Abstract
Exchange of information between the nucleus and cytosol depends on the metabolic state of the cell, yet the energy-supply pathways to the nuclear compartment are unknown. Here, the energetics of nucleocytoplasmic communication was determined by imaging import of a constitutive nuclear protein histone H1. Translocation of H1 through nuclear pores in cardiac cells relied on ATP supplied by mitochondrial oxidative phosphorylation, but not by glycolysis. Although mitochondria clustered around the nucleus, reducing the distance for energy transfer, simple nucleotide diffusion was insufficient to meet the energetic demands of nuclear transport. Rather, the integrated phosphotransfer network was required for delivery of high-energy phosphoryls from mitochondria to the nucleus. In neonatal cardiomyocytes with low creatine kinase activity, inhibition of adenylate kinase-catalyzed phosphotransfer abolished nuclear import. With deficient adenylate kinase, nucleoside diphosphate kinase, which secures phosphoryl exchange between ATP and GTP, was unable to sustain nuclear import. Up-regulation of creatine kinase phosphotransfer, to mimic metabolic conditions of adult cardiac cells, rescued H1 import, suggesting a developmental plasticity of the cellular energetic system. Thus, mitochondrial oxidative phosphorylation coupled with phosphotransfer relays provides an efficient energetic unit in support of nuclear transport.
Efficient communication between the cytosol and nucleus is essential in cellular homeostasis, regulating proper processing of genetic and metabolic information. Central in nucleocytoplasmic exchange is the transport of macromolecules across the nuclear envelope (1, 2), a multistep process that initially proceeds by signal-mediated recognition of the macromolecule to be transported, following by docking events and, ultimately, translocation through nuclear pores (1–5). In energy-depleted cells, molecules that are actively transported into the nucleus, such as the constitutive chromatin protein histone H1, tend to accumulate on the cytosolic surface of the nuclear membrane (2, 4). While formation and docking of the transported protein, complexed with a transport receptor, may be energy-independent, the actual translocation and accumulation of molecules in the nuclear compartment against a concentration gradient may, however, require an energy source (4).
Energy-consuming enzymes, including nucleoside triphosphatases, are associated with the nuclear envelope, and their activity is stimulated in the presence of the transported substrate (6, 7). Underscoring the energetic cost of nuclear transport, receptor cycling and continued signal processing mandate catalytic conversion of the guanine nucleotide-binding protein Ran from Ran-GTP to Ran-GDP, which is accomplished by the RanGTPase and the subsequent regeneration of GTP (1, 2). Yet, transport of macromolecules across the nuclear envelope that can proceed in an apparently energy-independent manner also has been reported (8, 9). This observation was, however, made in single-round in vitro experiments in the presence of an ATP/GTP regenerating system, such as the creatine kinase/creatine phosphate system, which supports energy-dependent processes even at very low residual nucleotide levels (10). In fact, when trace amounts of ATP were eliminated with apyrase, nuclear transport did not proceed (11). Thus, although nuclear transport is sensitive to alterations in cellular metabolic state, the energy dependence of nuclear transport remains controversial, and energy-supply pathways remain unknown (2, 4, 11–15).
Recently, the importance has been demonstrated of enzymatic phosphotransfer reactions catalyzed by creatine kinase and/or adenylate kinase in energy delivery from mitochondria to ATP-utilization sites and in regulating ATP-sensitive cellular components (10, 16–22). Here, we present evidence that in intact cells, mitochondrial oxidative phosphorylation and catalyzed phosphotransfer are both essential in providing energy and maintaining proper nucleotide ratios in support of active nuclear transport.
Methods
Microinjection of Fluorescent Macromolecules.
Hearts of 1- to 2-day-old rats were digested with collagenase II (0.5 mg/ml) and pancreatin (0.15 mg/ml) in 116 mM NaCl/20 mM Hepes/1 mM NaH2PO4/5.5 mM glucose/5.4 mM KCl/0.8 mM MgSO4/0.6 ml/liter phenol red, pH 7.35. Isolated cardiomyocytes were purified by using a two-layer Percoll gradient (4, 14), plated on laminin-coated coverslips, and cultured in a serum-containing medium (37°C, 5% CO2). Forty-eight hours after plating, cells were transferred to DMEM at 37°C and supplemented with 0.5% BSA/10 mM Hepes, pH 7.5/20 mM butanedione monoxime. Cytosolic injections of purified and fluorescein-isothiocyanate labeled histones H1 or 10-kDa dextrans were carried out with a nanometer-precision microinjector unit (5242, Eppendorf) coupled to a micromanipulator (5171, Eppendorf) mounted on a fluorescence microscope (Axiovert 100, Zeiss). To this end, pipettes were filled with a prewarmed (37°C) injection buffer (150 mM KCl/1 mM Pipes/0.1 mM EDTA/0.025 mM EGTA, pH 7.2) containing 0.07 mg/ml H1 or 5 mM 10-kDa dextrans. Microinjection quality was confirmed by comparing cellular fluorescence intensity to a dilution standard of carboxyfluorescein-dextran in intracellular buffer (4). Injection volume did not exceed 1–2% of the cell volume.
Quantitation of Nuclear Transport.
Microinjected cardiac cells were placed in 116 mM NaCl/4 mM KCl/2 mM MgCl2/2 mM NaH2PO4/4 mM NaHCO3/21 mM Hepes/1 mM CaCl2, pH 7.4 at 37°C and were imaged by laser confocal microscopy (410, Zeiss) using 40× (1.3 N.A.) or 63× (1.4 N.A.) objectives. The thickness of the optical sections was set at 1–2 μm to discriminate fluorescence emitted from nuclear vs. nonnuclear regions. Fluorescent probes were excited (at 488 nm) by using an argon-krypton visible laser (Omnichrome), and the emission envelope was collected by using a 510-nm pass dichroic beam splitter and a 515-nm pass emission filter. Two-dimensional confocal images (512 × 512 pixels) were acquired by scanning a field at 16 s per frame. Fluorescence intensity per unit area in the nucleus vs. cytosol was determined by using the ANALYZE software (Mayo Foundation). For nuclear fluorescence, the value was obtained from the total nuclear area. For cytosolic fluorescence, an area surrounding the nucleus and equivalent to the nuclear area was used. Nuclear accumulation was expressed as the ratio of nuclear over cytosolic fluorescence (4, 14).
Nucleotide Levels and Enzymatic Activities.
Cardiomyocytes, washed with ice-cold PBS and immersed into liquid nitrogen, were layered over with 0.6 M HClO4/1 mM EDTA, homogenized, and centrifuged (Hermline Z230 MA microcentrifuge, Labnet, Bournemouth, U.K.). Supernatant was neutralized with 2 M K2HCO3, and precipitate was removed by centrifugation (14,000 × g at 4°C). ATP, ADP, GTP, and GDP were determined by high performance liquid chromatography (HPLC System Gold, Beckman Coulter) using a QHR5/5 column (Amersham Pharmacia). Nucleotides were eluted with a linear gradient of triethylammonium bicarbonate buffer (4, 20). In addition, changes in ATP levels in intact cells were determined by measuring free Mg2+ concentration using the fluorescent indicator Magnesium Green (23). This technique, validated for assessment of ATP levels in cardiomyocytes, is based on the greater affinity of Mg2+ for ATP than ADP, resulting in an increase in cytosolic Mg2+ concentration upon ATP hydrolysis (23). Activities of adenylate kinase, creatine kinase, nucleoside diphosphate kinase, and pyruvate kinase were measured in cellular extracts by spectrophotometric procedures with a spectrophotometer (DU 7400, Beckman Coulter; refs. 4 and 24). Cells were extracted with 150 mM NaCl/60 mM Tris⋅HCl (pH 7.5)/5 mM EDTA/1 mM phenylmethylsulfonyl fluoride (PMSF)/10 μg/ml leupeptin, 1 μg/ml aprotinin/0.2% Triton X-100 and centrifuged (10,000 × g at 4°C). Adenylate kinase activity was determined in 100 mM K+-acetate/20 mM Hepes, pH 7.5/20 mM glucose/4 mM MgCl2/2 mM NADP+/1 mM EDTA/1 mM DTT/2 mM ADP plus 4.5 units/ml hexokinase and 2 units/ml glucose-6-phosphate dehydrogenase (24). Creatine kinase was measured in 100 mM Tris⋅acetate, pH 7.5/20 mM glucose/2 mM EDTA/10 mM MgCl2/2 mM DTT/2 mM NADP+/2 mM ADP/5 mM AMP/20 mM creatine phosphate plus 20 μM diadenosine pentaphosphate/4.5 units/ml hexokinase/2 units/ml glucose-6-phosphate dehydrogenase (4, 24). Nucleoside diphosphate kinase was measured in 100 mM KCl, 50 mM Tris⋅HCl, pH 7.4/10 mM MgCl2/0.7 mM thymidine diphosphate/4 mM phosphoenolpyruvate plus 2 units/ml pyruvate kinase and lactate dehydrogenase (25). Pyruvate kinase activity was determined in media containing 50 mM imidazole (pH 7.6), 20 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 0.1 mM NADH, 1 mM ADP, 4 mM phosphoenolpyruvate, and 4.5 units/ml lactate dehydrogenase (24).
Electron Microscopy.
Cardiomyocytes, fixed in 0.1 M PBS containing 1% (vol/vol) glutaraldehyde and 4% (vol/vol) formaldehyde (pH 7.2) were postfixed in phosphate-buffered 1% (vol/vol) OsO4, stained en bloc with 2% (vol/vol) uranyl acetate, dehydrated in ethanol and propylene oxide, and embedded in low-viscosity epoxy resin (14). Thin (90-nm) sections were cut on an ultramicrotome (Ultracut E, Reichert), placed on 200-μm mesh copper grids, and stained with lead citrate. Micrographs were taken on an electron microscope (1200 EXII, JEOL) operating at 60 kV.
Statistics.
Results are expressed as mean ± SEM. Statistical analysis was carried out by the Student's t test. Significant difference was accepted at the P < 0.05 level.
Results
Energetics of Nucleocytoplasmic Communication.
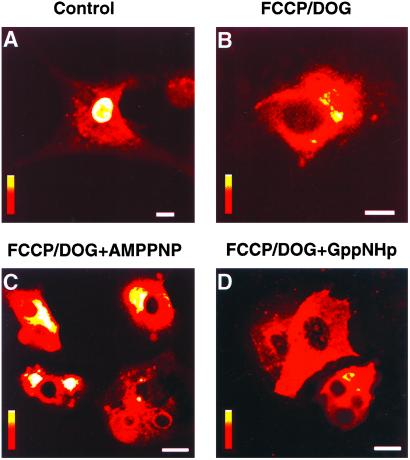
To assess energy requirements for nuclear transport in intact cells, fluorescein-tagged histone 1 (H1) was microinjected in the cytosol of neonatal cardiomyocytes and transport into the nucleus monitored by laser confocal microscopy. In control energetically competent cells, H1 was readily transported into the nucleus, as indicated by pronounced nuclear fluorescence (Fig. 1A). In contrast, in cells in which ATP and GTP were depleted by inhibitors of mitochondrial oxidative phosphorylation [carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP)] and glycolysis [(2-deoxyglucose (DOG)], nuclear transport of H1 was essentially abolished (Fig. 1B). Treatment with 1 μM FCCP plus 6 mM DOG reduced ATP by 86% (from 25.3 ± 1.8 to 3.6 ± 0.3 nmol/mg protein, n = 6; P < 0.001) and GTP by 88% (from 3.20 ± 0.2 to 0.42 ± 0.04 nmol/mg protein, n = 6; P < 0.001). Concomitantly, the nuclear/cytoplasmic fluorescence ratio, an index of nuclear transport (4), decreased from 4.17 ± 0.28 (n = 11) in control to 0.53 ± 0.04 (n = 12) in FCCP/DOG-treated cells, a reduction of 87% (P < 0.001). Microinjection of nonhydrolyzable ATP or GTP analogs, AMP-PNP (pipette solution 0.1 M; Fig. 1C) or GppNHp (pipette solution 0.1 M; Fig. 1D), into ATP/GTP-depleted cells did not rescue import of H1. Up to 3 mM of nonhydrolyzable analogs were introduced in this way into FCCP/DOG-treated cells, yet nuclear transport could not proceed in the absence of a usable energy source. Although discrete steps of nuclear transport may be energy-independent (8, 9), translocation of H1 through nuclear pores seems to require ATP/GTP generating pathways and is not supported by nonhydrolyzable nucleotide analogs.
Figure 1.
Nuclear import of histone H1 requires energy of ATP and is not supported by nonhydrolyzable ATP or GTP analogs. Nuclear transport of fluorescein-tagged histone H1 was monitored by laser confocal microscopy after microinjection of H1 into the cytosol of neonatal cardiomyocytes. Cellular ATP/GTP levels were depleted by a 30-min treatment (37°C) with the mitochondrial uncoupler, FCCP (1 μM), and the inhibitor of glycolysis, DOG (6 mM). H1 is transported in the nucleus of control (A), but not ATP/GTP-depleted (B) cells. Microinjection into ATP/GTP-depleted cells of nonhydrolyzable ATP and GTP analogs, AMPPNP (C) or GppNHp (D), failed to rescue nuclear import of H1. Vertical bars = fluorescence scale of 0–255 arbitrary units, with red indicating lowest and white indicating highest intensity. (A–D) Magnification = 10 μm.
Mitochondrial Oxidative Phosphorylation Required for Nuclear Transport.
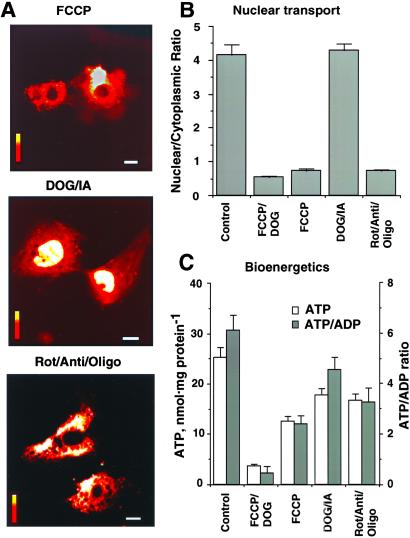
Cardiac cells rely primarily on the energy produced by mitochondrial oxidative phosphorylation (21, 26, 27). In addition, specialized functions including ion transport across the plasma membrane can be supported by ATP generated by glycolysis (16, 28). Although glycolytic enzymes have been identified in nuclei of several cell types where they may furnish a portion of energy requirement (28), the contribution of glycolysis vs. mitochondrial oxidative phosphorylation to nuclear energetics has not been established. To determine the energy supply pathway(s) supporting nuclear transport, transport of H1 into the nucleus was monitored in cardiomyocytes treated with inhibitors of mitochondrial function or glycolysis (Fig. 2A). An uncoupler of mitochondrial oxidative phosphorylation (1 μM FCCP) or a mixture of mitochondrial respiratory chain and F0F1-ATPase inhibitors (rotenone, antimycin, oligomycin, 1 μg/ml each) prevented nuclear accumulation of H1 (Fig. 2A Top and Bottom). Accordingly, the index of nuclear transport, the nuclear/cytoplasmic ratio, was 0.74 ± 0.04 and 0.73 ± 0.10 in FCCP (n = 10) and rotenone/antimycin/oligomycin-treated cells (n = 13), significantly lower from untreated controls (P < 0.001; Fig. 2B). Thus, even a moderate decrease in bulk levels of ATP induced by mitochondrial inhibitors (Fig. 2C) translated into a dramatic reduction in nuclear transport (Fig. 2B). In contrast, inhibitors of glycolysis (6 mM 2-deoxyglucose plus 0.5 mM iodacetate; DOG/IA), reduced intracellular ATP levels (Fig. 2C) but did not affect nuclear transport of H1 (Fig. 2A Middle). In DOG/IA-treated cells, the nuclear/cytoplasmic ratio remained at 4.28 ± 0.18 (n = 19), a value not significantly different from untreated cells (Fig. 2B). Thus, intact mitochondrial oxidative phosphorylation, rather than glycolytic metabolism, is required for nuclear transport of H1. Changes in the ATP/ADP ratio (Fig. 2C), which parallels alterations in the cellular phosphorylation potential (26, 27), indicate that nuclear import of macromolecules is compromised once this ratio drops below the value of 4 in FCCP/DOG, FCCP, or rot/anti/oligo-treated cells, suggesting the existence of an energetic threshold required in support of nuclear transport. Values of energy charge (ATP + 1/2ADP/ATP + ADP + AMP), reflecting the general energy state of a cell (27, 28), were 0.93 ± 0.12 in control vs. 0.43 ± 0.08 (P < 0.001), 0.70 ± 0.11 (P < 0.05), 0.86 ± 0.18, and 0.84 ± 0.14 in FCCP/DOG, FCCP, IA/DOG, and rot/anti/oligo-treated cells, respectively (n = 6 in each experimental condition). Moreover, values of the ATP/CrP ratio also did not correlate with inhibition of nuclear transport and were 2.66 ± 0.22 in control vs. 2.57 ± 0.21, 4.81 ± 0.26 (P < 0.05), 3.77 ± 0.18, and 5.76 ± 0.24 (P < 0.05) in FCCP/DOG, FCCP, IA/DOG, and rot/anti/oligo-treated cells, respectively (n = 6 in each experimental condition). Although normally functioning mitochondria and a maintained cellular phosphorylation potential seem to be a prerequisite for efficient translocation of H1 across the nuclear membrane, it was unclear why following mitochondrial inhibition remaining ATP levels were insufficient to support nuclear transport. This observation suggests a limited access for nucleotide triphosphates to nuclear pores and, ultimately, an inability to sustain proper nucleotide ratios at, and across, the nuclear envelope (14).
Figure 2.
Mitochondrial ATP production but not glycolysis is sufficient to support nuclear transport. (A) Nuclear import of H1 inhibited by an uncoupler of mitochondrial oxidative phosphorylation (Top, 1 μM FCCP) or by a mixture of mitochondrial respiratory chain and F0F1-ATPase inhibitors (Bottom, 1 μg/ml each of rotenone, antimycin, oligomycin; Rot/Anti/Oligo), but not by inhibitors of glycolysis (Middle, 6 mM 2-deoxyglucose plus 0.5 mM iodacetate, DOG/IA). Exposure time to inhibitors was 30 min. Horizontal bars = 10 μm; vertical bars = fluorescence scale of 0–255 arbitrary units as in Fig. 1. (B) Average nuclear/cytoplasmic fluorescence ratio for fluorescein-isothiocyanate-labeled H1 injected into the cytosol of controls and cells treated with FCCP/DOG, FCCP, DOG/IA, and Rot/Anti/Oligo. (C) Cellular ATP levels and ATP/ADP ratios in cardiomyocytes after treatments with mitochondrial or glycolytic inhibitors.
Energy Supply Routes from Mitochondria to the Nucleus.
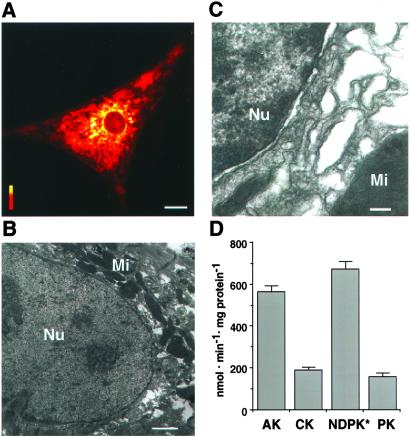
Once generated by mitochondria, ATP is exported into the cytosol and distributed through the cell to maintain adequate nucleotide triphosphate pools (16, 21, 26). Mitochondria, detected here by confocal (Fig. 3A) and electron (Fig. 3B) microscopy, were clustered around the nucleus, indicating the high-energy requirements of nuclear processes and the necessity to keep the distance of energy transfer as short as allowed by cellular architecture. Although mitochondria surrounded the nucleus, the perinuclear zone was filled with membrane structures and proteins (Fig. 3C), creating diffusional hindrances for energy-rich phosphoryl delivery. To reach the nuclear envelope, ATP produced by oxidative phosphorylation would have to traffic from narrow tubular mitochondrial cristae through a crowded perinuclear space. Diffusional restrictions could result in the inability to sustain local ATP/ADP and GTP/GDP ratios required for efficient nuclear transport. Evidence is accumulating that high-energy phosphoryl delivery can be enhanced by phosphotransfer reactions coupling mitochondrial ATP-production with distal cellular sites of ATP-consumption (10, 16–22, 26). Cardiomyocytes expressed a high catalytic activity of phosphotransfer enzymes, adenylate kinase, creatine kinase and pyruvate kinase (Fig. 3D). The respective catalytic activities were 565 ± 21, 191 ± 12, and 158 ± 19 nmol/min/mg protein (n = 5; Fig. 3D). It was noteworthy that, in neonatal cardiomyocytes used here, the activity of creatine kinase, which commonly plays a major role in energy transfer in adult tissue (10, 18, 22), displayed a lower activity than that of adenylate kinase. Although adenylate kinase activity is comparable in neonatal and adult cardiomyocytes, creatine kinase activity is roughly an order of magnitude higher in adult cells (10, 18, 24). In addition, cardiomyocytes possessed a high activity (2020 ± 75 nmol/min/mg protein, n = 5) of nucleoside diphosphate kinase, which is responsible for ATP to GTP conversion and maintenance of GTP/GDP ratios across the nuclear envelope (14). Therefore, clustering of mitochondria around the nucleus along with the high activity of phosphotransfer enzymes could facilitate energy delivery to the nucleus and maintain local ATP/ADP and GTP/GDP ratios required for efficient nuclear transport.
Figure 3.
Mitochondrial distribution and phosphotransfer enzyme activity in cardiac cells. (A) Mitochondria cluster around the nucleus as detected by the mitochondrial marker MitoTracker (0.5 μM, 20-min incubation) using confocal microscopy. Horizontal bar = 10 μm; vertical bar = fluorescence scale of 0–255 arbitrary units. (B and C) Electron micrographs of cardiac cells show mitochondria (Mi) around the nucleus (B, Nu) and a crowded perinuclear space filled with membranes (C). Bars = 1 μm (B) and 200 nm (C). (D) Average catalytic activity of phosphotransfer enzymes expressed in nmol/min/mg protein. AK, adenylate kinase; CK, creatine kinase; NDPK, nucleoside diphosphate kinase; PK, pyruvate kinase. * indicates that the value of NDPK activity should be multiplied by three.
Adenylate Kinase Phosphotransfer Facilitates Energetic Communication Between Mitochondria and the Nucleus.
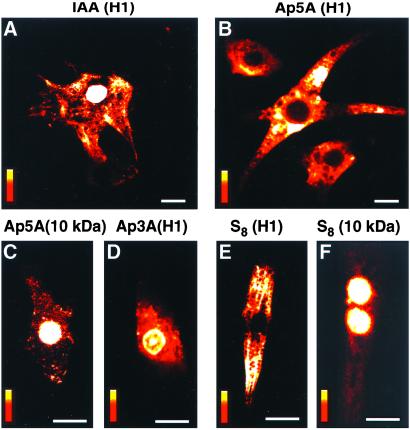
Creatine kinase and adenylate kinase are critical components of the intracellular phosphoryl transfer network responsible for cellular energetic economy and for facilitating signal communication between ATP-generating and ATP-consuming and/or -sensing processes (10, 18, 19, 29, 30). Although both creatine kinase and adenylate kinase are present in cytosolic and nuclear compartments (10, 15, 31, 32), whether these phosphotransfer reactions facilitate energy delivery to nuclear pores is unknown. Here, treatment of cardiomyocytes with iodoacetamide (IAA; 0.5 mM), which inhibits creatine kinase-catalyzed phosphotransfer (10), did not affect nuclear import of H1 (Fig. 4A). In IAA-treated cells, the nuclear/cytoplasmic ratio for H1 was 3.77 ± 0.29 (n = 11), which is not significantly different from untreated controls (Fig. 2B). However, cytosolic microinjection of diadenosine pentaphosphate (Ap5A, pipette solution 0.5 mM), a potent inhibitor of adenylate kinase (33), abolished nuclear transport of H1 (Fig. 4B). The nuclear/cytoplasmic fluorescence ratio for H1 in Ap5A-treated cells was 0.65 ± 0.0.03 (n = 9), indicating deficient nuclear transport. Such microinjection could produce up to 15 μM of intracellular Ap5A, sufficient to inhibit cytosolic and mitochondrial adenylate kinase isoforms (18, 19, 33) without reducing intracellular ATP, as deduced from the absence of increase in Magnesium Green fluorescence (data not shown). Ap5A did not affect passive diffusion of 10-kDa fluorescein-tagged dextrans (Fig. 4C, 10 kDa) and, accordingly, the nuclear/cytoplasmic ratio for 10 kDa was 1.98 ± 0.11 (n = 8), which is not different from untreated cells (2.11 ± 0.08, n = 10), thus ruling out a nonspecific effect of the adenylate kinase inhibitor on nuclear pores. In addition, microinjection of the Ap5A analog, diadenosine triphosphate (Ap3A, pipette solution 0.5 mM), that has no inhibitory effect on adenylate kinase, did not prevent nuclear import of H1 (Fig. 4D). In Ap3A-treated cells, the nuclear/cytoplasmic fluorescence ratio for H1 was 4.08 ± 0.16 (n = 12), which is not significantly different from untreated cells (Fig. 2B). However, treatment of cardiomyocytes with elemental sulfur (10 μM S8), which, like Ap5A, does inhibit adenylate kinase (34), suppressed nuclear transport of H1 (Fig. 4E), and the nuclear/cytoplasmic fluorescence ratio for H1 dropped to 0.78 ± 0.09 (n = 9). In contrast, S8 did not affect passive diffusion of 10-kDa dextrans, and the nuclear/cytoplasmic ratio for 10 kDa was maintained at 2.18 ± 0.12 (n = 7; Fig. 4F). Thus, nuclear transport of H1 is sensitive to structurally diverse agents that have in common the inhibition of adenylate kinase-catalyzed phosphotransfer. These results demonstrate that intact mitochondrial ATP production and simple diffusion-mediated exchange of nucleotides between the cytosol and nucleus are not sufficient for adequate energy support of nuclear processes. Rather, phosphotransfer relays, catalyzed by enzymes such as adenylate kinase, are the required mechanism for facilitated high-energy phosphoryl export from mitochondria, delivery to ATP-utilization sites, and maintenance of local nucleotide ratios across the nuclear envelope.
Figure 4.
Nuclear import of H1 abolished by inhibitors of adenylate kinase. (A) Treatment with 0.5 mM IAA, which inhibits creatine kinase, did not affect nuclear import of H1. (B) Cytosolic microinjection of diadenosine pentaphosphate (Ap5A, 0.5 mM in pipette solution), which inhibits adenylate kinase, abolished nuclear import of H1. (C) Ap5A did not affect transport of 10-kDa dextrans, which occurs by passive diffusion. (D) Microinjection of the Ap5A analog Ap3A (0.5 mM in pipette solution), which does not inhibit adenylate kinase, did not prevent import of H1. (E and F) Treatment with elemental sulfur (0.01 mM S8), which, like Ap5A, inhibits adenylate kinase, inhibited nuclear import of H1 (E) but not passive diffusion of 10-kDa dextrans (F). Bar = 10 μm (A, B) or 20 μm (C–F); vertical bars = fluorescence scale of 0–255 arbitrary units.
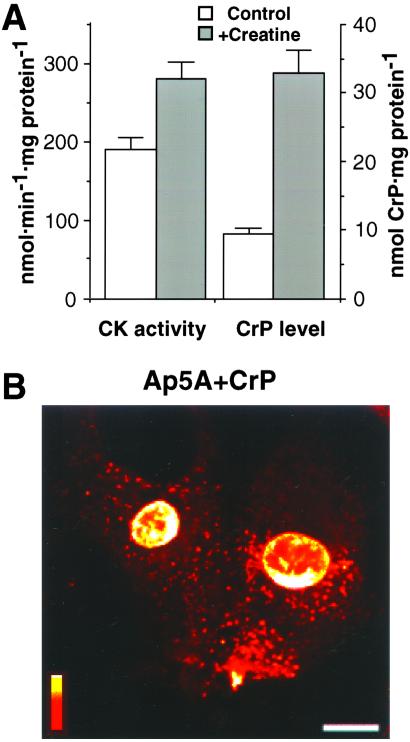
Suppressed Nuclear Transport Under Adenylate Kinase Deficit Rescued Through Up-Regulated Creatine Kinase Phosphotransfer.
Creatine kinase works in parallel with adenylate kinase to promote intracellular metabolic signal transduction (10, 16, 17, 22, 29). As neonatal cardiomyocytes have low creatine kinase activity (Fig. 3D) and a low creatine phosphate content (Fig. 5A), cells were grown for 48 h in media supplemented with 20 mM creatine, which crosses the cell membrane and induces creatine kinase phosphotransfer (10). This procedure up-regulated creatine kinase activity (from 191 ± 12 to 281 ± 23 nmol/min/mg protein in control vs. creatine-treated cardiomyocytes, n = 6) and increased intracellular creatine phosphate levels (from 9.5 ± 1.4 to 33.0 ± 1.7 nmol/mg protein; Fig. 5A) while maintaining ATP levels unchanged (23.2 ± 0.3 and 24.8 ± 0.8 nmol/mg protein), thus generating an adult-like energetic phenotype. In such creatine-treated cardiomyocytes, microinjection of the adenylate kinase inhibitor Ap5A alone or concomitantly with the substrate for creatine kinase creatine phosphate (CrP; 0.5 M in pipette solution) failed to inhibit nuclear transport of H1 (Fig. 5B). In fact, the nuclear/cytoplasmic ratio for H1 was 4.30 ± 0.14 (n = 12), indicating maintained nuclear import. Hence, whereas acute inhibition of adenylate kinase-mediated phosphotransfer compromised active nuclear transport (Fig. 4 B and E), nuclear import of H1 was rescued in cells with up-regulated creatine kinase phosphotransfer (Fig. 5). Therefore, impaired energy delivery and communication between mitochondria and the nucleus because of deficiency of a single phosphotransfer pathway can be rescued by activation of alternate phosphotransfer reactions overcoming perinuclear diffusional restrictions.
Figure 5.
Suppressed nuclear import under adenylate kinase deficit, rescued by activation of creatine kinase phosphotransfer. (A) Creatine kinase (CK) activity and creatine phosphate (CrP) levels in neonatal cardiomyocytes grown without (Control) and with 20 mM creatine (+Creatine). (B) Nuclear import of H1 is maintained in cells grown with creatine after microinjection of the adenylate kinase inhibitor, Ap5A (0.5 mM in pipette solution), in the presence of the creatine kinase substrate CrP (67 mM in pipette solution). Horizontal bar = 10 μm; vertical bar = fluorescence scale of 0–255 arbitrary units.
Discussion
Intense nuclear functions, including regulation of DNA replication and gene transcription, critically depend on efficient transport of molecules across the nuclear envelope (1, 2). Although active nuclear transport is a highly organized process sensitive to metabolic inhibition (2, 4, 14), the principles governing the energetics of nucleocytoplasmic communication are poorly understood (5, 13, 15). Here, we demonstrate that mitochondrial ATP production, rather than glycolysis, is required to support energy-consuming processes at the nuclear envelope. Mitochondrial clustering around the nucleus reduced the distance of energy transfer, yet oxidative phosphorylation and simple nucleotide diffusion were insufficient to meet the energy requirements for nucleocytoplasmic communication. By using nuclear translocation of histone H1 as a prototypic energy-dependent event at the nuclear pore, we identify that adenylate kinase phosphotransfer directed transmission of high-energy phosphoryls from mitochondria to the nucleus, maintaining optimal nucleotide ratios required for active nuclear transport. Adenylate kinase coupled with nucleoside diphosphate kinase secured phosphoryl transfer between ATP and GTP, as both nucleoside triphosphates are necessary for active nuclear transport. Inhibition of nuclear transport in neonatal cardiomyocytes by disruption of the adenylate kinase relay was rescued through up-regulation of the creatine kinase system, which is more active in adult cells, underscoring the fail-safe plasticity of the cellular energetic network. Thus, phosphotransfer reactions are essential in providing energy for nuclear processes that are spatially separated from mitochondrial sites of energy transduction.
A mechanistic basis for thermodynamically efficient coupling of cell energetics with nuclear pore function lies in the unique property of adenylate kinase catalysis (ATP + AMP ↔ 2ADP) which transfers both β- and γ-phosphoryls of ATP, doubling the energetic potential of ATP as an energy-carrying molecule (16–19, 29). Isoforms of adenylate kinase have been found in mitochondria, cytosol, and membranes (18, 19, 29, 31) and are apparently also present in the nucleus (32) creating an integrated phosphotransfer network. Phosphorelays composed of adenylate kinase isoforms facilitate high-energy phosphoryl delivery to sites of ATP-utilization, and thus increase the energetic efficiency of a cell (16, 18, 29, 30). An intimate relationship of adenylate kinase with mitochondrial respiration, myofibrillar and membrane ATPases, as well as metabolic sensors such as ATP-sensitive K+ channels, have been demonstrated in diverse cell types, including cardiomyocytes, where such tight coupling secures efficient regulation of energy metabolism, membrane excitability, and cell contraction (16–20, 29). Genetic deletion of adenylate kinase impedes ATP export from mitochondria, reduces the cellular energetic economy, and increases cell vulnerability to injury (30, 35, 36). The present study reveals a previously uncharacterized property of adenylate kinase phosphotransfer in catalyzing energy support of nuclear transport. This property is expected to be particularly significant in cell-types with low or absent creatine kinase activity, as shown here in neonatal cardiomyocytes.
Assigning such a role to adenylate kinase implicates phosphotransfer enzymes in the regulation of information exchange between the cytosol and nucleus. Specifically, the mitochondrial AK2 isoform, present in the inter-membrane space, facilitates both production and export of ATP by mitochondria (16, 26). In turn, the cytosolic AK1 isoform, through sequential phosphotransfers, is responsible for the transmission of ATP and the maintenance of the ATP/ADP ratio at ATP-utilization sites (18, 19, 29). Moreover, by providing ATP, adenylate kinase phosphotransfer would facilitate the nucleoside diphosphate kinase reaction (37, 38), which is required for generation of the GTP/GDP gradient across the nuclear membrane and regulation of GTP/GDP exchange, which is essential in nuclear transport (5, 39). In fact, the high activity of nucleoside diphosphate kinase, found here in cardiac cells, could provide effective phosphoryl exchange between ATP and GTP at the nuclear and mitochondrial compartment, where specific nucleoside diphosphate kinase isoforms are expressed (38). Disruption of the phosphoryl transfer network by acute inhibition of adenylate kinase by using Ap5A, an inhibitor of adenylate kinase isoforms (33), compromised active nuclear transport without significantly reducing cellular ATP levels. A structurally unrelated inhibitor of adenylate kinase, S8, but not the inactive analog of Ap5A, Ap3A, also arrested nuclear translocation of H1, suggesting that the action of Ap5A is ,indeed, caused by the inhibition of adenylate kinase. Defective nuclear transport in the presence of preserved ATP levels suggests that transport of macromolecules across the nuclear envelope does require maintained nucleotide ratios and a high free energy of ATP hydrolysis (22). Concerted hydrolysis of ATP at the nuclear pore is believed to drive vectorial conformational changes facilitating nuclear transport, with 8–10 molecules of ATP required for a single translocation (40–42). Moreover, the energy of ATP transformed into the chemical potential of the ran-GTP/GDP gradient across the nuclear envelope would contribute to the driving force for import and export of macromolecules (2, 39). Thus, adenylate kinase coupled with nucleoside diphosphate kinase assures proper adenine and guanine nucleotide ratios at the nuclear membrane, keeping the free energy of ATP hydrolysis above the threshold required for active nuclear transport.
Impaired nuclear import of H1 in neonatal cells with inhibited adenylate kinase was rescued by induction of the alternate phosphotransfer pathway catalyzed by creatine kinase, which is highly expressed in adult cells and regulates both adenine and guanine nucleotide ratios (10, 15). Indeed, whereas adenylate kinase is highly active in neonatal cardiomyocytes, in the adult myocardium, phosphotransfer is primarily catalyzed through creatine kinase (18, 27, 43). A remarkable plasticity of the phosphotransfer system has been recently recognized, where deficiency in an individual enzyme is compensated through remodeling of the cellular energetic network (16, 20, 27, 30, 36). Such complementation among phosphotransfer systems and tight integration with mitochondrial energetics is used under different stages of development and/or states of metabolic demand to provide adequate energy support for the fluctuating needs of cellular processes (10, 16, 26, 30, 43–45). Variations of phosphotransfer enzyme activity in the cytosol and nucleus correlate with the intensity of nuclear processes in normal and diseased conditions, underscoring the significance of maintained phosphotransfer in directing cellular energy flow (14, 15, 28, 32, 43, 45).
In summary, this study identifies the energy supply pathways that maintain efficient nucleocytoplasmic communication, a vital component in the regulation of cellular functions. We demonstrate that coupled mitochondrial oxidative phosphorylation with catalyzed phosphotransfer is the minimal energetic unit required to support nuclear transport. Disrupted phosphotransfer compromises macromolecular trafficking and, thereby, information exchange between the cytosol and nucleus. Thus, integration of the nuclear compartment with mitochondrial energetics to secure the energy demands of intense nuclear processes is accomplished through specialized enzymatic networks catalyzed by adenylate kinase and/or creatine kinase, the significance of which depends on the developmental stage of the cell.
Acknowledgments
This work was supported by the American Heart Association, National Institutes of Health, Clinician-Investigator Program at the Mayo Clinic, Miami Heart Research Institute, Bruce and Ruth Rappaport Program in Vascular Biology and Gene Delivery, and the Marriott Foundation. A.T. is an Established Investigator of the American Heart Association.
Abbreviations
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- DOG
2-deoxyglucose
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 2.Gorlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham D E. Science. 1996;273:1875–1877. doi: 10.1126/science.273.5283.1875. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Terzic C, Gacy A M, Bortolon R, Dzeja P P, Puceat M, Jaconi M, Prendergast F G, Terzic A. Circ Res. 1999;84:1292–12301. doi: 10.1161/01.res.84.11.1292. [DOI] [PubMed] [Google Scholar]
- 5.Talcott B, Moore M S. Trends Cell Biol. 1999;9:312–318. doi: 10.1016/s0962-8924(99)01608-6. [DOI] [PubMed] [Google Scholar]
- 6.Schroder H C, Rottmann M, Bachmann M, Muller W E G. J Biol Chem. 1986;261:663–668. [PubMed] [Google Scholar]
- 7.Schmitt C, von Kobbe C, Bachi A, Pante N, Rodrigues J P, Boscheron C, Rigaut G, Wilm M, Seraphin B, Carmo-Fonseca M, Izaurralde E. EMBO J. 1999;18:4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englmeier L, Olivo J C, Mattaj I W. Curr Biol. 1999;9:30–41. doi: 10.1016/s0960-9822(99)80044-x. [DOI] [PubMed] [Google Scholar]
- 9.Ribbeck K, Kutay U, Praskeva E, Gorlich D. Curr Biol. 1999;9:47–50. doi: 10.1016/s0960-9822(99)80046-3. [DOI] [PubMed] [Google Scholar]
- 10.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger H M. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer T. Histochem Cell Biol. 2000;113:455–465. doi: 10.1007/s004180000147. [DOI] [PubMed] [Google Scholar]
- 12.Hetzer M, Mattaj I W. J Cell Biol. 2000;148:293–303. doi: 10.1083/jcb.148.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izaurralde E, Adam S. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Terzic C, Gacy A M, Bortolon R, Dzeja P, Puceat M, Jaconi M, Prendergast F G, Terzic A. J Biol Chem. 2001;276:20566–20571. doi: 10.1074/jbc.M101950200. [DOI] [PubMed] [Google Scholar]
- 15.Manos P, Bryan G K. Dev Neurosci. 1993;15:271–279. doi: 10.1159/000111344. [DOI] [PubMed] [Google Scholar]
- 16.Dzeja P P, Zeleznikar R J, Goldberg N D. Mol Cell Biochem. 1998;184:169–182. [PubMed] [Google Scholar]
- 17.Bessman S P, Carpenter C L. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- 18.Dzeja P P, Vitkevicius K T, Redfield M M, Burnett J C, Terzic A. Circ Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco A J, Dzeja P P, Alekseev A E, Pucar D, Zingman L V, Abraham M R, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Proc Natl Acad Sci USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzeja P P, Zeleznikar R J, Goldberg N D. J Biol Chem. 1996;271:12847–12851. doi: 10.1074/jbc.271.22.12847. [DOI] [PubMed] [Google Scholar]
- 21.Saks V A, Tiivel T, Kay L, Novel-Chate V, Daneshrad Z, Rossi A, Fontaine E, Keriel C, Leverve X, Ventura-Clapier R, et al. Mol Cell Biochem. 1996;160–161:195–208. doi: 10.1007/BF00240050. [DOI] [PubMed] [Google Scholar]
- 22.Tian R, Ingwall J S. Am J Physiol. 1996;270:H1207–H1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 23.Leyssens A, Nowicky A V, Patterson L, Crompton M, Duchen M R. J Physiol. 1996;496:111–128. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzeja P P, Pucar D, Redfield M, Burnett J C, Terzic A. Mol Cell Biochem. 1999;201:33–40. doi: 10.1023/a:1007016703229. [DOI] [PubMed] [Google Scholar]
- 25.Biggs J, Hersperger E, Steeg P S, Liotta L A, Shearn A. Cell. 1990;63:933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- 26.Dzeja P P, Redfield M, Burnett J C, Terzic A. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- 27.Saupe K W, Spindler M, Tian R, Ingwall J S. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 28.Ottaway J H, Mowbray J. Curr Top Cell Reg. 1977;12:107–208. doi: 10.1016/b978-0-12-152812-6.50010-x. [DOI] [PubMed] [Google Scholar]
- 29.Dzeja P P, Kalvenas A, Toleikis A, Praskevicius A. Biochem Int. 1985;10:259–265. [PubMed] [Google Scholar]
- 30.Janssen E, Dzeja P P, Oerlemans F, Simonetti A W, Heerschap A, de Haan A, Rush P S, Terjung R R, Wieringa B, Terzic A. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanabe T, Yamada M, Noma T, Kajii T, Nakazawa A. J Biochem. 1993;113:200–207. doi: 10.1093/oxfordjournals.jbchem.a124026. [DOI] [PubMed] [Google Scholar]
- 32.Criss W E. J Biol Chem. 1970;245:6352–6356. [PubMed] [Google Scholar]
- 33.Lienhard G E, Secemski I I. J Biol Chem. 1973;248:1121–1123. [PubMed] [Google Scholar]
- 34.Conner J, Russell P J. Biochem Biophys Res Commun. 1983;113:348–352. doi: 10.1016/0006-291x(83)90472-2. [DOI] [PubMed] [Google Scholar]
- 35.Pucar D, Janssen E, Dzeja P P, Juranic N, Macura S, Wieringa B, Terzic A. J Biol Chem. 2000;275:41424–41429. doi: 10.1074/jbc.M007903200. [DOI] [PubMed] [Google Scholar]
- 36.Bandlow W, Storable G, Zoglowek C, Oechner U, Magdolen V. Eur J Biochem. 1988;178:451–457. doi: 10.1111/j.1432-1033.1988.tb14469.x. [DOI] [PubMed] [Google Scholar]
- 37.Mathews C K. Prog Nucl Acid Res Mol Biol. 1993;44:167–203. doi: 10.1016/s0079-6603(08)60220-2. [DOI] [PubMed] [Google Scholar]
- 38.Ouatas T, Selo M, Sadji Z, Hourdr J, Denis H, Mazabraud A. Int J Dev Biol. 1998;42:43–52. [PubMed] [Google Scholar]
- 39.Nachury M V, Weis K. Proc Natl Acad Sci USA. 1999;96:9622–9627. doi: 10.1073/pnas.96.17.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahin V, Danker T, Enss K, Ossig R, Oberleithner H. FASEB J. 2001;15:1895–1901. doi: 10.1096/fj.00-0838com. [DOI] [PubMed] [Google Scholar]
- 41.Breeuwer M, Goldfarb D S. Cell. 1990;60:999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- 42.Hanover J A. FASEB J. 1992;6:2288–2295. doi: 10.1096/fasebj.6.6.1312045. [DOI] [PubMed] [Google Scholar]
- 43.Pucar D, Dzeja P P, Bast P, Juranic N, Macura S, Terzic A. J Biol Chem. 2001;276:44812–44819. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- 44.Weiss J N, Korge P. Circ Res. 2001;89:108–110. [PubMed] [Google Scholar]
- 45.Dzeja P P, Holmuhamedov E L, Ozcan C, Pucar D, Jahangir A, Terzic A. Circ Res. 2001;89:744–746. [PubMed] [Google Scholar]