ms. (B) CZP (10 μM) reversibly antagonizes nerve activity induced by 1 μM BK in 16 fibers (circles). Vehicle (filled squares) does not antagonize the activity (n = 7). (C) Antagonists of the PLA2/lipoxygenase/VR1 cascade significantly (P < 0.01) reduce the number of action potentials induced by 1 μM BK compared with the control response to BK before application of antagonists. INDO has no significant effect on the response to BK. Numbers in parentheses represent the number of experiments.
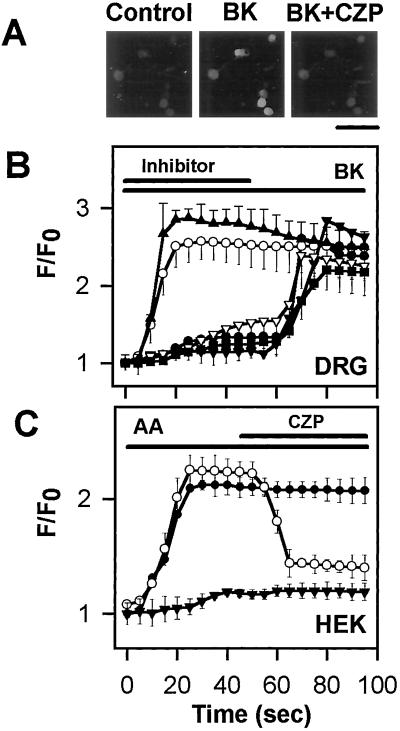
In addition to depolarizing neurons, BK exerts some of its cellular effects by increasing the [Ca2+]i. Therefore, we tested the hypothesis that this action of BK is also mediated by the cascade of PLA2, lipoxygenase, and capsaicin receptor. As shown in Fig. 4, BK (1 μM) induced a [Ca2+]i increase in 26% (24/84) of cultured DRG neurons (Fig. 4B), and capsaicin (1 μM) induced a [Ca2+]i increase in 60% (50/84) of neurons. BK-induced increases in [Ca2+]i depended on influx of external Ca2+ because they were eliminated in Ca2+-free medium (data not shown). Furthermore, CZP (5 μM) reduced the BK-induced [Ca2+]i transients by 85.8 ± 7.4% (n = 13), and QN (10 μM) reduced the BK responses by 64.4 ± 1.9% (n = 8). Both NDGA (30 μM) and baicalein (10 μM), a specific inhibitor of 12-lipoxygenase, also reduced the BK response by 91.5 ± 4.7% (n = 9) and 87.8 ± 5.4% (n = 7), respectively (Fig. 4B). INDO (10 μM), a cyclooxygenase inhibitor, failed to reduce the BK-induced increase in [Ca2+]i.
Figure 4.
BK-evoked calcium influx is mediated by the PLA2/12–lipoxygenase/VR1 pathway. (A) Fluorescence photomicrographs show that 1 μM BK-induced [Ca2+]i increase in cultured sensory neurons is blocked by 5 μM CZP. Control represents a fluorescence photomicrograph of cultured sensory neurons preincubated with Fluo-3/AM before BK and BK and CZP (BK+CZP) application. (Bar = 100 μm.) (B) Increase in [Ca2+]i induced by 1 μM BK in sensory neurons (filled triangles, n = 15) and inhibition by an inhibitor of PLA2 (QN, open triangles), inhibitors of 12-lipoxygenase (NDGA, filled inverted triangles and baicalein, filled circles), and CZP (filled squares). A cyclooxygenase inhibitor, INDO (open circles) fails to inhibit the BK-induced increase in [Ca2+]i. (C) The 12-lipoxygenase/VR1 cascade can be reconstituted in HEK cells. AA (10 μM) evokes a large [Ca2+]i increase in HEK cells transfected with both 12-lipoxygenase and VR1 (filled circles, n = 72), but not in cells transfected with cyclooxygenase-2 and VR1 (filled triangles, n = 54). The AA-induced [Ca2+]i increase in HEK cells transfected with 12-lipoxygenase and VR1 (open circles, n = 67) is blocked by 10 μM CZP.
Further experiments were performed to confirm that 12-lipoxygenase is the critical metabolic enzyme for activating VR1. HEK cells were transfected with cDNA encoding VR1. Application of 10 μM AA did not induce an increase in [Ca2+]i in these cells (data not shown). However, expression of 12-lipoxygenase in the VR1-transfected HEK cells enabled them to produce [Ca2+]i transients in response to AA application (n = 72), a response that was blocked by 5 μM CZP by 91 ± 13% (n = 67) (Fig. 4C). In contrast, expression of cyclooxygenase-2 in VR1-transfected HEK cells failed to confer responsiveness to AA (n = 54). Cells transfected with 12-lipoxygenase or cyclooxygenase-2 alone did not increase [Ca2+]i in response to AA application (data not shown).
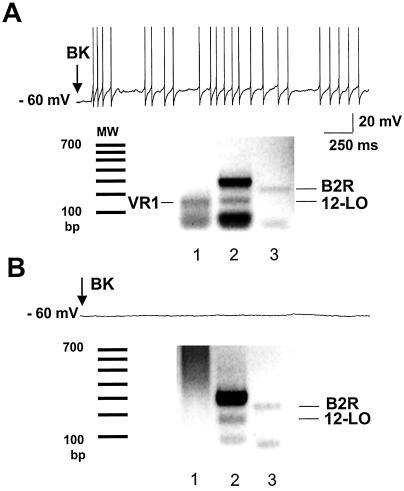
To verify that the necessary elements of the PLA2/lipoxygenase/capsaicin receptor pathway in fact coexist in sensory neurons that respond to BK, single-cell RT-PCR was performed to detect the coexpression of mRNAs encoding B2 receptor, 12-lipoxygenase, and VR1 in adult cultured DRG neurons. Among neurons that responded electrophysiologically to 10 μM BK, 75% (6/8) coexpressed detectable levels of the three mRNAs (Fig. 5A). In contrast, among neurons that were not responsive to BK, only 5% (1/19) coexpressed all three mRNAs. Of note, five neurons that expressed both B2 receptor and 12-lipoxygenase, but lacked detectable VR1, failed to respond to BK (Fig. 5B).
Figure 5.
B2 BK receptor, VR1, and 12-lipoxygenase are coexpressed in most BK-responsive sensory neurons. (A) Single-cell RT-PCR products of VR1 (lane 1), 12-lipoxygenase (12-LO, lane 2), and B2 receptor (B2R, lane 3) are coexpressed in a single sensory neuron that fires action potentials (upper trace) in response to BK. MW denotes the molecular weight standards. (B) In a cell that does not respond to BK (upper trace), RT-PCR products of 12–lipoxygenase and B2R are detected, but VR1 is undetectable (lower trace).
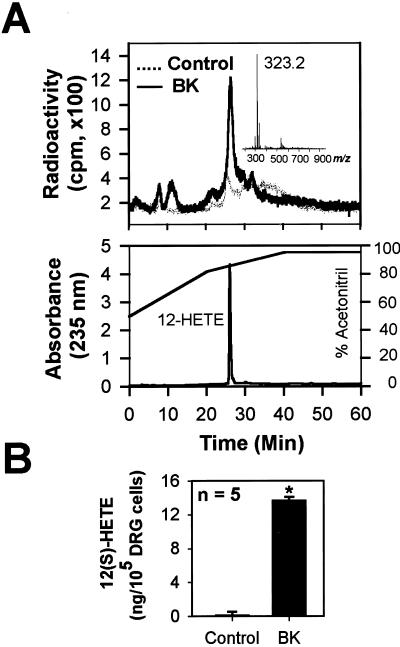
To determine whether BK can, in fact, stimulate synthesis of 12-lipoxygenase products in sensory neurons, we used high-performance liquid chromatography coupled with radioisotope detection of chemicals released from primary cultures of rat sensory neurons. As shown in Fig. 6A, preincubation with 14C-AA elicited a weak radio-labeled peak. This peak was increased by a factor of 5.6 ± 2.0 (n = 5, P < 0.05) with application of 1 μM BK (Fig. 6A). The retention time of the radio-labeled peak was identical to that of 12-HETE, a downstream metabolite of 12-HPETE, which itself is an immediate metabolite of 12-lipoxygenase and the most potent known lipid agonist of VR1 (5). The chemical identity of the peak as C14-12-HETE (molecular weight = 322.5) was further confirmed by mass spectrometric analysis (Fig. 6A Inset). The production of 12-HETE after BK application was also confirmed by an enzyme immunoassay by using a polyclonal antibody specific to 12(S)-HETE (14). As shown in Fig. 6B, application of 1 μM BK elicited about 11-fold (n = 9, P < 0.001) increase in the level of 12(S)-HETE in the primary cultures. Although primary cultures of DRGs also contained nonneuronal cells, the BK-induced 12-HETE production was likely of neuronal origin because after depletion of sensory neurons from the DRG culture, the 12-HETE peak was not detected (data not shown).
Figure 6.
BK causes the production of 12-lipoxygenase metabolites of AA in sensory neurons. (A) Application of 1 μM BK to cultured sensory neurons preincubated with 14C-AA evokes a radio-labeled peak that is identical to 12-HETE in its retention time when detected with a high-performance liquid chromatography at 235-nm wavelength (Lower). A mass spectral analysis of the radio-labeled peak (Inset) further confirms the molecular ion peak (m/z 323.5 [M+H]+) of 14C-12-HETE (molecular weight = 322.5). Concentration of acetonitrile in the mobile solution is indicated as a solid line. (B) 12(S)-HETE specific enzyme immunoassay also detects the increase in 12(S)-HETE after BK application in sensory neurons (*, P < 0.0001).
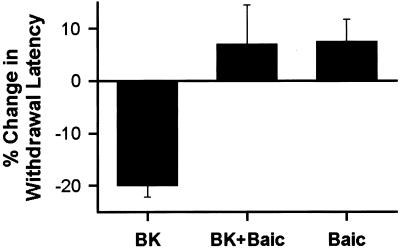
Behavioral experiments were performed to determine whether 12-lipoxygenase contributes in vivo to BK-induced thermal hyperalgesia (Fig. 7). Intradermal injection of BK (1 μg/2.5 μl) into the rat hind paw caused a significant decrease (P < 0.05) in the latency for withdrawal from a radiant heat stimulus. Coadministration of baicalein (1 μg/2.5 μl) prevented this BK-induced hyperalgesia, restoring threshold to a level not significantly different from the baseline. Thus, 12-lipoxygenase activity is required for BK-induced thermal hyperalgesia.
Figure 7.
BK-induced thermal hyperalgesia requires 12-lipoxygenase activity. Injection of 1 μg of BK in the hind paw reduces the latency of the heat-evoked withdrawal reflex (n = 8). Coadministration of 1 μg of baicalein (BK + Baic) prevented the effect of BK (n = 8). Baicalein (Baic) alone had no significant effect on the paw withdrawal latency (n = 4).
Discussion
This study demonstrates that BK can act directly on sensory neurons to stimulate the production of the endogenous capsaicin receptor agonist, 12-HPETE (5) via the 12-lipoxygenase pathway. Our in vitro electrophysiological experiments suggest that the pathway operates entirely within sensory neurons, consistent with evidence that 12-HPETE may bind to the capsaicin-binding site (5), which is located in the cytosolic domain of VR1 (9, 19). The present data also demonstrate that VR1 can be activated by BK, and that CZP, the competitive antagonist of VR1, blocks the BK effects.
BK both activates (17) and sensitizes (20) sensory neurons. In the present study, it appears that capsaicin receptors are activated by BK, which would be consistent with the suggestion that 12-HPETE acts as an agonist of VR1 (5). However, the present data cannot exclude the possibility that BK sensitizes heat-activated capsaicin receptors to such a degree that they become active at ambient temperature. This would be compatible with the recent report that BK activates C-fibers in vivo by reducing their heat threshold to a level below body temperature (21). If so, the elevated temperature of inflamed tissue (calor) could exacerbate this effect of BK in vivo.
There is evidence that BK can sensitize capsaicin receptors by mechanisms that may not involve AA metabolites (22). Specifically, protein kinase C is known to contribute to BK-induced thermal sensitization of sensory neurons (23), and recent studies have implicated phospholipase C and protein kinase C in BK-induced activation of vanilloid receptors (6, 7). In view of such data, it seems likely that interactions between the protein kinase C and PLA2/12-lipoxygenase pathways could play a role in BK-induced effects on capsaicin receptors. One possibility is that protein kinase C activity contributes to BK-induced calcium influx (24) that is required for BK-induced mobilization of AA (25).
Because vanilloid receptors might act as heat transducers (1, 2, 26) in inflamed tissue (2, 3), we hypothesized that BK-induced activation of capsaicin receptors would produce thermal hyperalgesia. Supporting this idea, BK-induced thermal hyperalgesia was completely blocked by an inhibitor of 12-lipoxygenase. Furthermore, BK-induced thermal hyperalgesia is much reduced in animals deficient in VR1 (6). This finding is consistent with previous reports that enhancement of heat sensitivity is a direct effect of BK on sensory nerves (27, 28), whereas BK-induced mechanical hyperalgesia is an indirect result of BK acting on sympathetic nerves (29) that release factors (e.g., cytokines, prostaglandins, and epinephrine) (30, 31) that then sensitize sensory nerve endings to mechanical stimuli.
In summary, this study demonstrates that the inflammatory mediator BK can activate capsaicin receptors via an intracellular second messenger pathway involving mobilization of AA by PLA2 and generation of the 12-lipoxygenase product, 12-HPETE. Furthermore, the 12-lipoxygenase pathway is required for BK-induced thermal hyperalgesia. These insights suggest new strategies in the development of treatments for inflammatory pain and perhaps for other pathologies related to inflammation-induced activation of vanilloid receptors in sensory nerve fibers, e.g., airway hyperreactivity (32).
Acknowledgments
This work was supported by National Creative Research Initiatives, BK21 program of Korea, and National Institutes of Health Grant NS21647.
Abbreviations
- 12-HETE
12-hydroxyeicosatetraenoic acid
- 12-HPETE
12-hydroperoxyeicosatetraenoic acid
- NDGA
nordihydroguaiaretic acid
- PLA2
phospholipase A2
- RTX
resiniferatoxin
- HEK
human embryonic kidney
- DRG
dorsal root ganglion
- RT-PCR
reverse transcriptase–PCR
- BK
bradykinin
- [Ca2+]i
intracellular Ca2+ concentration
- CZP
capsazepine
- QN
quinacrine
- INDO
indomethacin
- AA
arachidonic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Petersen-Zeitz K R, Koltzenburg M, Basbaum A I, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Davis J B, Gray J, Gunthorpe M J, Hatcher J P, Davey P T, Overend P, Harries M H, Latcham J, Clapham C, Atkinson K, et al. Nature (London) 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 5.Hwang S W, Cho H, Kwak J, Lee S Y, Kang C J, Jung J, Cho S, Min K H, Suh Y G, Kim D, Oh U. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang H H, Prescott E D, Kong H, Shields S, Jordt S E, Basbaum A I, Chao M V, Julius D. Nature (London) 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 7.Premkumar L S, Ahern G P. Nature (London) 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 8.Thayer S A, Perney T M, Miller R J. J Neurosci. 1988;8:4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung J, Hwang S W, Kwak J, Lee S Y, Kang C J, Kim W B, Kim D, Oh U. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steen K H, Steen A E, Reeh P W. J Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichling D B, Levine J D. Proc Natl Acad Sci USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acs G, Lee J, Marquez V E, Blumberg P M. Mol Brain Res. 1996;35:173–182. doi: 10.1016/0169-328x(95)00204-6. [DOI] [PubMed] [Google Scholar]
- 13.Szallasi A, Blumberg P M, Annicelli L L, Krause J E, Cortright D N. Mol Pharmacol. 1999;56:581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Nunez D, Claria J, Rivera F, Poch E. Hypertension. 2001;37:334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- 15.Levine J D, Taiwo Y O, Collins S D, Tam J K. Nature (London) 1986;323:158–160. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- 16.Baron R, Janig W, Kollmann W. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- 17.Lang E, Novak A, Reeh P W, Handwerker H O. J Neurophysiol. 1990;63:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- 18.Reeh P W. Prog Brain Res. 1988;74:271–276. doi: 10.1016/s0079-6123(08)63024-1. [DOI] [PubMed] [Google Scholar]
- 19.Welch J M, Simon S A, Reinhart P H. Proc Natl Acad Sci USA. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumazawa T, Mizumura K, Minagawa M, Tsujii Y. J Neurophysiol. 1991;66:1819–1824. doi: 10.1152/jn.1991.66.6.1819. [DOI] [PubMed] [Google Scholar]
- 21.Liang Y F, Haake B, Reeh P W. J Physiol. 2001;532:229–239. doi: 10.1111/j.1469-7793.2001.0229g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stucky C L, Abrahams L G, Seybold V S. Neuroscience. 1998;84:1257–1265. doi: 10.1016/s0306-4522(97)00572-1. [DOI] [PubMed] [Google Scholar]
- 23.Cesare P, Dekker L V, Sardini A, Parker P J, McNaughton P A. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 24.Burgess G M, Mullaney I, McNeill M, Dunn P M, Rang H P. J Neurosci. 1989;9:3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gammon C M, Allen A C, Morell P. J Neurochem. 1989;53:95–101. doi: 10.1111/j.1471-4159.1989.tb07299.x. [DOI] [PubMed] [Google Scholar]
- 26.Kirschstein T, Greffrath W, Busselberg D, Treede R D. J Neurophysiol. 1999;82:2853–2860. doi: 10.1152/jn.1999.82.6.2853. [DOI] [PubMed] [Google Scholar]
- 27.Koltzenburg M, Kress M, Reeh P W. Neuroscience. 1992;46:465–473. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- 28.Cesare P, Moriondo A, Vellani V, McNaughton P A. Proc Natl Acad Sci USA. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taiwo Y O, Heller P H, Levine J D. Neuroscience. 1990;39:523–531. doi: 10.1016/0306-4522(90)90288-f. [DOI] [PubMed] [Google Scholar]
- 30.Green P G, Luo J, Heller P H, Levine J D. Neuroscience. 1993;55:1037–1043. doi: 10.1016/0306-4522(93)90317-9. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira S H, Lorenzetti B B, Poole S. Br J Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veronesi B, Oortgiesen M, Roy J, Carter J D, Simon S A, Gavett S H. Toxicol Appl Pharmacol. 2000;169:66–76. doi: 10.1006/taap.2000.9040. [DOI] [PubMed] [Google Scholar]