Abstract
Background
Fistula formation has been reported in intraductal papillary-mucinous neoplasms (IPMNs) with or without invasion of the adjacent organs. The presence or absence of invasion is mostly determined by postoperative histological examination rather than by preoperative work-up.
Case presentation
A 72 year-old Japanese woman showed remarkable dilatation of the main pancreatic duct (MPD) in the distal region of the pancreas. Subsequent ERCP also showed MPD dilatation, after which the patient suffered moderate pancreatitis. A subsequent gastroscopy revealed a small ulceration that had not been observed in a gastroscopy performed 3 months prior. Mucinous discharge from the ulceration suggested it might be the orifice of a fistula connected to the MPD. En bloc resection including the distal region of the pancreas, spleen, stomach and part of the transverse colon was performed under the pre- and intraoperative diagnosis of an invasive malignant IPMN. However, histopathology revealed the lesion to be of "borderline malignancy" without apparent invasion of the stomach. Light microscopy showed inflammatory cellular infiltrates (mainly neutrophils) around the pancreatogastric fistula, but there was no evidence of neoplastic epithelia lining the fistulous tract.
Conclusion
This case highlights that a pancreatogastric fistula can develop after acute inflammation of the pancreas in the absence of cancer invasion. Further information regarding IPMN-associated fistulae is necessary to clarify the pathogenesis, diagnosis, appropriate surgical intervention and prognosis for this disorder.
Background
Intraductal papillary-mucinous neoplasms (IPMNs) of the pancreas have unique clinico-pathological characteristics and show a wide spectrum of histological types, ranging from adenomatous hyperplasia to invasive cancer. While non-invasive IPMNs show slow growth and good prognosis after resection, the outcome can become poor if they transform into invasive ductal cancers. There are three types of IPMN: main duct,, branch, and combined. The main duct and combined types are associated with higher rates of invasive cancer than the branch type [1].
A feature of IPMNs is occasional fistula formation with surrounding organs such as the duodenum, bile ducts, stomach and even the peritoneal or pleural cavity [2-10]. While such fistula formation is generally thought to be associated with invasive IPMNs, some non-invasive IPMNs also form fistulas. Thus, IPMN fistula formation raises intriguing issues concerning pathogenesis, diagnosis and patient prognosis.
We report here a rare non-invasive "borderline-malignant" IPMN that formed a pancreatogastric fistula. Fistula formation was triggered by acute pancreatitis following endoscopic retrograde cholangiopancreatography (ERCP).
Case presentation
A 72 year-old Japanese woman underwent abdominal ultrasonography (US) as part of a routine physical checkup in April 2001. This procedure incidentally detected marked dilatation of the main pancreatic duct (MPD) in the distal region of the pancreas. Magnetic resonance cholangiopancreatography (MRCP) and abdominal computed tomography (CT) showed MPD dilatation and a multi-lobular cystic lesion. ERCP confirmed these findings, and no extraluminal leakage of the contrast medium was observed (Figure 1). Cytology on pancreatic fluid indicated non-neoplastic epithelia. Endoscopic ultrasonography (EUS) identified 8 mm papillary projections in the cystic lesion, which was a key indication for surgery. A routine gastroscopy performed simultaneously with EUS showed no significant findings.
Figure 1.
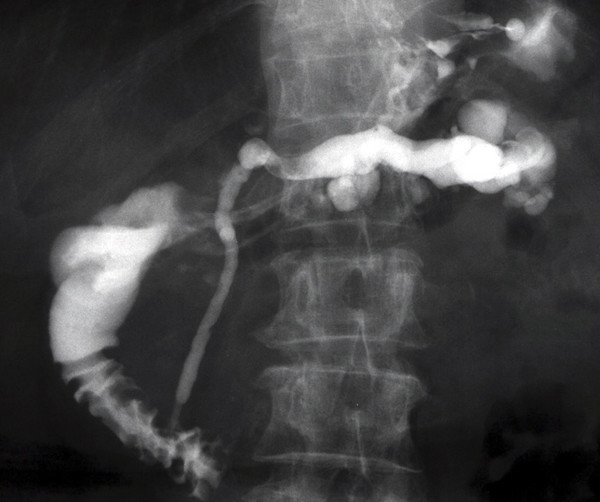
ERCP on presentation showing a dilated MPD and cystic lesions in the distal region of the pancreas.
Following the ERCP, the patient complained of abdominal pain and showed hyperamylasemia and leukocytosis. Distinct swelling of the pancreas and thickening of the gastric wall were subsequently observed by abdominal CT (Figure 2), which indicated a mild post-ERCP pancreatitis. Gastroscopy performed for the assessment of gastric wall thickness revealed a small ulceration in the body of the stomach (Figure 3), which was accompanied by a mucinous discharge suggesting the presence of a fistula opening into the MPD. These findings, along with abdominal angiography data indicating encasement of the splenic artery suggested a malignant invasion into the stomach by the primary lesion. All other laboratory data, including tumor marker expression (e.g., CEA and CA19-9), were within normal limits.
Figure 2.
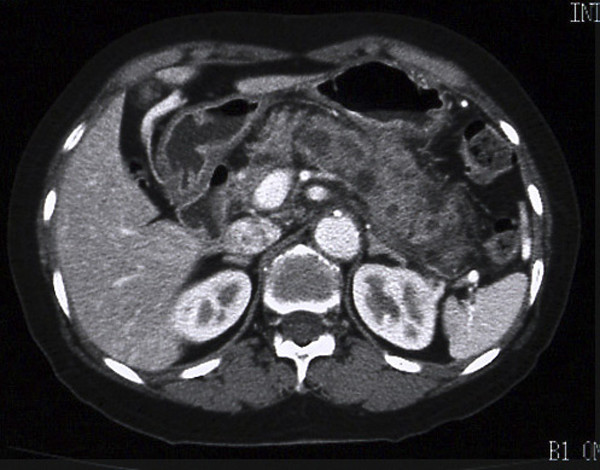
Abdominal CT just prior to surgery showing distinct swelling of the pancreas and the gastric wall, causing the border between the two organs to be unclear.
Figure 3.
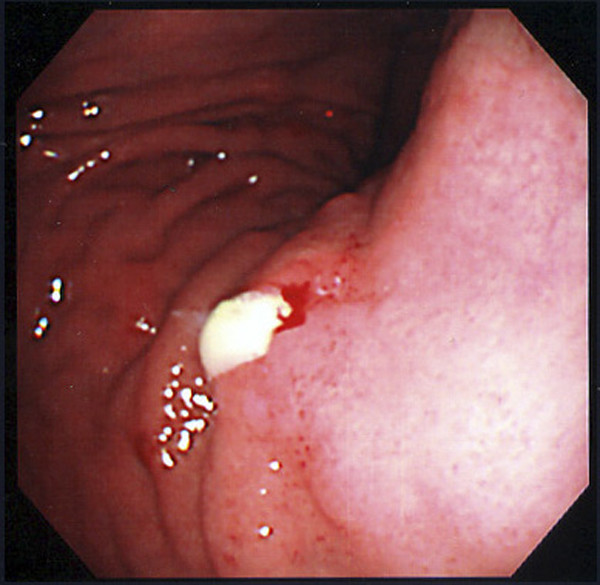
Gastroscopy just prior to surgery showing a small ulcer producing a mucinous discharge into the body of the stomach.
A laparotomy was performed 1 month after the ERCP. Firm fibrous tissue between the pancreas and the mesenterium of the transverse colon suggested the pancreatic neoplasm was of an invasive nature. An en bloc resection of the distal pancreas, spleen, stomach and part of the transverse colon was performed. Postoperative histopathology showed the distal pancreas had an IPMN whose lining epithelia showed only "borderline-malignancy" and no apparent invasion of adjacent tissues or organs. Light microscopy examination revealed inflammatory cellular infiltration consisting mainly of neutrophils around a pancreatogastric fistula which had no neoplastic epithelia (Figure 4). The patient was discharged after an uneventful postoperative recovery and showed no signs of recurrence 3 years after surgery.
Figure 4.
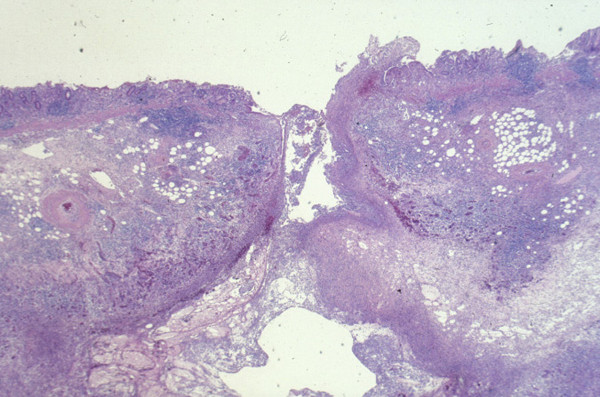
A cross-section of the pancreatogastric fistula (Hematoxylin and Eosin stain × 40). The fistula was significantly infiltrated with neutrophils and showed no evidence of neoplastic infiltration.
Discussion
Fistula formation associated with an IPMN was first reported by Ohhashi et al in 1980 as a pancreatobiliary fistula [2]. Since then there have been 41 cases reported between 1980 and 2004 (Table 1) [3-9]. According to these reports, the organs most frequently affected by fistula formation were the duodenum (24 cases, 59%), common bile duct (21 cases, 51%) and stomach (7 cases, 17%). Apparent cancer invasion was the main cause of the fistulae in 16 cases (37%), while similar to the present case, 17 cases (41%) showed fistulae into neighboring organs in the absence of tumor invasion (Table 2). Five of the six previous IPMN cases associated with pancreatogastric fistulae were linked to apparent cancer invasion.
Table 1.
Published cases of developing fistulae from IPMNs (1980–2004)
| No. | Author | Year | Affected Organs | Cancer Invasion | Pathological Diagnosis |
| 1 | Ohhashi [2] | 1980 | C | - | cystadenocarcinoma |
| 2 | Koike [3] | 1980 | C, D | unknown | pap |
| 3 | Sukisaki [3] | 1984 | S, D | + | pap (+ muc) |
| 4 | Usui [3] | 1985 | D | - | pap |
| 5 | Suyama [3] | 1986 | C | + | pap |
| 6 | Suyama [3] | 1986 | D | + | pap |
| 7 | Yamao [3] | 1986 | D | unknown | pap |
| 8 | Yamao [3] | 1986 | C | unknown | pap |
| 9 | Yamao [3] | 1986 | C | unknown | pap |
| 10 | Miyagawa [3] | 1988 | C, D | + | pap (+ muc) |
| 11 | Kuga [3] | 1988 | D | - | pap |
| 12 | Tohyama [3] | 1989 | C | - | pap |
| 13 | Nakajima [3] | 1989 | C | - | pap |
| 14 | Ohnuma [3] | 1990 | S | + | pap (+ muc) |
| 15 | Sakimoto [3] | 1991 | C | + | pap (+ muc) |
| 16 | Mayumi [3] | 1991 | C, D | + | muc (+ pap) |
| 17 | Hayashi [3] | 1991 | C | unknown | cystadenocarcinoma |
| 18 | Oda [3] | 1993 | C, D | - | pap |
| 19 | Saitoh [3] | 1993 | C | - | pap |
| 20 | Kobayashi [4] | 1993 | S | + | muc (+ pap) |
| 21 | Kobayashi [4] | 1993 | S, D | + | muc (+ pap) |
| 22 | Kobayashi [4] | 1993 | C, D | unknown | pap |
| 23 | Kobayashi [4] | 1993 | D | unknown | pap |
| 24 | Kobayashi [4] | 1993 | D | unknown | muc (+ pap) |
| 25 | Mori [3] | 1994 | C | + | pap |
| 26 | Ieda [3] | 1995 | D | + | tub |
| 27 | Yago [5] | 1995 | S | - | pap |
| 28 | Takekuma [3] | 1996 | D | - | pap |
| 29 | Nakamura [3] | 1996 | C, D | + | pap |
| 30 | Murata [3] | 1996 | C, D | - | pap |
| 31 | Hirota [3] | 1996 | D | - | pap |
| 32 | Tadokoro [6] | 1996 | D | - | unknown |
| 33 | Nakatsuka [3] | 1997 | S, D | + | muc |
| 34 | Matsubayashi [7] | 1998 | D | + | pap |
| 35 | Kawaharada [3] | 1999 | D | + | pap |
| 36 | Kawaharada [3] | 1999 | D | + | pap |
| 37 | Shiroko [8] | 2000 | C, D | - | unknown |
| 38 | Kurihara [9] | 2000 | C | - | pap |
| 39 | Kurihara [9] | 2000 | C | - | pap |
| 40 | Fujisawa [3] | 2001 | C | - | pap |
| 41 | Koizumi(present case) | 2004 | S | - | low grade malignancy |
Affected Organ; C: Common bile duct, D: Duodenum, S: Stomach
Pathological Diagnosis; pap: papillary carcinoma, muc: mucinous carcinoma
tub: tubular adenocarcinoma
Table 2.
Organs developing IPMN-associated fistulae (Revised from Table 1)
| Cancer invasion | ||||
| Affected organs | Case | + | - | unknown |
| C | 13 | 3 | 7 | 3 |
| D | 13 | 5 | 5 | 3 |
| C and D | 8 | 3 | 3 | 2 |
| S | 4 | 2 | 2 (this case) | 0 |
| S and D | 3 | 3 | 0 | 0 |
| Total | 41 | 16 | 17 | 8 |
Affected Organ; C: Common bile duct, D: Duodenum, S: Stomach
To our knowledge, the present report is only the second describing a case of IPMN associated with pancreatogastric fistulae without invasive cancer. Yago et al reported a rare case of non-invasive intraductal papillary adenocarcinoma of the pancreas that was associated with development of a pancreatogastric fistula in the absence of cancer invasion [5]. In that case, neoplastic epithelia were observed only on the surface of the fistula and gastric mucosa, while the structure of the gastric wall beside the fistula was maintained without invasive cancer. Those authors speculated that the pancreatogastric fistula developed due to high pressure in the MPD. Baek et al and Watanabe et al reported the same phenomenon in mucinous cystic tumors (MCTs) [11,12]. The mechanism of such fistula formation without cancer spread could be explained by a combination of high pressure in the MPD and inflammatory stimulation [3,4,13].
In the present case, ERCP played a key role in fistula formation. To our knowledge, this is the first report to show an absence of a pancreatogastric fistula immediately prior to ERCP, and then the presence of a fistula following post-ERCP pancreatitis. Post-ERCP pancreatitis, which is reported to occur in approximately 1.8–10% of patients, should be carefully investigated in IPMN cases [14,15].
We emphasize here that development of an IPMN-associated fistula does not necessarily represent underlying invasive cancer. Fistula formation can result in an incorrect preoperative diagnosis of invasive cancer and lead to the undertaking of unnecessary surgical procedures. Although the lesion in the present case was a non-invasive tumor, surgery was appropriate given the indications of invasive cancer. While non-invasive IPMNs have a good prognosis, there remains the possibility of dissemination by tumor penetration. When treating IPMNs associated with fistula, the extent of resection should depend on the extent of cancer invasion. To avoid dissemination, the fistula should be removed independent to cancer invasion. The extent of resection of affected organs should be individually determined based on the results of preoperative images and precise intraoperative histological examinations.
Conclusion
The present case highlights that a pancreatogastric fistula can develop in the absence of invasive cancer. Further data regarding IPMN-associated fistulae are necessary in order to clarify the pathogenesis, diagnosis, appropriate surgical intervention and prognosis for this disorder.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MK, NS, KY, MT, KK, YY and HN were involved in performing surgery, undertook the literature search and drafted the manuscript for submission.
HN supervised preparation of the manuscript and edited the final version for publication. All authors read and approved the manuscript.
Acknowledgments
Acknowledgements
Patient consent was obtained for publication of case records, MRCP, abdominal CT, ERCP, US, gastroscopy and histopathological images.
Contributor Information
Masaru Koizumi, Email: mkoizumi@jichi.ac.jp.
Naohiro Sata, Email: sata@jichi.ac.jp.
Koji Yoshizawa, Email: k-yoshi@jichi.ac.jp.
Munetoshi Tsukahara, Email: m-tsuka@jichi.ac.jp.
Katsumi Kurihara, Email: kkurihar@jichi.ac.jp.
Yoshikazu Yasuda, Email: yyasuda@jichi.ac.jp.
Hideo Nagai, Email: nagaihd@jichi.ac.jp.
References
- Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228:685–691. doi: 10.1097/00000658-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhashi K, Tajiri H, Gondo M, Yokoyama Y, Maruyama M, Takekoshi T, Matsuura Y, Kasumi F, Takagi K, Kato Y. A case of cystadenocarcinoma of the pancreas forming bilio-pancratic fistula. Progress of Digestive Endoscopy. 1980;17:261–264. [Google Scholar]
- Fujisawa T, Osuga T, Mita M, Sakamoto N, Sakaguchi K, Onishi Y, Toyoda M, Maeda M, Kawase Y, Nishigami T. Intraductal papillary adenocarcinoma of the pancreas accompanied by biliopancreatic fistula; report of a case. Ganno Rinsyo (Jpn J Cancer Clin) 2001;47:144–150. [Google Scholar]
- Kobayashi G, Fujita N, Noda Y, Kimura K, Watanabe H, Chonan A, Matsunaga A, Yuki T, Ando M, Sato Y, Tominaga G, Mochizuki F, Yamazaki T. Study of cases of mucin producing tumors of the pancreas showing penetration of other organs. Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterology) 1993;90:3081–3089. [PubMed] [Google Scholar]
- Yago A, Fujita N, Noda Y, Kobayashi G, Kimura K, Mochizuki F, Yamazaki T, Ikeda T. A case of non-invasive intraductal papillary adenocarcinoma of the pancreas showing penetration of the stomach. Fukubu Gazo Shindan. 1995;15:496–500. [Google Scholar]
- Tadokoro H, Toki F, Yoshida K, Nishino T, Kojima S, Shiratori K, Watanabe S, Kozu T, Takeuchi T, Hayashi N, Imaizumi T, Suzuki S. Two cases of pancreaticoduodenal fistula. Gastroenterological Endoscopy. 1996;38:1208–13. [Google Scholar]
- Matsubayashi H, Kokuno S, Itoi T, Mizumura Y, Niki S, Takeda K, Onoda K, Ogiwara M, Ohno H, Horibe T, Miwa K, Shinohara Y, Magami Y, Niido T, Seki T, Saitoh T. A case of intraductal papillary adenocarcinoma of the pancreas with pancreatico-duodenal fistula. Progress of Digestive Endoscopy. 1998;52:180–181. [Google Scholar]
- Shiroko J, Takai S, Yoshikawa T, Ohnishi T, Nakai M, Asano H, Tanabashi S, Kametani M, Okamoto K. A case of Mucin-producing pancreatic cancer penetrating to the common bile duct and the duodenum. Ganno Rinsyo (Jpn J Cancer Clin) 2000;46:967–971. [Google Scholar]
- Kurihara K, Nagai H, Kasahara K, Kanazawa K, Kanai N. Biliopancreatic fistula associated with intraductal papillary-mucinous pancreatic cancer: institutional experience and review of the literature. Hepato-Gastroenterology. 2000;47:1164–1167. [PubMed] [Google Scholar]
- Inoue M, Ikeda Y, Kikui M, Ogawa T, Yasumitsu T. Mucin-producing tumor of the pancreas associated with pyothorax: report of a case. Surg Today. 2001;31:538–541. doi: 10.1007/s005950170118. [DOI] [PubMed] [Google Scholar]
- Baek Y, Midorikawa T, Nagasaki H, Kikuchi H, Kitamura N, Takeuchi S, Koh Y, Yagi H, Yoshizawa Y, Kumada N. A case report of pancreatic mucinous cystadenocarcinoma with penetration to the stomach. Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterology) 1999;96:685–690. [PubMed] [Google Scholar]
- Watanabe N, Hasegawa H, Tsuneya Y, Kumazawa M, Baba M, Kusano I, Watanabe M, Yamao Y. A case of mucinous cystadenocarcinoma with a fistula between cyst and stomach. Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterology) 2003;100:349–353. [PubMed] [Google Scholar]
- Fukushima N, Mukai K. Mechanism of invasion through the duct wall by intraductal papillary adenocarcinoma of the pancreas. Tan to Sui (J Bil Panc) 1999;20:17–20. [Google Scholar]
- Pannu HK, Fishman EK. Complications of endoscopic retrograde cholangiopancreatography: Spectrum of abdominalities demonstrated with CT. Radio Graphics. 2001;21:1441–1453. doi: 10.1148/radiographics.21.6.g01nv101441. [DOI] [PubMed] [Google Scholar]
- Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A, Prada A, Passoni GR, Testoni PA. Complications of diagnostic and therapeutic ERCP: A prospective multicenter study. Am J Gastroenterol. 2001;96:417–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]


