Abstract
Polycystin-1 (PC-1) is the product of the PKD1 gene, which is mutated in autosomal dominant polycystic kidney disease. We show that the Na,K-ATPase α-subunit interacts in vitro and in vivo with the final 200 amino acids of the polycystin-1 protein, which constitute its cytoplasmic C-terminal tail. Functional studies suggest that this association may play a role in the regulation of the Na,K-ATPase activity. Chinese hamster ovary cells stably expressing the entire PC-1 protein exhibit a dramatic increase in Na,K-ATPase activity, although the kinetic properties of the enzyme remain unchanged. These data indicate that polycystin-1 may contribute to the regulation of Na,K-ATPase activity in kidneys in situ, thus modulating renal tubular fluid and electrolyte transport.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a common human genetic disorder. ADPKD is characterized by the expansion of fluid-filled cysts within the renal tubules, causing progressive impaired renal function, and ultimately kidney failure (Gabow, 1993). Mutations in the PKD1 gene account for most (85–90%) of the cases of ADPKD.
The predicted product of the PKD1 gene is polycystin-1 (PC-1), which is an extremely large glycoprotein with a molecular mass exceeding 460 kDa (Wilson, 2004a,b). The polycystin-1 protein includes a very long N-terminal extracellular domain, which contains several motifs that may play a role in cell–cell or cell–matrix interactions. The fulllength PC-1 protein is predicted to span the membrane 11 times and contains an ∼200-amino acid cytosolic C-terminal domain (Wilson, 2004). PC-1 exhibits a complicated spatial and temporal pattern of expression in the kidney (Chauvet et al., 2002). Its subcellular localization includes, although is not limited to, the basolateral plasma membrane domains of renal epithelial cells (Wilson, 2004).
The short cytoplasmic tail of the PC-1 protein may participate in multiple signaling pathways. PC-1 expression has been shown to affect signaling through both the Wnt (Kim et al., 1999) and AP-1 pathways (Arnould et al., 1998). In addition, studies using overexpression systems suggest that the polycystin-1 C-terminal region may directly participate in the regulation of ion transport processes. For example, expression of the most distal fragment of the PC-1 cytosolic tail protein in Xenopus oocytes up-regulates a calcium-permeable cation channel (Vandorpe et al., 2001). In addition, overexpression of the entire cytosolic tail in cortical collecting duct cells prolongs the duration of ATP-stimulated chloride conductance and the associated intracellular calcium response (Wildman et al., 2003).
The renal cyst expansion characteristic of ADPKD is currently thought to involve increased cell proliferation. Fluid accumulation in the lumen of the cysts seems to be associated with the conversion of the phenotype of solute reabsorption observed in normal renal kidney epithelial cells to one of net ion secretion in the cyst lining cells (Sullivan et al., 1998). Although the events leading to cyst formation and expansion in ADPKD have been studied extensively, the precise cellular and molecular mechanisms through which mutations in the PKD-1 gene induce these transformations remain largely unclear.
Solute and fluid transport in the kidney is intimately related to the establishment of ion gradients. These gradients are primarily achieved through the action of an ubiquitous heterodimeric ion pump, the Na,K-ATPase (Lingrel, 1992). During its catalytic cycle, this pump uses the energy derived through the hydrolysis of ATP to transport sodium and potassium ions against their electrochemical gradients (Dunbar and Caplan, 2001). The α-subunit of the Na,K-ATPase constitutes the catalytic core of the enzyme. This subunit spans the membrane 10 times, and its cytoplasmic domains are involved in ATP binding and hydrolysis.
The expression of the sodium pump in renal polarized epithelial cells is restricted to the basolateral membrane, where its activity generates the driving force for net renal sodium reabsorption. Regulation of the activity and membrane distribution of the Na,K-ATPase is of fundamental importance to the maintenance of tubular epithelial cell function. Any perturbation in these parameters of pump function might be expected to lead to profound pathological changes.
Previous work has found that the Na,K-ATPase is mislocalized to the apical membranes of the epithelial cells that line cysts derived from patients carrying polycystin-1 mutations (Wilson et al., 2000). These data suggest that the Na,K-ATPase resides on the luminal side of cyst lining renal epithelial cells, representing a complete reversal of the basolateral localization of the Na,K-ATPase that is seen in normal kidney epithelia. These observations have led to the suggestion that apical pump-mediated sodium export contributes to transepithelial fluid secretion in these cells. However, in other studies of intact cysts (Brill et al., 1996) and animal models of ADPKD (Kawa et al., 1994; Takahashi et al., 1997; Thomson et al., 2003) this mislocalization was not confirmed. Moreover, studies conducted on intact cysts have shown that application of the Na,K-ATPase inhibitor ouabain from the basolateral but not the apical side markedly inhibits fluid secretion (Grantham et al., 1995). Together, these studies suggest the possibility of a link between the genetic defect in polycystic kidney disease and the regulation of the Na,K-ATPase in the development of the cystic phenotype.
We have found in two separate yeast two-hybrid screens that the C-terminal tail of the PC-1 protein interacts with two different regions of the Na,K-ATPase α-subunit: the large cytosolic loop connecting the fourth to the fifth transmembrane domain (N/P domain), and a construct containing the N-terminal tail linked to the cytosolic loop between transmembrane domain 2 and 3 (A domain) (Pagel et al., 2003). Based upon the structural information derived from studies of the closely related Ca-ATPase (Toyoshima et al., 2000, 2004), these domains are very likely to undergo dramatic movements relative to one another during the course of pump catalysis.
In the present study, we report for the first time that a physical association between the endogenous Na,K-ATPase α-subunit and the PC-1 protein occurs in vivo in MadinDarby canine kidney (MDCK) cells and in situ in the kidneys of transgenic mice that overexpress PC-1.
We have also investigated the physiological role that this interaction plays using Chinese hamster ovary (CHO) cells that stably express the entire PC-1 protein. These cell lines demonstrate a dramatic increase in the total activity of the endogenous Na,K-ATPase pump. We propose that a functional association occurs between the C-terminal tail of the PC-1 protein and the Na,K-ATPase α-subunit and that this association may contribute to the regulation the Na,K-ATPase pump activity.
MATERIALS AND METHODS
Constructs
The A domain (residue 1 to 85) and N/P domain (residues 137 to 280) of the rat Na,K-ATPase α-subunit were amplified by PCR (primer sequences available upon request) (Pagel et al., 2003). These constructs were subcloned as BamHI/EcoRI and EcoRI/NotI fragments, respectively, into the pGEX-4T-3 vector (GE Healthcare, Piscataway, NJ) to produce the cDNAs encoding glutathione S-transferase (GST)-fusion proteins. Cloning of the murine PC-1 full-length construct carrying N-terminal FLAG and C-terminal hemagglutinin (HA) epitope tags into pcDNA3.1 vector has been described previously (Grimm et al., 2003). Subcloning of the C-terminal tail of the PC-1 protein (p200 construct) into pcDNA4 has also been described previously (Chauvet et al., 2004).
Cell Culture and Transfections
MDCK and CHO cells were grown in a 5% CO2, 95% air humidified incubator at 37°C in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin.
MDCK and CHO cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Selection and maintenance of stable clones was performed in media containing 1.2 g/l of the antibiotic G418 (Invitrogen). Clones were screened for expression by immunofluorescence and Western blotting.
Recombinant Protein Expression and GST-Pull-Down
The Escherichia coli strain BL21 (DE3) (Novagen/EMD Biosciences, San Diego, CA) was transformed with cDNAs encoding GST and GST-fusion proteins. A single colony was grown overnight in 50 ml of LB media supplemented with ampicillin (100 μg/ml). This culture was used to inoculate 500 ml of LB supplemented with ampicillin to an A600 of 0.1. The bacterial cells were grown at 37°C until an A600 of 0.8–1 was attained. Protein synthesis was induced with 0.1 mM isopropyl-1-thio-β-d-galactopyranoside, and the cells were grown for four additional hours. Cells were then pelleted (5000 × g for 15 min) and resuspended in 10 ml of ice-cold phosphate-buffered saline (PBS) (150 mM NaCl, 15 mM NaH2PO4) supplemented with protease inhibitor mix (1 μg/ml leupeptin, 1 μg/ml pepstatin A, 5 μg/ml phenylmethylsulfonyl fluoride, 0.5 μg/ml aprotinin). The cells were lysed by sonication and after addition of Triton X-100 (1% final concentration), the mixture was allowed to incubate for 30 min at 4°C. Soluble proteins were separated from cellular debris by centrifugation (12,000 × g for 10 min at 4°C). The amount of GST fusion protein in each preparation was determined by incubating 20 μl of glutathione-Sepharose 4 B (GE Healthcare) with a dilution series of the cleared lysate, and saturating amounts of protein were estimated by SDS-PAGE and visualization with a Coomassie Brilliant Blue stain.
For the pull-down assay, the C-terminal tail (p200 construct) of PC-1 was in vitro translated in rabbit reticulocyte lysate (Promega, Madison, WI) primed with [35S]methionine. Aliquots of 10 μl of labeled lysate were diluted in 1.5 ml of binding buffer (50 mM K-phosphate, 150 mM KCl, 1 mM MgCl2, 10% glycerol, 1% Triton) and incubated overnight at 4°C with beads loaded with GST alone or with GST-fusion proteins. After washing three times in ice-cold PBS and incubation in SDS sample buffer in ice for 10 min, the samples were analyzed by SDS-PAGE and autoradiography.
Biochemical Procedures
For coimmunoprecipitation experiments, each 3.5-cm dish of MDCK cells was incubated in 250 μl of lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA) supplemented with 1% Triton X-100 or 1 mg/ml C12E10 (decaethylene-glycoldodecyl-monoether; Sigma-Aldrich, St. Louis, MO). The lysate was cleared by centrifugation (10,000 × g for 30 min at 4°C) and incubated with 2 μg of polyclonal anti-HA antibody (Covance, Princeton, NJ) and protein A agarose beads (Pierce Chemical, Rockford, IL) overnight at 4°C. The HA antibody reacts with the HA epitope tag at the C terminus of the PC-1 construct expressed in these cells. Beads were washed three times with cold lysis buffer and once with cold PBS. The washed beads were incubated in SDS sample buffer containing 100 mM dithiothreitol (DTT) and heated to 65°C for 10 min.
For coimmunoprecipitation experiments from wild-type and PKD1 transgenic mice, membranes were isolated from whole kidney homogenates. The generation and characterization of the PKD1 transgenic mice have been described previously (Chauvet et al., 2004). Kidney tissues were resuspended in a solution containing 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, and 250 mM sucrose (1 ml/100 mg tissue), subjected to Dounce homogenization, and centrifuged for 10 min at 800 × g. The supernatant was then centrifuged for 30 min at 100,000 × g. The pellet was resuspended in a buffer containing 10 mM Tris-HCl and 1 mM EDTA, aliquoted and stored at –80°C until membranes were prepared, as described below. Membranes (50 μg) from wildtype and transgenic mice were resuspended in 1 ml of lysis buffer, incubated with anti-polycystin-1 polyclonal BD3 serum (1:100 dilution) (kindly provided by Dr. O. Ibraghimov-Beskrovnaya, Genzyme, Framingham, MA) (Ibraghimov-Beskrovnaya et al., 1997), and protein A agarose beads (Pierce Chemical) overnight at 4°C. Beads were washed three times in cold PBS, incubated in SDS sample buffer containing 100 mM DTT and heated to 65°C for 10 min.
Samples were resolved in 10% SDS-PAGE using standard protocols. The proteins were transferred to nitrocellulose membranes and blocked with milk solution [150 mM NaCl, 20 mM Tris, 5% (wt/vol) powdered milk, 0.1% Tween], incubated with monoclonal anti-α-subunit antibody 6H (1:500) (Pietrini et al., 1992) and subsequently with horseradish peroxidase-conjugated anti-mouse antibody (1:20,000; Jackson ImmunoResearch Laboratories, West Grove, PA). Bands were visualized with the enhanced chemiluminescence kit (GE Healthcare).
For biotinylation experiments, CHO cells were biotinylated as described previously (Gottardi et al., 1995), lysed in lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA), and incubated overnight at 4°C with streptavidin-conjugated agarose beads (Pierce Chemical). Precipitated proteins were eluted from the beads as described for coimmunoprecipitation experiments and analyzed by standard SDS-PAGE and Western immunoblotting. To assess the level of PC-1 expression, equal amounts of total lysates were subjected to SDS-PAGE and Western immunoblotting using an anti-FLAG polyclonal antibody (1:2000; Sigma-Aldrich). The bands were quantified using a GS-800 densitometer.
Preparation of Plasma Membranes
The following procedure was performed entirely on ice. It has been modified from the method published by Vilsen (1992). After CHO cells were grown to confluence in 10-cm tissue culture dishes, they were washed twice with PBS. Each dish of cells was scraped into 500 μl of a solution containing 250 mM sucrose, 20 mM Tris·HCl, and 1 mM EDTA at pH 7.5. The cells were then passed 10 times through a 27-gauge needle and spun in a Beckman tabletop centrifuge at 3000 × g for 10 min. The pellet was discarded, and the supernatant was combined with an equal volume of a solution containing 1 M NaI, 5 mM MgCl2, 20 mM EDTA, and 160 mM Tris at pH 8.3. This membrane suspension was incubated for 10 min on ice before the membranes were pelleted by ultracentrifugation at 48,000 × g for 90 min. The supernatant was aspirated, and the pellet was resuspended and washed in Tris-EDTA solution (10 mM Tris, 1 mM EDTA, pH 7.4). The membranes were pelleted a second time by ultracentrifugation and resuspended in the same Tris-EDTA solution. To homogenize the membrane suspension, it was passed through a gauge needle, and the yield was determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Membranes were stored at –80°C.
ATPase Assay
ATPase assays were carried out using a colorimetric procedure for measuring Pi released from ATP (Mense et al., 2000). Before the assay, the crude plasma membranes were permeabilized by incubation in 0.65 mg/ml deoxycholic acid, 2 mM EDTA, and 20 mM imidazole for 30 min at room temperature. Assays were initiated by adding 25 μl of the membrane suspension to 475 μl of assay solution (3 mM MgCl2, 3 mM Tris-ATP, 25 mM imidazole, 3 mM KCl). For sodium titration experiments, the assay solution also contained 20 mM KCl, NaCl and N-methyl-d-glucamine (NMDG). The combined concentration of NaCl and NMDG was maintained at 130 mM to ensure consistent ionic strength. For ouabain titration experiments the solution contained 130 mM NaCl and 20 mM KCl. To assess the maximal Na,K-ATPase activity, the assay was performed in a solution containing 130 mM NaCl and in the absence or presence of 1 mM ouabain. The amount of protein assayed was modified in each experiment to ensure that each assay measured similar levels of ATPase activity. During 2 h of incubation at 37°C at least 4%, but no more than 10% of the total ATP (3 mM), was hydrolyzed under Vmax conditions. After 2 h of incubation, the reaction was stopped by adding 1 ml of freshly prepared ice-cold stop solution (0.5 M HCl, 3% ascorbic acid, 0.5% ammonium molybdate, 1% SDS). All tubes were then transferred to an ice-cold water bath, and 1.5 ml of a solution containing 2% sodium metaarsenite, 2% sodium citrate, and 2% acetic acid was added to each tube. For color development of the phosphate assay, the test tubes were incubated at 37°C for 10 min. Free Pi was determined by reading the optical density of each test tube at 850 nm on a Beckman DU-640 spectrophotometer. The Na,K-ATPase specific activity was determined as the difference between tubes without ouabain and those containing 1 mM ouabain. Individual experiments were carried out in triplicate. They were averaged and normalized to the maximal enzymatic activity (Vmax), which was computed by fitting the data to a Hill function, V = Vmax/{1 + (K1/2/[Na])n}. Each experiment was performed at least three times. Data were averaged and again fitted to the Hill function. The values shown are the means of at least three experiments, and the error bars correspond to the SD between the values obtained in individual experiments. The data are presented as a percentage of maximal activity (Vmax). The apparent K1/2 (Na) and Ki (ouabain) values were also obtained from the same data fits.
RESULTS
PC-1 Interacts In Vitro with Two Different Regions of the Na,K-ATPase α-Subunit
Previous work in this laboratory has determined that the last 200 amino acids of the extreme C terminus of polycystin-1 interact with both the A and N/P domains of the Na,K-ATPase α-subunit in a yeast two-hybrid analysis (Pagel et al., 2003).
To confirm the results of the two-hybrid screenings, we used GST fusion proteins for in vitro binding experiments. GST fusion proteins incorporating the A domain and N/P domain of the Na,K-ATPase α-subunit were produced in bacteria and immobilized on glutathione-Sepharose 4B beads. A polypeptide encoding the C-terminal 200 amino acids of PC-1 labeled with [35S]methionine was produced by in vitro translation. The charged glutathione beads were incubated with the radiolabeled PC-1 polypeptide and precipitated material was analyzed by SDS-PAGE and then visualized by autoradiography.
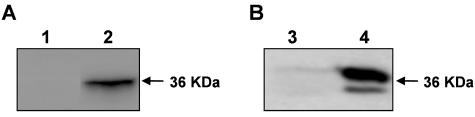
As shown in Figure 1 (lanes 2 and 4), both the Na,K-ATPase α-subunit A domain and N/P domain fusion proteins interact strongly, and to a similar extent, with the C-terminal tail of PC-1. The molecular weight of the band detected in the interaction with the A and N/P domains of the Na,K α-subunit (∼36 kDa, as reported in Chauvet et al., 2004) is consistent with the expected size of the C-terminal cytosolic tail, which contains 200 residues (Chauvet et al., 2004).
Figure 1.
The C-terminal tail of PC-1 interacts in vitro with the A domain (A) and N/P domain (B) of the Na,K-ATPase α-subunit. GST alone (lane 1, A; lane 3, B), GST fused to the A domain (lane 2, A) or to the N/P domain (lane 4, B) of the Na,K-ATPase α-subunit were conjugated to glutathione-Sepharose 4B beads and incubated with the product of the in vitro translation of the PC-1 tail labeled with [35S]methionine. The size of the C-terminal tail of PC-1 is indicated by the arrow. PC-1 is precipitated by both isolated pump domains, but not by GST alone.
Endogenous Na,K ATPase α-Subunit Interacts In Vivo and In Situ with PC-1 Protein
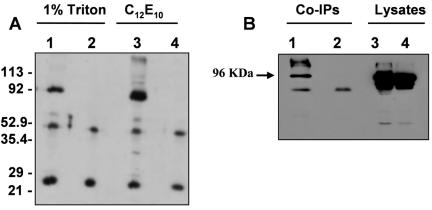
To investigate whether the physical association between the Na,K-ATPase α-subunit and the PC-1 protein occurs in vivo, we established an MDCK cell line that stably expresses the entire PC-1 protein. The PC-1 protein carries a FLAG epitope tag on its N terminus and an HA epitope tag on its C terminus (Chauvet et al., 2004). MDCK cells stably expressing the tagged construct were subjected to immunoprecipitation using a polyclonal anti-HA antibody. The immunoprecipitate was separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with the monoclonal antibody (mAb) 6H (Pietrini et al., 1992), which recognizes the first 23 N-terminal amino acids of the sodium pump. This analysis reveals a band of 96 kDa (Figure 2A, lanes 1 and 3) corresponding to the Na,K-ATPase α-subunit, demonstrating that endogenous Na,K-ATPase α-subunit specifically coprecipitates with the PC-1 protein. Na,K-ATPase α-subunit was not detected in the Western blot analysis of the proteins coimmunoprecipitated from the untransfected cells.
Figure 2.
(A) Endogenous Na,K-ATPase α-subunit coimmunoprecipitates with fulllength PC-1 protein. MDCK cells stably expressing the HA-tagged full-length PC-1 protein (lanes 1 and 3) or wild-type MDCK cells (lanes 2 and 4) were lysed in 1% Triton (lanes 1 and 2) and in 1 mg/ml C12E10 (lanes 3 and 4) and immunoprecipitated with polyclonal anti-HA antibody. Precipitated proteins were immunoblotted with anti-Na,K-ATPase α-subunit mAb (6H). The α-subunit is present in the HA precipitates from PC-1-expressing cells but not untrasfected cells. (B) Endogenous Na,K-ATPase α-subunit coimmunoprecipitates with PC-1 from kidneys of PC-1 transgenic mice. Kidney membranes from transgenic mice expressing full-length PC-1 (lane 1) and from wild-type mice (lane 2) were solubilized in 1% Triton and immunoprecipitated using anti-PC1 polyclonal antibody BD3. The immunoprecipitated proteins were detected with anti Na,K-ATPase α-subunit mAb 6H. Total membrane lysates (50 μg) from wild-type (lane 3) and transgenic mice (lane 4) were loaded as controls. The band corresponding to the Na,K ATPase α-subunit (96 kDa) is indicated by the arrows on the left.
We find that the endogenous Na,K-ATPase α-subunit and the overexpressed PC-1 proteins are able to coimmunoprecipitate when the MDCK cells are solubilized in different detergents, including 1% Triton (Figure 2A, lane 1) and C12E10 (Figure 2A, lane 3). This latter detergent has been used successfully to solubilize functionally intact sodium pump, and it has been shown that after solubilization >90% of the enzyme retains its kinetic and conformational properties (Garty et al., 2002). These results show that the association between PC-1 and the endogenous Na,K-ATPase α-subunit occurs in vivo and is preserved when the pump is solubilized in its active state.
To further investigate a possible interaction between PC-1 and Na,K-ATPase α-subunit in situ, we performed coimmunoprecipitation experiments from kidney membranes isolated from either wild-type mice or transgenic mice that overexpress the native full-length PC-1 protein. We found that the expression of the full-length PC-1 is increased approximately fourfold over wild-type levels in this mouse model (our unpublished data). The data are in agreement with the previously reported levels of mRNA detected in these animals (Chauvet et al., 2004). Crude membrane preparations were subjected to immunoprecipitation using a polyclonal antibody (BD3 serum) raised against the C-terminal tail of the PC-1 protein (Ibraghimov-Beskrovnaya et al., 1997) (kind gift of Dr. Ibraghimov-Beskrovnaya, Genzyme, Framingham, MA). Western blot analysis of the immunoprecipitated proteins using the 6H mAb demonstrates that the endogenous Na,K-ATPase α-subunit coprecipitates with PC-1, as demonstrated by the 96-kDa band visualized in Figure 2B, lane 1. We were unable to detect the sodium pump in BD3 immunoprecipitates from adult wild-type mice. It has been reported that the expression of PC-1 dramatically decreases during development (Geng et al., 1996). It is possible that the association of PC-1 with the Na,K ATPase α-subunit occurs predominantly during early stages of embryonic development in wild-type animals.
Total Ouabain-sensitive ATPase Activity in Cells Expressing the PC-1 Protein
To assess whether its association with the C-terminal tail of the PC-1 protein affects the activity of the endogenous Na,K-ATPase, we measured the ouabain-sensitive ATPase activity of the endogenous pump in CHO cells expressing the fulllength PC-1 protein. For these experiments, crude plasma membrane preparations from different CHO cell lines stably expressing the PC-1 protein or transfected with the empty vector were used. These experiments were carried out at saturating sodium and potassium concentrations to ensure that the pump's activity was measured at its maximal velocity (Vmax). In all of the assays of the Na,K ATPase activity reported, we found that the ouabain-insensitive fraction of the activity of these membrane preparations was ∼20% of the total activity. These data are in agreement with the previously reported measurements conducted on the pump expressed in different cell types and membrane preparations (Blostein et al., 1999; Mense et al., 2000).
We observed an increase in the total ouabain-sensitive Na,K-ATPase activity in CHO cells that express the entire PC-1 protein, compared with control cells that are stably transfected with empty vector. The experiments presented in Figure 3 show the Na,K-ATPase activity measured in two different cell lines expressing PC-1 compared with Na,K-ATPase activity measured in two different control cell lines that are transfected with empty vector alone. The activity of the endogenous pump measured in the control cells is very similar between the two cell lines (1.12 μmol Pi/mg protein/h, MOCK 3, and 1.34 μmol Pi/mg protein/h, MOCK 2; Figure 3A). We found that pump activity is significantly higher in cells lines that express PC-1 compared with the control cell lines. One PC-1-expressing cell line demonstrated Na,K-ATPase activity more than fivefold higher than the control cells (6.08 μmol Pi/mg/h, clone 71; Figure 3A), and the other PC-1-expressing cell line demonstrated Na,K-ATPase activity approximately threefold higher than the control cells (3.19 μmol Pi/mg protein/h, clone 93; Figure 3A). It should be noted that the conditions used in the immunoprecipitations from renal tissue (Figure 2B) are very similar to those used in the assay of ATPase activity. Thus, the interaction between PC-1 and the Na,K ATPase should persist under the ATPase assay regimen. Finally, Western immunoblotting analyses conducted on cell membranes from cells overexpressing PC-1 incubated for 2 h at 37°C (our unpublished data) did not reveal any change in the quantity of the pump or the PC-1 protein over the course of this incubation period.
Figure 3.
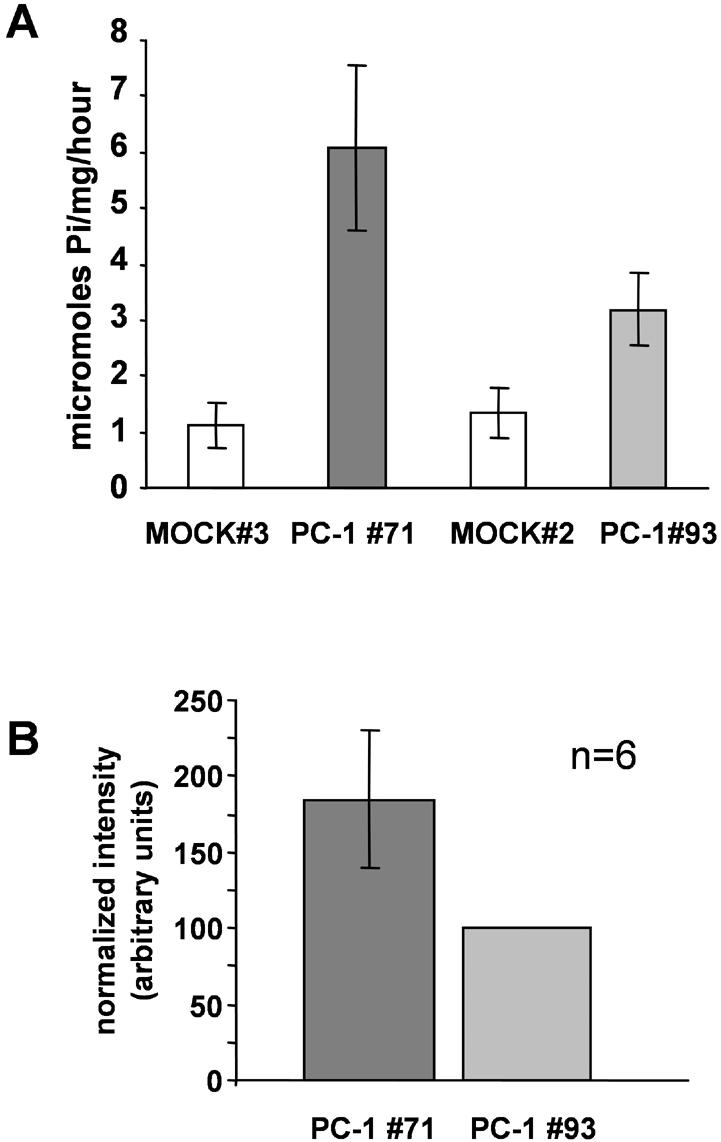
(A) Total ouabain-sensitive Na,K-ATPase activity is elevated in CHO cells expressing the PC-1 protein. Total ouabainsensitive Na,K-ATPase activity was measured as described in Materials and Methods. Values of maximal activity in MOCK cell lines were 1.12 (MOCK 3) and 1.34 μmol Pi/mg protein/h (MOCK 2). In cells expressing the PC-1 protein, the activity was 6.08 (A, PC-1 clone 71) and 3.2 (B, PC-1 clone 93) μmol Pi/mg protein/h. Each value was calculated as the difference between total activity and ouabain-sensitive activity. Each bar is the average of three independent experiments (±SD), repeated in triplicate (*p < 0.01). (B) Relative PC-1 expression in total cell lysates. Total cell lysates (20 μg) from CHO cells expressing the PC-1 protein (clones PC-1 93 and 71) were subjected to SDS-PAGE and immunoblotted with anti-FLAG antibody. Signal intensity of each band was quantified by densitometry. Six independent experiments were performed. Data are represented as the intensity of the Na,K-ATPase α-subunit band detected in cell line PC-1 71(dark gray bar) normalized to the expression associated with cell line PC-1 93 (light gray bar).
We then asked whether these differences in activity correlate with differences in the level of expression of the PC-1 protein. We analyzed PC-1 expression in cell lysates prepared from the PC-1-expressing cell lines by immunoblotting, using the anti-FLAG antibody to recognize the N-terminal region of the PC-1 protein. Western immunoblotting analyses performed with an antibody raised against the native PC-1 protein (our unpublished data) conducted on control cells revealed that wild-type CHO cells do not endogenously express detectable PC-1 protein. Quantification of exogenous PC-1 expression (Figure 3B) in these cell lines demonstrates that the cell line with higher total Na,K-ATPase activity expresses ∼85% more PC-1 protein (Figure 3B, PC-1 #71) than the cell line with lower ATPase activity (Figure 3B, PC-1 #93). These data are consistent with a correlation between the differences in activity observed in the two cell lines and the level of expression of the total PC-1 protein.
To rule out the possibility that the increase in Na,K-ATPase activity observed in the PC-1 cells lines is due to higher levels of expression of the Na,K-ATPase α-subunit in these lines, and to determine whether CHO cells expressing the PC-1 protein show an increase in the total number of the Na,K-ATPase α-subunit molecules expressed at the plasma membrane, we determined the total amount of Na,K-ATPase α-subunit present in these cells. Western blot analysis of sodium pump in total cell lysates (Figure 4A) shows that no significant difference in total pump expression is detectable between control cells and cells that express the PC-1 protein. To ascertain the amount of α-subunit at the cell surface, we subjected cells expressing either the PC-1 protein or the empty vector to cell-surface biotinylation experiments. CHO cells transfected with empty vector (Figure 4B, lanes 1–4) or with the entire PC-1 protein (Figure 4B, lanes 5–8) were biotinylatated in quadruplicate. The biotinylated cells were then lysed and incubated with streptavidin beads. The precipitated proteins were separated by SDS-PAGE, and the membranes were probed with the monoclonal 6H anti-Na,K-ATPase α-subunit antibody. The amount of α-subunit at the cell-surface is similar in cell lines that express the PC-1 protein and mock-transfected cells. These results indicate that the total quantity of the pump protein that is expressed at the plasma membrane in cells expressing PC-1 is unaffected by the overexpression of the PC-1 protein and that the observed increase in Na,K-ATPase activity is not attributable to changes in the level of the pump protein expression.
Figure 4.
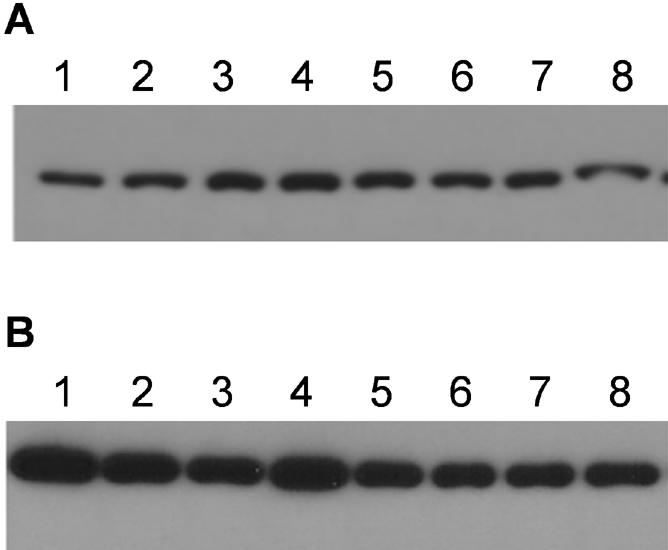
Expression of total and plasma membrane-associated Na,K-ATPase α-subunit is not increased in cells expressing fulllength PC-1 protein. (A) Immunoblot of total protein lysates (5 μg) from MOCK CHO cells (clone 3, lanes 1, 2, 3, 4) or CHO cells expressing FL-PC1 (clone 71, lanes 5, 6, 7, 8) probed with anti-Na,K-ATPase α-subunit antibody (6H antibody). (B) CHO cells transfected with empty vector and CHO cells expressing PC-1 were biotinylated with biotin-N-hydroxysuccimide ester and incubated with streptavidin-conjugated agarose beads. The precipitated proteins from CHO cells expressing empty vector (clone 3, lanes 1–4) and CHO cells expressing FLAG-PC-1 (clone 71, lanes 5–8) were immunoblotted with anti-Na,K-ATPase α-subunit antibody (6H).
Sodium and Ouabain Dependence of the Na,K-ATPase Activity
To determine whether the change in Na,K-ATPase activity was due to alterations in kinetic properties of the pump, we examined the sodium dependence of the Na,K-ATPase activity as well as the pump's sensitivity to its specific inhibitor ouabain. We first assessed the sodium dependence of the endogenous Na,K-ATPase activity in CHO cells stably expressing the PC-1 protein and in cells transfected with the empty vector. The sodium titration experiments were performed in solutions containing 20 mM potassium and different sodium concentrations. These experiments (Figure 5A) showed that the apparent affinity of the pump for sodium in cells stably expressing the PC-1 protein is not significantly different from the sodium affinity measured in cell lines transfected with the empty vector. We find that the K1/2 (Na) of the endogenous Na,K-ATPase in cells transfected with the empty vector is 21.4 mM, which is somewhat higher than values that have been previously reported for the wild-type enzyme (Mense et al., 2000). In cells expressing the PC-1 protein, the apparent K1/2 (Na) of the pump is 20.8 mM (Figure 5B), which is not significantly different from the value calculated in the control cells. The comparatively high values for the apparent K1/2 (Na) may be characteristic of the hamster Na,K ATPase or may be due in part to the relatively high concentration of potassium present in the assay. These data indicate that in cells expressing the PC-1 protein, the apparent affinity of the Na,K-ATPase α-subunit for sodium remains essentially unchanged.
Figure 5.
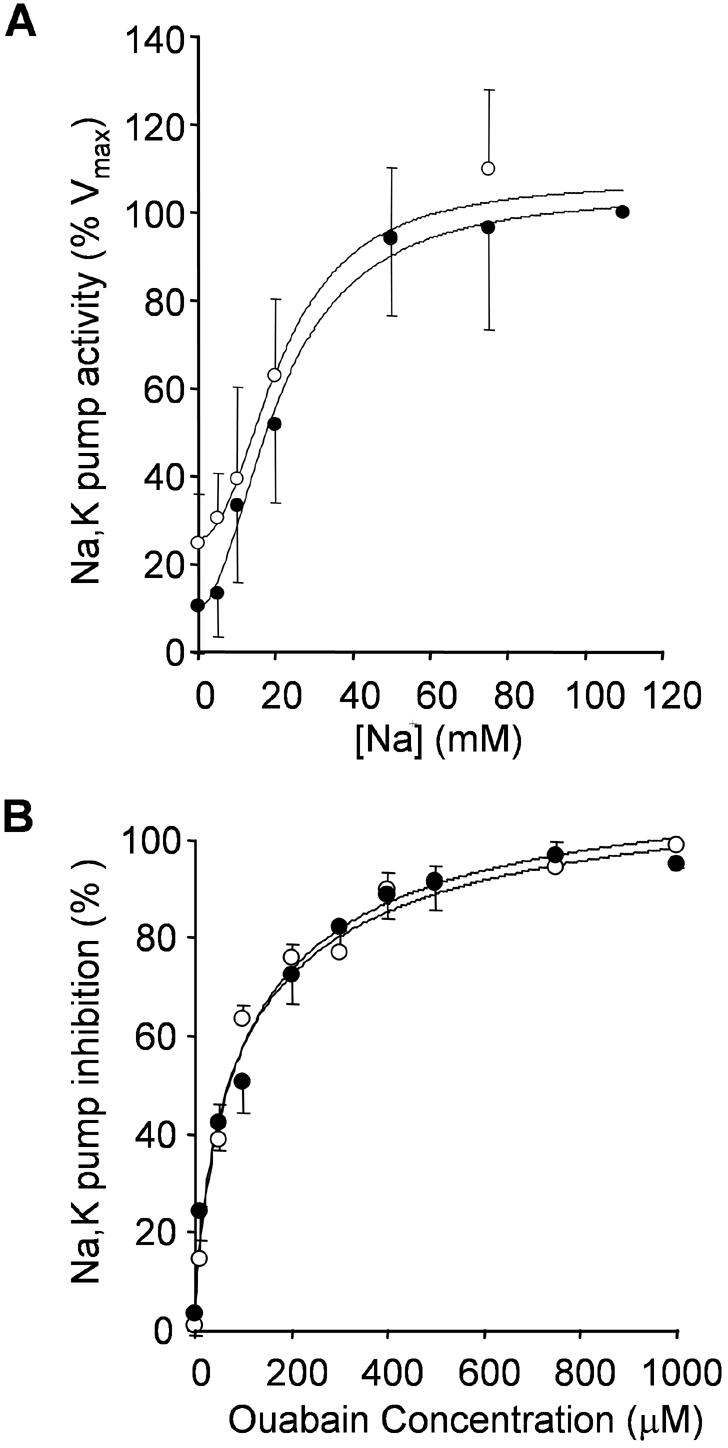
Determination of the apparent Na affinity and ouabain sensitivity of the Na,K-ATPase. Sodium and ouabain titration measurements of the endogenous Na,K-ATPase activity were carried out as described in Materials and Methods. Membranes were isolated from CHO cells transfected with the empty vector (open circles) and cells stably expressing the PC-1 protein (closed circles). The Na,K-ATPase activity was measured at different sodium (A) and ouabain (B) concentrations. Each data point is the average (±SD) of at least three independent assays, each of which was performed in triplicate. For sodium titration experiments (A), the percentage of total activity was plotted against its respective sodium concentration and fitted to the Hill equation: V = V0/{Vmax /{1 + (K1/2 /[Na]) n}, with n = 1. of Values K1/2[Na] thus obtained were 21.4 MOCK for mM cells and 20.8 mM for cells expressing PC-1 (n = 12, p > 0.2). For ouabain titration experiments (B), the percentages of inhibition of the Na,K-ATPase-specific activity were plotted against their respective ouabain concentration and fitted to the Hill equation V = Vmax/{1 + (Ki/[ouabain])n}. Values of Ki(ouabain) thus obtained were 103 μM for MOCK cells and 100 μM for cells expressing PC-1 (n = 9, p < 0.1).
We then assessed whether the sensitivity of the enzyme to its specific inhibitor ouabain was affected in cells expressing the PC-1 protein compared with cells expressing the empty vector. Figure 5B shows the ouabain titration experiments conducted in these cell lines. We found that the calculated Ki of the pump for ouabain in the cell line transfected with the empty vector was identical to the Ki in the cell line stably expressing the PC-1 protein. Together, these data indicate that in these cells the expression of the PC-1 protein does not correlate with a change in the measured kinetic properties of the pump activity.
DISCUSSION
In the present work, we describe a previously uncharacterized interaction between the α-subunit of the Na,K-ATPase and the C-terminal cytosolic tail of the polycystin-1 protein. We show that the physical association between the endogenous Na,K-ATPase α-subunit and the PC-1 protein occurs both in cells in culture and in transgenic mice that overexpress PC-1. We also characterize the functional consequences of this interaction in CHO cells that stably express the entire PC-1 protein, demonstrating that endogenous sodium pump activity is higher in these cells.
Several studies (Peters et al., 1999; Ong, 2000) have shown that the levels and pattern of polycystin-1 expression can vary dramatically during development. Although the precise stoichiometry of the interaction between the sodium pump and the PC-1 protein has not yet been determined, we have demonstrated that high levels of expression of the PC-1 protein are required for the association, as shown by the fact that in adult wild-type mice the low endogenous levels of polycystin-1 are not sufficient to form a detectable complex with the Na,K-ATPase α-subunit. It is possible, therefore, that this interaction is favored in the early stages of renal development, which are associated with relative high levels of expression of PC-1.
Previous in vivo and in situ work (Chauvet et al., 2004) has shown that polycystin-1 undergoes a cleavage that releases its C-terminal tail, which then translocates to the nucleus. This fragment has been identified as the last ∼200 amino acids of the PC-1 protein. We observed that when lysates are prepared from cells expressing the last 200 amino acids of PC-1 as a soluble fragment, the Na,K-ATPase α-subunit can be immunoprecipitated with this PC-1 polypeptide construct (our unpublished data). The experiments presented here do not allow us to conclude whether the Na,K-ATPase α-subunit interacts preferentially with the full-length PC-1 protein or with its cleaved C-terminal tail fragment. Furthermore, it remains to be investigated whether the cleavage event modulates the effects of the interaction of the α-subunit with the PC-1 C-terminal tail on the functional properties of the pump.
In this study, we have also demonstrated that increasing the level of expression of PC-1 protein results in a corresponding increase in the activity of the endogenous Na,K-ATPase in a cell culture model. These data suggest the possibility that the PC-1 protein may participate in the physiological regulation of the Na,K-ATPase's activity. It is possible that the different levels of expression of the PC-1 protein observed during development may contribute to differential regulation of pump activity during renal morphogenesis. None of the individual kinetic properties that we investigated were altered by PC-1 expression. These data are consistent with the possibility that PC-1's effects on the pump may occur through an increase in the turnover rate of the Na,K-ATPase. Additional experiments will be required to clarify this issue.
The present work may provide some insight that could be relevant to the mechanism of renal cyst development. In ADPDK, transepithelial fluid accumulation inside the lumen of the cysts is a requirement for cyst expansion. The precise mechanisms involved in converting normally absorptive renal epithelial cells into secretory epithelia are still unknown. The current model (Sullivan et al., 1998) of secretion inside the luminal space of the cysts involves the up-regulation of the apical cystic fibrosis transmembrane conductance regulator channel, whereas the Na,K-ATPase is thought to act at its normal basolateral localization. Altered levels of PC-1 expression in ADPKD may affect the activity of the Na,K-ATPase, which may in turn disturb the balance among the cell's transport activities, ultimately converting the tubule cell from an absorptive to a secretory phenotype and leading to cyst expansion. Further study will be required to determine whether the interaction we observed plays any role in preventing or fostering the development of ADPKD.
Acknowledgments
We are grateful to all the members of the Caplan laboratory for valuable discussions and advice. We thank Oxana Ibraghimov-Beskrovnaya for the kind gift of the BD3 serum. We also thank Dr. S. Somlo and T. Xian for generously providing renal tissue from PC-1 transgenic mice. Special thanks to Gilles Crambert, Biff Forbush, Peter Aronson, and Gary Rudnick for helpful discussions. This work was supported by Grants GM-42136 and DK-57328 from the National Institutes of Health (to M.J.C.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0200) on August 17, 2005.
Abbreviations used: ADPKD, autosomal dominant polycystic kidney disease; CHO, Chinese hamster ovary; MDCK, Madin-Darby canine kidney; HA, hemagglutinin; PBS, phosphate-buffered saline; PC-1, polycystin-1.
References
- Arnould, T., Kim, E., Tsiokas, L., Jochimsen, F., Gruning, W., Chang, J. D., and Walz, G. (1998). The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273, 6013–6018. [DOI] [PubMed] [Google Scholar]
- Blostein, R., Dunbar, L., Mense, M., Scanzano, R., Wilczynska, A., and Caplan, M. J. (1999). Cation selectivity of gastric H,K-ATPase and Na,K-ATPase chimeras. J. Biol. Chem. 275, 18374–18381. [DOI] [PubMed] [Google Scholar]
- Brill, S. R., Ross, K. E., Davidow, C. J., Ye, M., Grantham, J. J., and Caplan, M. J. (1996). Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc. Natl. Acad. Sci. USA 93, 10206–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet, V., et al. (2002). Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 160, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet, V., et al. (2004). Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Investig. 114, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, L. A., and Caplan, M. J. (2001). Ion pumps in polarized cells: sorting and regulation of the Na+,K+ and H+,K+-ATPases. J. Biol. Chem. 276, 29617–29620. [DOI] [PubMed] [Google Scholar]
- Gabow, P. A. (1993). Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 329, 332–342. [DOI] [PubMed] [Google Scholar]
- Garty, H., Lindzen, M., Scanzano, R., Aizman, R., Fuzesi, M., Goldshleger, R., Farman, N., Blostein, R., and Karlish, S. J. (2002). A functional interaction between CHIF and Na-K-ATPase: implication for regulation by FXYD proteins. Am. J. Physiol. 283, 607–615. [DOI] [PubMed] [Google Scholar]
- Geng, L., et al. (1996). Identification and Localization of Polycystin, the PKD1 Gene Product. J. Clin. Investig. 98, 2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi, C. J., Dunbar, L. A., and Caplan, M. J. (1995). Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am. J. Physiol. 268, 285–295. [DOI] [PubMed] [Google Scholar]
- Grantham, J. J., Ye, M., Gattone V. H., 2nd, and Sullivan, L. P. (1995). In vitro fluid secretion by epithelium from polycystic kidneys. J. Clin. Investig. 95, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, D. H., Cai, Y., Chauvet, V., Rajendran, V., Zeltner, R., Geng, L., Avner, E. D., Sweeney, W., Somlo, S., and Caplan, M. J. (2003). Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J. Biol. Chem. 278, 36786–36793. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O., et al. (1997). Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. USA 94, 6397–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa, G., Nagao, S., Yamamoto, A., Omori, K., Komatz, Y., Takahashi, H., and Tashiro, Y. (1994). Sodium pump distribution is not reversed in the DBA/2FG-pcy, polycystic kidney disease model mouse. J. Am. Soc. Nephrol. 4, 2040–2049. [DOI] [PubMed] [Google Scholar]
- Kim, E., Arnould, T., Sellin, L. K., Benzing, T., Fan, M. J., Gruning, W., Sokol, S. Y., Drummond, I., and Waltz, G. (1999). The polycystic kidney disease 1 gene product modulates Wnt signaling. J. Biol. Chem. 274, 4947–4953. [DOI] [PubMed] [Google Scholar]
- Lingrel, J. B. (1992). Na,K-ATPase: isoform structure, function, and expression. J. Bioenerg. Biomembr. 24, 263–270. [DOI] [PubMed] [Google Scholar]
- Mense, M., Dunbar, L. A., Blostein, R., and Caplan, M. J. (2000). Residues of the fourth transmembrane segments of the Na,K-ATPase and the gastric H,K-ATPase contribute to cation selectivity. J. Biol. Chem. 275, 1749–1756. [DOI] [PubMed] [Google Scholar]
- Ong, A. C. (2000). Polycystin expression in the kidney and other tissues: complexity, consensus and controversy. Exp. Nephrol. 8, 208–214. [DOI] [PubMed] [Google Scholar]
- Pagel, P., Zatti, A., Kimura, T., Duffield, A., Chauvet, V., Rajendran, V., and Caplan, M. J. (2003). Ion pump-interacting proteins: promising new partners. Ann. N. Y. Acad. Sci. 986, 360–368. [DOI] [PubMed] [Google Scholar]
- Peters, D. J., van de Wal, A., Spruit, L., Saris, J. J., Breuning, M. H., Bruijn, J. A., and de Heer, E. (1999). Cellular localization and tissue distribution of polycystin-1. J. Pathol. 188, 439–446. [DOI] [PubMed] [Google Scholar]
- Pietrini, G., Matteoli, M., Banker, G., and Caplan, M. J. (1992). Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA 89, 8414–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, L. P., Wallace, D. P., and Grantham, J. J. (1998). Epithelial transport in polycystic kidney disease. Physiol. Rev. 78, 1165–1191. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Tsuchiya, K., Komatsu, Y., and Nihei, A. (1997). A role for Na/K adenosine triphosphatase in the pathogenesis of cyst formation in experimental polycystic kidney disease. J. Lab. Clin. Med. 129, 517–526. [DOI] [PubMed] [Google Scholar]
- Thomson, R. B., Mentone, S., Kim, R., Earle, K., Delpire., E., Somlo, S., and Aronson, P. S. (2003) Histopathological analysis of renal cystic epithelia in the Pkd2WS25/mouse model of ADPKD. Am. J. Physiol. 285, F870–F880. [DOI] [PubMed] [Google Scholar]
- Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000). Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405, 647–655. [DOI] [PubMed] [Google Scholar]
- Toyoshima, C., Nomura, H., and Tsuda, T. (2004). Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368. [DOI] [PubMed] [Google Scholar]
- Vandorpe, D. H., Chernova, M. N., Jiang, L., Sellin, L. K., Wilhelm, S., Stuart-Tilley, A. K., Walz, G., and Alper, S. L. (2001). The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J. Biol. Chem. 276, 4093–4101. [DOI] [PubMed] [Google Scholar]
- Vilsen, B. (1992). Functional consequences of alterations to Pro328 and Leu332 located in the 4th transmembrane segment of the alpha-subunit of the rat kidney Na+,K(+)-ATPase. FEBS Lett. 314, 301–307. [DOI] [PubMed] [Google Scholar]
- Wildman, S. S., Hooper, K. M., Turner, C. M., Sham, J. S., Lakatta, E. G., King, B. F., Unwin, R. J., and Sutters, M. (2003). The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl– conductance through increased Ca2+ entry. Am. J. Physiol. 285, F1168–F1178. [DOI] [PubMed] [Google Scholar]
- Wilson, P. D. (2004a). Polycystic kidney disease: new understanding in the pathogenesis. Int J. Biochem. Cell Biol. 36, 1868–1873. [DOI] [PubMed] [Google Scholar]
- Wilson, P. D. (2004b). Polycystic kidney disease. N. Engl. J. Med. 350, 151–164. [DOI] [PubMed] [Google Scholar]
- Wilson, P. D., Devuyst, O., Li, X., Gatti, L., Falkenstein, D., Robinson, S., Fambrough, D., and Burrow, C. R. (2000). Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform. Am. J. Pathol. 156, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]