Abstract
The Wnt signaling pathway is important in the formation of neural crest cells in many vertebrates, but the downstream targets of neural crest induction by Wnt are largely unknown. Here, we examined quantitative changes in gene expression regulated by Wnt-mediated neural crest induction using quantitative PCR (QPCR). Induction was recapitulated in vitro by adding soluble Wnt to intermediate neural plate tissue cultured in collagen, and induced versus control tissue were assayed using gene-specific primers at times corresponding to premigratory (18 and 24 h) or early (36 h) stages of crest migration. The results show that Wnt signaling up-regulates in a distinct temporal pattern the expression of several genes normally expressed in the dorsal neural tube (slug, Pax3, Msx1, FoxD3, cadherin 6B) at “premigratory” stages. While slug is maintained in early migrating crest cells, Pax3, FoxD3, Msx1 and cadherin 6B all are down-regulated by the start of migration. These results differ from the temporal profile of these genes in response to the addition of recombinant BMP4, where gene expression seems to be maintained. Interestingly, expression of rhoB is unchanged or even decreased in response to Wnt-mediated induction at all times examined, though it is up-regulated by BMP signals. The temporal QPCR profiles in our culture paradigm approximate in vivo expression patterns of these genes before neural crest migration, and are consistent with Wnt being an initial neural crest inducer with additional signals like BMP and other factors maintaining expression of these genes in vivo. Our results are the first to quantitatively describe changes in gene expression in response to a Wnt or BMP signal during transformation of a neural tube cell into a migratory neural crest cell.
INTRODUCTION
The vertebrate neural crest is a migratory embryonic cell population that forms at the border between the neural plate and future epidermis. Neural crest cells delaminate from the dorsal neural tube in a rostrocaudal wave and migrate throughout the embryo to form a wide range of derivatives. They build much of the craniofacial skeleton and, together with cranial placodes, the peripheral nervous system. Specific derivatives include sensory, sympathetic and enteric neurons and glia, melanocytes, smooth muscle, dermis, connective tissue, cartilage and bone (LeDouarin and Kalcheim, 1999). Because of its prolific contribution to various tissues, the neural crest has been intensively studied as an important player in vertebrate embryogenesis.
Neural crest cells form at the border between the neural plate and the nonneural ectoderm, which lies above the border between the dorsal (axial) and ventrolateral (paraxial and lateral plate) mesoderm. Thus, several tissue interactions are potentially involved in the induction of neural crest cells. In avian embryos, Wnts appear to be necessary and sufficient for neural crest induction both in vivo and in vitro. Wnt-6 is expressed in the correct spatial and temporal pattern to mediate neural crest induction, being present in the ectoderm adjacent to the neural folds but not in the neural folds or neural plate (Garcia-Castro et al., 2002). Inhibition of Wnt signaling adjacent to the open neural plate or the closing neural tube blocks neural crest production as assayed by expression of the neural crest markers slug and the HNK-1 epitope (Garcia-Castro et al., 2002). Furthermore, addition of Wingless (Wg) conditioned medium, a soluble form of the Wnt signal, is sufficient to induce migratory neural crest cells in naive intermediate neural plate in the absence of other factors (Garcia-Castro et al., 2002). This result, together with studies in Xenopus, zebrafish and mouse, support an important role for Wnt signaling in neural crest formation throughout vertebrates.
The Wnt signaling pathway is highly conserved, from worms and flies to vertebrates (Cadigan and Nusse, 1997). This pathway is particularly important in specifying cell fates during development, and plays multiple roles in neural crest development, from the initial induction phase (Garcia-Castro et al., 2002) to the subsequent process of delamination (Burstyn-Cohen et al., 2004). Wnt signaling is also involved in differentiation of the neural crest (Saint-Jeannet et al., 1997; Lee et al., 2004), expansion of precursors (Ikeya et al.,1997), and specification of pigment cells, sensory neurons and dorsal interneurons (Dorsky et al., 1998; Dunn et al., 2000; Jin et al., 2001; Muroyama et al., 2002). Experiments in Xenopus have helped define the role of Wnts and their responsive genes during neural crest development. Neural crest cell markers such as the transcription factors krox-20, AP-2 and slug are up-regulated in neuralized (noggin-expressing) Xenopus animal caps treated with Xwnt-1 and Xwnt-3a; similarly, overexpression of Xwnt-1 or Xwnt-3a in embryos dramatically increases the number of slug-positive and krox-20-positive cells. Furthermore, inhibiting Wnt signaling by overexpressing GSK-3β leads to the reduction or absence of krox-20-expressing neural crest cells from rhombomere 5 (Saint-Jeannet et al., 1997; LaBonne and Bronner-Fraser, 1998). Thus, Wnts and the genes they regulate establish an environment that fosters the development of the neural crest.
Other growth factors besides Wnt play an important role in the development of the neural crest. In particular, the BMP signaling pathway has been reported to be involved in the processes of neural crest induction and delamination (for review see Knecht and Bronner-Fraser, 2002; Huang and Saint-Jeannet, 2004; Kalcheim and Burstyn-Cohen, 2005). Inhibition of BMP signaling by the secreted molecules chordin, noggin and follistatin is necessary for neural differentiation in Xenopus. Experiments in Xenopus support a model in which inhibition of BMP expression is required for neural crest induction, accompanied by at least one additional signal (LaBonne and Bronner-Fraser, 1998). However, this may or may not be the case in other organisms such as chick and mouse, as issues such as the timing and the relative expression of these BMP inhibitors indicate that the presence of such inhibitors may not be necessary to generate neural fates. Furthermore, experiments by Garcia-Castro et al. show that the induction of migratory neural crest cells by BMP signals (as demonstrated in previous reports (Liem et al., 1995; Liu and Jessell, 1998)) was medium-dependent, most likely due to the presence of additional factors besides BMPs in the medium (Garcia-Castro et al., 2002). Evidence suggests, however, that BMPs play a pivotal role in later events in neural crest development, such as the process of delamination, (Sela-Donenfeld and Kalcheim, 1999; Sela-Donenfeld and Kalcheim, 2000) and the maintenance of the precursor pool (Kleber et al., 2005). Thus, cross-talk between many growth factor signaling pathways, such as Wnts and BMPs, initiates neural crest cell development, in particular the induction process, and the utilization of such pathways may be different among various organisms.
Given the role of Wnt signaling in avian neural crest induction, further elucidation of Wnt downstream targets and analysis of interactions with other signaling cascades (e.g., BMPs) is the next logical step in understanding, at a molecular level, the nature of neural crest induction. To this end, we examined quantitatively the temporal changes in the responsiveness of selected candidate genes, expressed by premigratory neural crest cells, to Wnt and BMP signaling. Because these markers are expressed in the domain from which neural crest cells emigrate (dorsal neural tube), they represent potential downstream targets of neural crest induction. The results of this study reveal dynamic and differential responses to Wnt and BMP signaling of these potential targets as a function of time and an interesting profile where many of the transcription factors (e.g., FoxD3, Pax3 and Msx1) are rapidly up-regulated in response to Wnt signaling in premigratory neural crest but then down-regulated as neural crest cells initiate migration. In contrast, BMP4 maintains gene expression at 36 h of incubation. This is the first study to quantitatively examine changes in gene expression in response to Wnt and BMP signaling, as all previous studies have used in situ hybridization only, which is inherently nonquantitative, as a method to demonstrate gene responsiveness.
MATERIALS AND METHODS
Chick Embryo Culture
Fertilized chicken eggs were obtained from SPAFAS Laboratories (Charles River, Illinois) and incubated on their side at 37°C in humidified rocking incubators (Lyon Electric Co., Chula Vista, CA). Ethanol was used to clean the eggs before dissection. The embryo was removed from the yolk sac using forceps and scissors. A microscope was used to determine the stage of the embryo. The stage was identified by counting the number of somites according to Hamburger and Hamilton. Embryos were treated in dispase for 15 min at 37°C, and then intermediate neural plate tissue from 8 to 12 somite state (ss) embryos was dissected away from surrounding ectoderm and mesoderm tissues using tungsten needles in PB-1 standard medium. Intermediate neural plate tissue was explanted to collagen (BD Sciences) and grown directly in collagen in plastic tissue culture dishes.
Preparation of Control and Wnt Conditioned Medium and Other Reagents
S2 and Wg cells were obtained from Dr. Roel Nusse (Stanford University). Cells were grown as described (Leeuwen et al., 1994), and media was collected in one-tenth volume serum-free DMEM + penicillen/streptomycin (GIBCO). Control and Wnt-3a conditioned media was prepared as described (Shibamoto et al., 1998) and diluted in 5% serum before use in assays. For some experiments, recombinant mouse noggin (R&D Systems) was added to the intermediate neural plate cultures in the presence or absence of Wnt at a concentration of 0.3 μg/ml, the high end of the recommended ED50 for this inhibitor. Recombinant human BMP4 protein (R&D Systems) was used at a concentration of 50 ng/ml. Unless indicated otherwise, control or Wg conditioned medium and recombinant BMP4 were diluted in serum-free medium before addition to collagen explants.
RNA and cDNA Preparation by RT-PCR
Total RNA was prepared from the explants using either the RNA Isolation Kit (Stratagene) or the RNAqueous Total RNA Isolation Kit (Ambion) and the methods described therein. DNAse I (Roche) treatment was performed at 37°C for 1 h, and a standard 1:1 phenol/chloroform:isoamyl alcohol (24:1) extraction and ethanol precipitation at–20°C in the presence of 1 μl 25 mg/ml glycogen and 1/10th volume 5M ammonium acetate was performed to isolated the DNAse-treated total RNA. After washing with 75% ethanol and air drying, the RNA pellet was resuspended in 20 μl DEPC water. The RNA was converted to cDNA in the presence of random hexamers (IDT) and dNTPs (Roche) using SuperScript II RNase H-reverse transcriptase (Promega) and the accompanying buffers; the reaction was carried out at 42°C for 50 min. The enzyme was subsequently heat inactivated at 70°C for 15 min, and the cDNA was quantified using a spectrophotometer. PCR was performed on a Hybaid PCRExpress thermalcycler using the following parameters: 94°C, 6 min; 94°C, 45 s, 56°C, 45 s, 72°C, 2 min, for a total of 35 cycles; 72°C, 10 min. Forward and reverse primers for slug and GAPDH were designed as follows: Slug-F: 5′-ACC CCA TTA CTG TGT GGA CTA CAA-3′; Slug-R: 5′-TTG GAT CTG TCT GCG AAA GCC CTG-3′; GAPDH-F: 5′-AGT CAT CCC TGA GCGAA TG-3′; GADPH-R: 5′-AGG ATC AAG TCC ACA ACA CG-3′.
Quantitative Polymerase Chain Reaction (QPCR)
QPCR was performed using the 96 well-plate ABI 7000 QPCR machine in a TaqMan assay (Applied Biosciences). Gene-specific primers and probes were designed using the Primer Express program (Applied Biosystems) and obtained from Applied Biosystems. 50 μl reactions were performed using the 2X TaqMan master mix in the presence of ∼500–750 ng of cDNA, 300 nM of each primer, and 450 nM of each probe. Each sample was also run in three to five replicates to reduce any potential errors. After each run, baseline and threshold levels were set according to the protocols described (Applied Biosystems). Gene expression was then assessed using the standard curve assay method as described in the Applied Biosystems literature. Briefly, each QPCR run was performed to determine the relative expression of the gene of interest and a normalizer gene (chick ribosomal 18S) in our experimental sample populations, as well as the expression of the gene of interest and normalizer in a standard cDNA population that has been previously shown to already express the gene of interest and normalizer in measurable quantities. This standard cDNA population was typically total RNA prepared from a mixture of 8–12 ss embryos. A standard curve for the gene of interest and normalizer was then prepared by graphing the threshold cycle (Ct) values obtained for various amounts of the standard cDNA versus the log of the quantity of these amounts. From this curve and their respective Ct values, the quantity of the gene of interest (amount of gene product) and of the normalizer was extrapolated. The average quantity for the gene of interest and normalizer was calculated and then a normalized relative expression value was obtained by dividing the average quantity for the gene of interest by the average quantity for the normalizer. Fold induction was then determined by dividing the relative expression value for the gene of interest in the presence of Wg CM by the relative expression value for the gene of interest in the presence of the control CM.
Immunostaining
HNK-1 immunostaining was performed as described (Bronner-Fraser, 1987; Sechrist et al., 1995). Briefly, explants were fixed for 30 min at room temperature in the presence of 4% paraformaldehyde. Explants were then washed 2 × 5 min each in PTW (phosphate-buffered saline (PBS), 0.1% Tween). HNK-1 primary antibody was diluted 1:10 into PBS + 10% goat serum + 0.1% Triton X-100 + 0.1% bovine serum albumin (BSA) and incubated on the explants overnight at 4°C on a nutator. The explants were then washed 3 × 30 min each and incubated in a 1:1000 dilution of goat anti-mouse IgM-Alexa green (488 nm) secondary antibody into PBS + 10% goat serum + 0.1% Triton X-100 + 0.1% BSA. The explants were washed again as described above and imaged using an Axiocam Zeiss microscope. Images were manipulated in Adobe Photoshop.
Preparation of Riboprobes
Briefly, SP6 and T7 polymerases (Promega) were used to transcribe the antisense and sense riboprobes, respectively, in the presence of digoxygenin-11-UTP (Enzo) and 10X transcription buffer (Boehringer Mannheim) for two hours at 37°C. DNase I (Roche) treatment was performed for 15 min at 37°C. Probes were precipitated using 100 mM lithium chloride in the presence of 100% ethanol at -20°C for one hour. RNA was collected by centrifugation and washed two times with 70% ethanol. The pellet was dried and resuspended in DEPC water and stored at -80°C. The Pax3 probe was constructed in the same manner from a lab stock of quail Pax3 with 5Y deletion fragment. All solutions were made with DEPC water and are considered RNase-free.
In Situ Hybridization
Embryos or intermediate neural plate explants were rehydrated from methanol to PTW, and treated with 10 μg/ml Proteinase K (Roche) (incubation time in Proteinase K equals the stage of the embryo). The samples were postfixed in 4% paraformaldehyde/0.1% gluteraldehyde/PTW for 20 min and then washed three times in PTW. The samples were rinsed with PTW/hybridization mix (50% Formamide, 1.3X SSC, 5 mM EDTA, 50 μg/ml yeast tRNA, 0.2% Tween-20, 0.5% CHAPS, 100 μg/ml heparin) and then incubated in hybridization mix for two hours at 65°C. 40 μl of probe was added to 2 ml hybridization mix and incubated overnight to hybridize at 65°C. On the next day the samples were rinsed two times with prewarmed (65°C) hybridization mix, and then washed at room temperature with 1X MABT (maleic acid disodium salt, 0.1% Tween-20). Embryos or explants were incubated for one hour at room temperature in 1X MABT + 2% Boehringer Blocking Reagent (BBR) (Boehringer Mannheim). Samples were then incubated ≥ one hour at room temperature with 1X MABT + 2% BBR + 20% heat-treated sheep serum. Fresh 1X MABT + 2% BBR + 20% serum was added to the samples, along with a 1/2000 dilution of anti-digoxygenin-AP Fab fragments (Roche), and the samples were incubated overnight at 4°C in the dark. The samples were then rinsed three times with 1X MABT and then washed five times each for an hour with 1X MABT. The samples were then washed two times, ten minutes each, with NTMT (5M NaCl, 2M Tris-Cl (pH 9.5), 2M MgCl2, 0.1% Tween-20). To develop the in situs, embryos or explants were incubated with 1.5–2.0 ml NTMT + 3.5 μl BCIP + 4.5 μl NBT (Roche) at room temperature until desired color was reached. At this point, samples were rinsed and washed with PTW and refixed with 4% paraformaldehyde/0.1% gluteraldehyde/PTW for two hours. Embryos and explants were rinsed with PTW, and explants were embedded in collagen. Embryos were dehydrated through a methanol series and stored in 100% methanol.
Imaging In Situs
Photographs were taken with a Zeiss Axioscope. Images were processed and assembled using Adobe Photoshop.
RESULTS
Numerous genes with localized expression in the dorsal-most portion of the neural tube have been suggested to play a role in neural crest induction, migration and/or differentiation. Slug is a zinc finger transcription factor originally identified as a gene marking premigratory and early migrating neural crest cells (Nieto et al., 1994). It has broad functions in epithelial/mesenchymal transitions (Bronner-Fraser, 2002) and has been shown to be responsive to Wnt signals in other systems (Vallin et al., 2001). Pax3 is a paired box-containing transcription factor expressed in the dorsal neural tube and necessary for proper neural crest cell migration (Serbedzija and McMahon, 1997). Msx1 is a transcription factor expressed in the dorsal neural tube that is a target of BMP4 signaling, and has recently been suggested to be a Wnt target (Willert et al., 2002). RhoB is a small GTP binding protein of the Ras superfamily that is expressed in the dorsal neural tube and later in premigratory and migratory neural crest cells. It has been implicated in regulating neural crest delamination (Liu and Jessell, 1998) and has been identified as Wnt responsive in an immortalized mouse mammary tissue culture cell line (Taneyhill and Pennica, 2004). FoxD3, a member of the winged-helix or forkhead transcription factor family, marks premigratory and migratory neural crest cells, is genetically downstream of Pax3 (Dottori et al., 2001), and has been shown to be a target of Wnt signaling in Xenopus (Pohl and Knochel, 2001). Cadherin 6B is expressed in the dorsal neural tube in the premigratory crest, and its expression is subsequently down-regulated upon migration (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998).
Based on the ability of Wnt signaling to induce neural crest migration and up-regulate the crest marker slug, we hypothesized that these dorsal neural tube marker genes are potential responsive downstream targets of Wnt signaling, thus establishing a pathway by which neural crest induction may be mediated. To analyze this process under defined conditions, we took advantage of an in vitro culture system in which intermediate regions of the open neural plate were dissected and cultured in the presence or absence of Wnt protein. The dissected tissue is “naïve” in that it does not express any characteristic dorsal neural tube/premigratory neural crest markers like slug, SoxE genes or FoxD3 (Figure 1). To examine relationships between candidate genes and Wnt-mediated neural crest induction, we used QPCR to assess quantitative changes in the response of several genes in induced versus control neural plates as a function of time.
Figure 1.
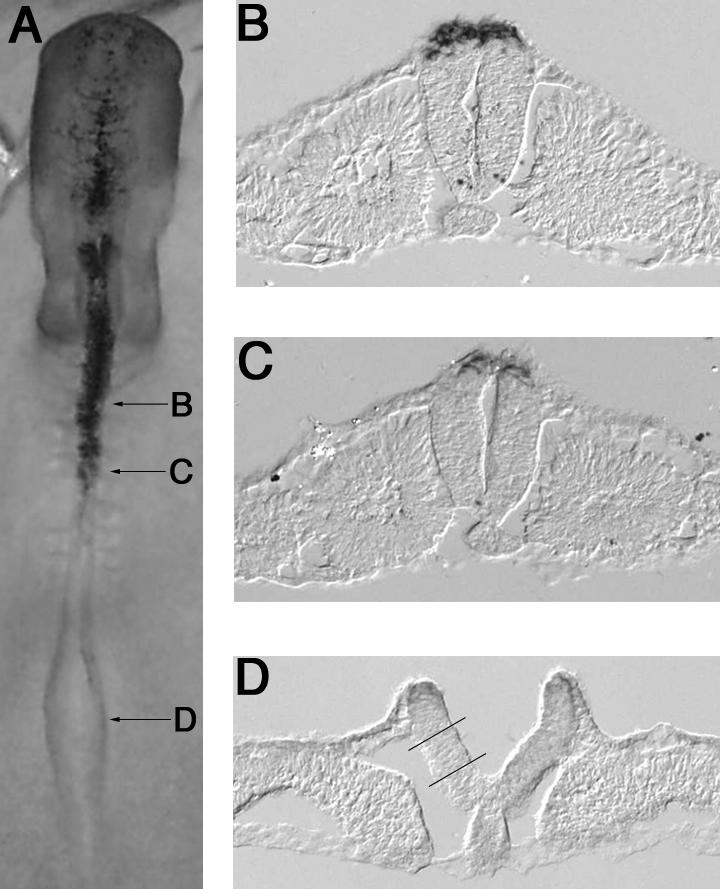
Whole mount (A) and sections (B-D) of a stage 10 embryo processed for in situ hybridization with the neural crest marker gene FoxD3. Lines in (D) indicate region of intermediate plate dissected and used in experimental assays.
Wnt Signaling Induces Intermediate Neural Plate Tissue to Generate Neural Crest
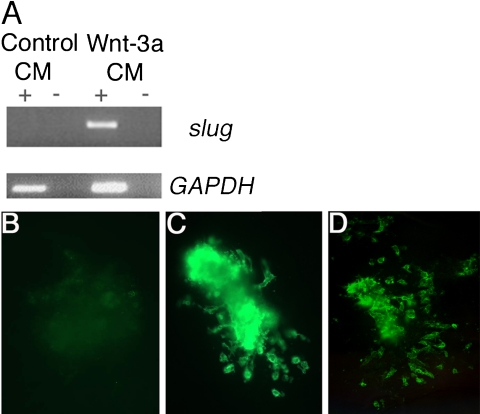
It has been previously shown that Drosophila Wg protein, as a source of soluble Wnt, induces expression of neural crest markers (e.g., slug and HNK-1 expression) and migratory cells in intermediate neural plate tissue (Garcia-Castro et al., 2002). To test whether vertebrate Wnt proteins have similar effects to fly Wg, we compared the ability of conditioned medium prepared from a cell line expressing a vertebrate Wnt, Wnt-3a, to substitute for Wg conditioned medium in this assay (Figure 2). Treatment of intermediate neural plate explants with Wnt-3a conditioned medium up-regulates slug after 24 h of culture (Figure 2A). As previously shown, treatment with Wg CM for 48 h results in the production of migratory neural crest cells that bear the HNK-1 epitope as determined by immunostaining (Figure 2C), and vertebrate Wnt-3a conditioned medium functions analogously to promote neural crest migration (Figure 2D). These results show that Wnt-3a functions similarly to Wg, suggesting that both invertebrate and vertebrate sources of Wnt can mediate neural crest induction from intermediate neural plates.
Figure 2.
Wnt signaling induces intermediate neural plate tissue to express the neural crest marker slug (A) and to generate migratory neural crest cells (B). In (A), RT-PCR for slug shows that a vertebrate Wnt signal, Wnt-3a, can up-regulate slug expression after 24 h. GAPDH is shown as a control, + = +RT, - = -RT, and CM = conditioned medium. In (B-D), immunostaining for the migratory neural crest cell surface marker HNK-1 shows that only migrating neural crest cells express HNK-1 (after 48 h of incubation in Wg or Wnt conditioned medium). B = Control conditioned medium, C = Wg conditioned medium, D = Wnt-3a conditioned medium.
Wnt and BMP Signaling Differentially Regulate Expression of Dorsal Neural Tube Marker Genes in a Temporal Manner
To assess the responsiveness of various candidate genes to Wnt and BMP signaling in vitro, explanted intermediate neural plate tissues from 8 to 12 ss chick embryos were cultured in collagen in the presence of Wg (or Wnt-3a), recombinant human BMP4 (50 ng/ml) or control medium diluted into serum-free DMEM. No differences were noted between the Wg or Wnt-3a medium, so the results are presented for Wg conditioned medium. Cultures were maintained from 6 to 36 h after which times RNA was isolated and cDNA prepared; the most informative time points were at 18, 24 and 36 h. QPCR was then performed using primers designed to a particular candidate gene of interest. Within each QPCR experiment, three to five replicates were run to ensure precision and accuracy in the data set, and each experiment was performed at least twice. The results presented represent an average of two to four experiments for each gene. Figure 3 and Figure 4 show the fold induction of each gene in response to Wnt or BMP treatment, respectively. In most instances, genes normally expressed in the dorsal neural tube showed very little expression in intermediate neural plate tissue cultured in control conditioned medium.
Figure 3.
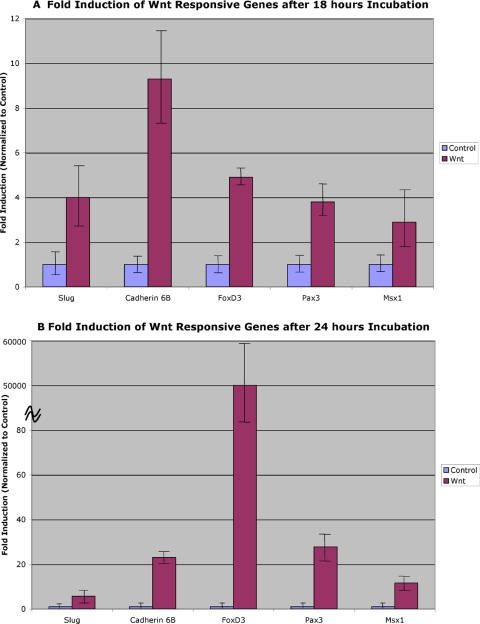
Fold up-regulation of dorsal neural tube genes in response to Wnt after 18 h (A) and 24 h (B) of incubation. Values represent the calculated fold induction of various dorsal neural tube genes in control- or Wnt-treated samples determined after normalization to 18S expression, and then to control expression, in the respective samples. Additional information on calculations is available in the MATERIALS AND METHODS.
Figure 4.
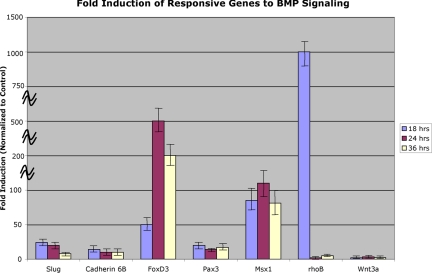
Fold up-regulation of dorsal neural tube genes in response to BMP4 after 18, 24 and 36 h of incubation. Values represent the calculated fold induction of various dorsal neural tube genes in BMP4-treated samples determined after normalization to 18S expression, and then to control expression, in the respective samples. Calculated standard deviations for control-treated samples were <0.4 (data not shown). Additional information on calculations is available in the MATERIALS AND METHODS.
Slug. Slug is one of the earliest known markers of the premigratory neural crest (Nieto et al., 1994). In response to Wnt-mediated induction, slug was found to be induced ∼fivefold after 18 h of culture, and this induction persisted at 24 and 36 h of culture, reaching a maximum of ∼11-fold after 24 h (Figures 3A, 3B). Interestingly, no slug was detected in either control or experimentally induced neural plates at either 6 or 12 h of incubation. In the presence of BMP4, the response of slug was more robust, with an up-regulation of ∼24-fold, 20-fold, and eightfold after 18, 24, and 36 h of culture, respectively (Figure 4).
Cadherin 6B. Cadherin 6B is expressed in the dorsal neural tube before neural crest emigration and is, therefore, an early marker of premigratory crest (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998). It is expressed before the onset of slug expression in the folds of the open neural plate but is down-regulated at the onset of emigration of neural crest cells from the dorsal neural tube (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998). After 18 and 24 h of culture with Wnt, cadherin 6B is induced 11- and 60-fold, respectively (Figure 3A, 3B), over levels in control explants, indicating that cadherin 6B is a robust Wnt responsive gene. Interestingly, no cadherin 6B expression can be detected by 36 h postinduction. Similarly, no cadherin 6B was detected in either control or experimentally induced neural plates at either 6 or 12 h of incubation. Thus, cadherin 6B is rapidly up-regulated via Wnt signaling by 18 h, persists for 24 h, but then is down-regulated. Because this appears to be one of the earliest neural crest markers, these results suggest that the response to Wnt signaling in this in vitro system begins around 18 h. In comparison, cadherin 6B is up-regulated 14-fold at 18 h and 10-fold at both 24 and 36 h of culture in the presence of BMP4 (Figure 4). Thus, cadherin 6B appears to be regulated by both Wnt and BMP signals.
FoxD3. FoxD3 is a marker of both premigratory and migratory neural crest, and its overexpression in the neural tube increases the numbers of migratory neural crest cells (Kos et al., 2001; Pohl and Knochel, 2001). FoxD3 is elevated after 18 h of Wnt-mediated neural crest induction (Figure 3A). However, it shows remarkable responsiveness to Wnt signaling by 24 h of incubation when compared with the control (Figure 3B). To date, this is the most dynamic response we have noted for all the candidate genes tested in our assay system. The decline in expression is equally marked. At 36 h, there is no detection of FoxD3 expression in our in vitro assay. FoxD3 is also highly up-regulated in response to BMP4 treatment (Figure 4), with an initial increase of ∼50-fold observed at 18 h, followed by an up-regulation of ∼500-fold at 24 h, then decreasing to ∼200-fold at 36 h. Thus, FoxD3 represents a robust responsive gene to both growth factors in this in vitro assay.
Pax3. Pax3 is an important gene involved in the regulation of neural crest migration and its expression overlaps with the premigratory neural crest domain in the dorsal neural tube (Serbedzija and McMahon, 1997; Mansouri et al., 2001). Pax3 is up-regulated ∼4- and 30-fold after 18 and 24 h of incubation, respectively, by Wnt signaling (Figure 3A, 3B). It is important to note that in the presence of control conditioned medium, Pax3 is expressed at a basal level. However, similar to cadherin 6B, Wnt signaling up-regulates Pax3 expression well above this basal level. At 36 h of incubation, however, Pax3 is not detected in this assay. Pax3 expression is also increased in response to BMP4 treatment. At 18 h, Pax3 is up-regulated ∼20-fold, with a slight decrease in expression at 24 h (14-fold up-regulation), followed by continued expression at 36 h (17-fold increase in expression) (Figure 4).
Msx1. Msx1 is expressed in the dorsal neural tube and ectoderm of early embryos (Timmer et al., 2002; Bach et al., 2003) and has been shown to be regulated by both BMP and Wnt signaling (Willert et al., 2002). After both 18 and 24 h of culture in the presence of Wnt signals in our experiments, Msx1 is up-regulated ∼fivefold (Figure 3A, 3B). Again, at 36 h of incubation, Msx1 is not detected in this assay. Msx1 expression, however, shows consistent up-regulation in response to BMP signals at all time points examined, ranging from an approximate 80- to 100-fold increase in expression (Figure 4).
RhoB. RhoB is a marker of premigratory and migratory neural crest and has been identified as a Wnt target gene in other systems (Taneyhill and Pennica, 2004). Surprisingly, rhoB transcript levels are lower in samples exposed to Wnt signals, with an approximate twofold down-regulation of rhoB in response to Wnt signaling in our assay (data not shown). RhoB shows marked up-regulation in response to BMP signaling, however, for all time points tested, with an approximate 1000-fold increase in expression at 18 h, followed by a subsequent decrease in gene expression at the 24 and 36 h time points (only an approximate twofold and fivefold up-regulation, respectively) (Figure 4). As rhoB is required for delamination of cells from the dorsal neural tube (Liu and Jessell, 1998), it is possible that the gene may be kept off or at low levels during times before delamination. It is interesting to note the differential regulation of rhoB by BMP and Wnt, as both signals are implicated in aspects of neural crest induction, migration and differentiation.
Wnt3a. As Wnt-3a is expressed in the dorsal neural tube later in chick development and is involved in the expansion of the neural crest (Ikeya et al., 1997), we examined its expression in response to treatment with Wnt or BMP4. No detectable Wnt3a expression was apparent in our assay in response to Wnt signaling (data not shown). However, BMP4 signaling up-regulated Wnt3a expression ∼2- to threefold at all time points examined (Figure 4). This observation is very interesting in light of the recent findings by Burstyn-Cohen et al. that demonstrate that BMP regulates expression of Wnt1 during the process of neural crest delamination (Burstyn-Cohen et al., 2004).
BMP4. BMP4 is expressed in the dorsal neural tube of the developing chick, and is particularly important later, after induction, for the onset of neural crest emigration from the dorsal neural tube (Sela-Donenfeld and Kalcheim, 1999). Therefore, we examined if Wnt signaling up-regulates BMP4 expression in our assay. No detectable BMP4 expression, however, is observed in our assay, either in the presence or absence of Wnt signals. Thus, Wnt signaling does not appear to regulate BMP4 expression in our in vitro assay. Similarly, addition of recombinant mouse Noggin (0.3 μg/ml) plus Wnt did not alter expression of slug, cadherin 6B, or Pax3 at 18 or 24 h (data not shown).
Addition of Wnt Six Hours after Explantation Leads to Further Increases in Gene Expression
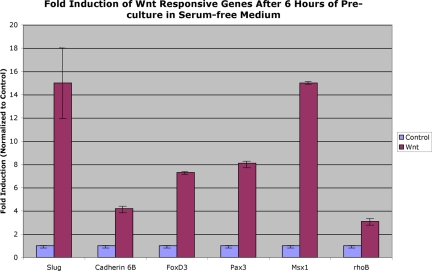
The earliest up-regulation of gene expression in response to Wnt signaling is observed at 18 h of culture in Wnt conditioned medium. No detectable gene expression was observed for the early premigratory crest markers slug or cadherin 6B at 6 and 12 h of culture when Wnt was added at the time of explantation. To better understand the effects and timing of Wnt signaling, we grew our collagen explants in serum-free media for 6 h before adding Wnt and cultured for an additional 18 h (24 h total culture time). The resulting gene expression for these experiments (effective time with Wnt = 18 h) demonstrates that gene expression is up-regulated to levels approximately two- to fourfold higher than that seen without pretreatment at 18 h for all genes examined with the exception of cadherin 6B (compare Figure 5 with Figure 3A). Interestingly, pretreatment with serum-free media before the addition of Wnt resulted in the up-regulation of rhoB by ∼threefold, which is in striking contrast to that seen for rhoB in the previous experiments.
Figure 5.
Fold up-regulation of dorsal neural tube genes after 6 h of culture followed by 18 h of Wnt treatment. Values represent the calculated fold induction of various dorsal neural tube genes in control- or Wnt-treated samples determined after normalization to 18S expression, and then to control expression, in the respective samples. Additional information on calculations is available in the MATERIALS AND METHODS.
In Situ Hybridization of Intermediate Neural Plate Explants Treated with Wnt In Vitro Confirms Up-Regulation of Candidate Genes Observed by QPCR Experiments
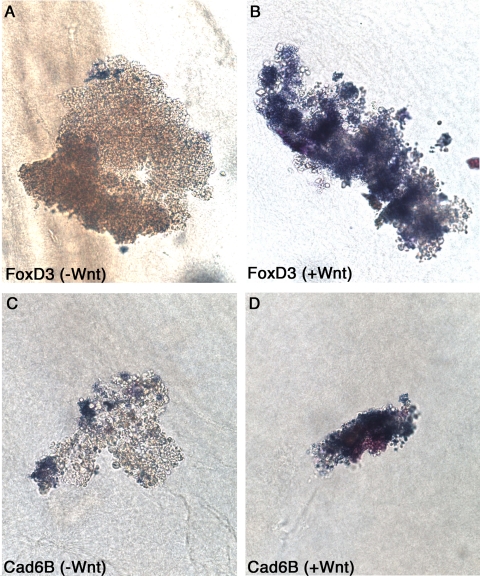
To provide an independent, albeit nonquantitative, verification of our QPCR data, we performed in situ hybridization on intermediate neural plate explants cultured in collagen in the presence or absence of Wnt for 24 h with representative neural crest markers. Figure 6 illustrates the result for two of the most robust Wnt responsive genes, FoxD3 (6A and 6B) and cadherin 6B (6C and 6D), demonstrating proof of principle of our QPCR assay. Only a few FoxD3- and cadherin 6B-positive cells are observed in the control (Figure 6A and 6C, respectively), whereas the number of positively-staining cells (and intensity of staining) is significantly increased in those explants treated with Wnt (Figure 6B and 6D, respectively). In situ hybridizations on explants for other genes after 18 and 24 h of culture showed similar up-regulation in the presence of Wnt (data not shown). These results nicely corroborate our in vitro QPCR data and provide further evidence that these genes are bona fide Wnt responsive genes.
Figure 6.
Whole mount in situ hybridization on intermediate neural plate explants for candidate genes confirms QPCR data. In situ hybridization was performed after 24 h of culture in collagen in the presence (B and D) or absence (A and C) of Wnt for FoxD3 (A and B) and cadherin 6B (C and D). Note increased staining in the presence of Wnt for both genes.
In Vivo Expression of Wnt Responsive Genes
QPCR on Embryonic Chick Neural Tubes. As an additional means by which to correlate the in vitro QPCR assay data with in vivo gene expression, QPCR was performed on embryonic chick stage 16 neural tubes. Two different axial levels were chosen for analysis to approximate the age of the intermediate neural plate explants used in the in vitro collagen assay: 1) at the level of the future forelimb where the process of neural crest emigration from the neural tube is beginning and 2) at the level of the future hindlimb were neural crest cells remain premigratory at this stage. These time points approximately correlate with the status of neural crest formation at 36 and 24 h, respectively, when recapitulating neural crest formation in vitro. QPCR was performed on these tissues for cadherin 6B, FoxD3, Msx1, Pax3 and slug. In agreement with the in vitro data, the results show that the expression of these genes in the dorsal neural tube in vivo is markedly higher in the region of the embryo corresponding to the premigratory crest, ranging from 1.5-to fourfold up-regulation (an average of two independent experiments, each done with four replicates per sample; data not shown) in comparison with gene expression at more rostral levels, correlating with the onset of neural crest migration.
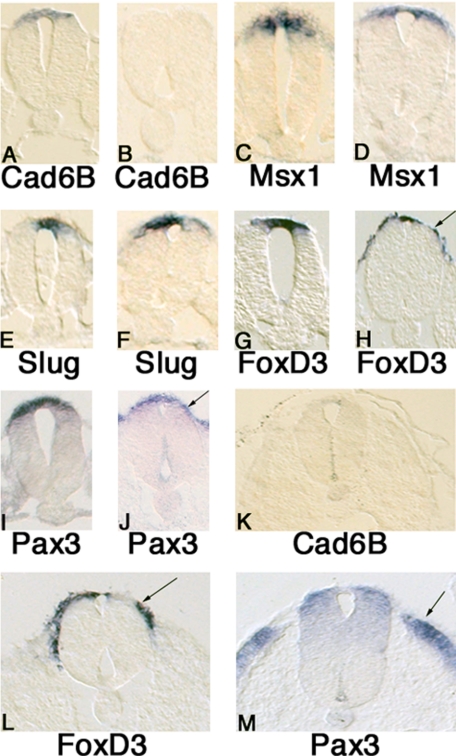
Distribution of Marker Genes in Premigratory and Early Migrating Neural Crest Cells. To further correlate the in vitro QPCR assay data with gene expression in vivo, we used whole mount in situ hybridization followed by histological analysis to assess the expression of the Wnt responsive genes during the phases of chick development corresponding to when crest cells are premigratory and at early stages of migration. Whole mount in situ hybridization was performed on stage 10 and 16 chick embryos, followed by sectioning at 14 microns in order to visualize gene expression at various axial levels: before neural crest migration adjacent to newly formed somites (caudal trunk), early neural crest migration (midtrunk) and advanced neural crest migration (rostral trunk).
Consistent with the in vitro QPCR assay data, highest levels of neural crest gene expression are observed in the premigratory neural crest of stage 16 embryos. For example, cadherin 6B is strongly expressed in the dorsal neural tube in areas of the embryo in which the neural crest has not yet started to migrate. Its expression is extinguished in the dorsal neural tube during neural crest migration (Figure 7A, 7K). For most of the genes examined, levels of expression appeared lower in regions corresponding to early migrating and advanced neural crest migration (Msx1, slug Figure 7C-F). However, an important difference between in vitro recapitulation of neural crest induction and the patterns observed in the embryo is that gene expression for all genes but cadherin 6B is maintained at early migratory stages in vivo, albeit at a lower level than at premigratory stages. This is particularly striking in the case of FoxD3 (Figure 7G,H; Figure 7L), where high levels are observed both in premigratory and migrating neural crest cells. Pax3 also is maintained in the dorsal neural tube at all stages examined; however, the levels of expression appear more robust at premigratory than at migratory stages (Figure 7I,J; Figure 7M). These results suggest that, for maintenance of expression of neural crest marker genes in vivo, additional signals other than Wnt are required, and that these signals may be absent when neural crest induction is recapitulated in vitro by Wnt signaling.
Figure 7.
Transverse sections through the trunk of stage 16 embryos at axial levels before neural crest migration (A,C,E,G,I), during early stages of neural crest migration (B,D,F,H,J), and through rostral regions (K,L,M). A,B) Cad6B is expressed in premigratory neural crest cells but is down-regulated as they migrate from the neural tube. C,D) Msx1 is expressed in the dorsal neural tube at both premigratory and early migratory stages, but at lower levels at the latter stage. E,F) Slug is expressed in the dorsal neural tube during both premigratory and migratory stages. G,H) FoxD3 is expressed in the neural tube at both premigratory and migratory stages and expression continues on migrating neural crest cells (arrow), though dorsal neural tube expression is lower at later stages. I,J) Pax3 is expressed in the dorsal neural tube at premigratory and early migratory stages (arrow), though at somewhat lower levels at the later stage. K) At the anterior-most trunk level where neural crest cells are actively migrating, no cad6B expression is observed. L) At the midtrunk level during a time of active neural crest migration, FoxD3 is expressed strongly on migrating neural crest cells (arrows) and at low levels in the dorsal neural tube. M) At rostral levels of the anterior trunk, Pax3 expression is present in the dorsal half of the neural tube, though at lower levels than at earlier times of neural crest migration (arrow).
DISCUSSION
By recapitulating neural crest induction in vitro using an intermediate neural plate collagen explant assay system in the presence of a Wnt or BMP signal, we have generated the first quantitative description of the temporal response to Wnt or BMP signaling of various genes that are normally expressed in the premigratory neural crest/dorsal neural tube. In addition, we have correlated this temporal expression with the in vivo expression patterns of these genes at points in development corresponding to premigratory and early migratory neural crest stages by examining gene expression through QPCR and in situ hybridization.
Wnt signaling plays multiple roles in neural crest development in various organisms, from the initial induction phase to the subsequent processes of delamination and the specification of sensory neurons and pigment cells. In the chick, Wnt-6 is expressed in the nonneural ectoderm adjacent to the neural plate, while the neural folds express BMP4 (Garcia-Castro et al., 2002). Subsequently, Wnt-1 and Wnt-3a are expressed in the dorsal neural tube, where they appear to be required for the expansion of dorsal neural precursors in mouse (Ikeya et al., 1997), and the specification of cell types for dorsal interneurons (Muroyama et al., 2002). In addition, Xenopus Wnt-1 and Wnt-3a regulate the dorsal/ventral patterning of the neural tube and function in neural crest differentiation through the regulation of crest marker genes such as krox-20, slug, and AP-2 (Saint-Jeannet et al., 1997). Expression of both Pax3 and Msx1, both markers of the lateral neural plate and the region from which the neural crest will subsequently arise, is blocked by expression of dominant negative XWnt-8 (Bang et al., 1999). Pax3 and Zic1 genes are also involved in the differentiation of the neural crest in Xenopus through a mechanism that requires Wnt signaling (Sato et al., 2005). Slug has long been studied as an early marker of the neural crest in Xenopus, and current evidence implicates it as a target of Wnt signaling (LaBonne and Bronner-Fraser, 1998; Vallin et al., 2001). Monsoro-Burq and colleagues recently demonstrated that Wnt signaling initiates slug expression through Pax3, and that Pax3 acts downstream of Msx1 during the process of neural crest induction (Monsoro-Burq et al., 2005). Wnt signaling activates early expression of the Xenopus homolog of FoxD3, and the overexpression of FoxD3 in Xenopus leads to a loss of neural crest formation (Pohl and Knochel, 2001). Finally, Wnt signaling has been observed to specify the development of melanocytes in chick, with a concomitant decrease in glial and neuronal lineages (Jin et al., 2001). This observation can be extended across species; in mice, Wnt1 signaling results in the expansion and differentiation of the melanocyte lineage (Dunn et al., 2000), and in zebrafish, Wnt signaling enhances pigment cell formation by the medial neural crest (Dorsky et al., 1998). Thus, there appears to be a cascade of events such that the same signaling pathway is used reiteratively in neural crest development. Given these multiple, temporally-regulated functions, it is not surprising that the downstream responsive genes involved during these developmental processes are different, as gene expression is very much context- and temporally-dependent.
Recently, Burstyn-Cohen et al. showed that inhibition of Wnt signaling in the neural tube of developing chick embryos (at the trunk level of 16- to 23-somite stage embryos) results in a down-regulation of cadherin 6B, Pax3 and Msx1 expression in the dorsal neural tube (Burstyn-Cohen et al., 2004). These authors hypothesize the Wnt acts downstream of BMP signaling during the process of neural crest delamination. This point is an important distinction between their study and ours. Neural crest delamination is an event that occurs after induction, at a later time point during neural crest development, such that the starting material and timing for each process are different. Hence, differences in gene expression occur upon exposure to Wnt during the processes of induction versus delamination because each process utilizes its own unique repertoire of genes. For example, Burstyn-Cohen et al. do not find any changes in slug and FoxD3 expression upon inhibition of Wnt signaling, whereas we find that the expression of these genes is up-regulated during Wnt-mediated neural crest induction.
We noted a consistent temporal expression profile of Wnt responsive genes. Initial gene expression was observed at 18 h after explantation, peaking at 24 h and declining thereafter. In addition, we found that two early markers of the premigratory neural crest (slug and cadherin 6B) are not detected in QPCR assays conducted after 6 and 12 h of incubation with control or Wnt conditioned medium. These results imply that 18 h of in vitro incubation may represent a time of early response to signaling events following recapitulation of neural crest induction in vitro. This lag in response time is not surprising, given that the physical dissection process itself might result in such a delay. Furthermore, it is well known that cultured tissue is generally delayed in comparison to that left in situ (Selleck and Bronner-Fraser, 1995; Gammill and Bronner-Fraser, 2002). The placement of tissue that would normally not form neural crest in collagen, an environment that is devoid of additional in vivo developmental cues, could explain the lag in the observed induction response. Such signaling molecules involved in neural induction in avian embryos include FGFs, Wnts, and BMPs, and the downstream pathways that they activate (Heeg-Truesdell and LaBonne, 2004). Interestingly, when we cultured explanted intermediate neural plates in collagen in the presence of serum-free media before the addition of Wnt, the resulting gene expression was actually higher than that observed in the absence of preculture. The results suggest that changes and/or patterns of gene expression begin after explantation of tissue to collagen, and that the addition of Wnt alters these expression patterns.
After one day in culture, substantial increases in gene expression in response to Wnt were noted for many of the candidate genes tested. This robust response to Wnt signaling at 24 h may signify a time during which enough downstream factor(s) become activated to permit accumulation of the transcripts of these candidate genes. Alternatively, it is possible that, at this 24 h time point, other downstream interactions set into motion by the Wnt signaling pathway have fostered the stabilization of these Wnt responsive transcripts, and the read-out assessed by QPCR reflects the posttranscriptional stabilization of such transcripts rather than true transcriptional up-regulation. Another possible explanation of the delayed response is that the induction of these candidate genes by Wnt is indirect. We are unable to carry out our experiments in the presence of the protein synthesis inhibitor cycloheximide because all canonical Wnt signaling (signaling through β-catenin) is sensitive to cycloheximide, precluding such an analysis (R. Nusse, pers. comm.; see also Willert et al., 2002). Thus, it is not possible at this time to discriminate between direct and indirect Wnt targets with our current assay system. However, Wnt does not appear to up-regulate BMP4, and Noggin does not appear to alter early Wnt responsive genes.
Like Wnt, BMP signaling also results in increases in neural crest marker gene expression. Gene expression in response to BMP4 was remarkably robust, with candidate genes up-regulated after 18 h of culture, and with up-regulation persisting throughout the 36 h time course. The differences in levels of up-regulation in gene expression by each pathway may possibly be attributed to the concentration of the growth factor in the media and the ability of the tissue to respond by initiating gene expression. Thus, caution must be used before drawing direct comparisons between the response levels to Wnt and BMP, in particular because we have used purified recombinant human BMP4 compared with Wnt conditioned medium, such that the effective concentrations of Wnt and BMP4 may be quite different. In our assays both Wnt and BMP were added to neural plate tissue in serum-free medium. Previously our lab showed that explants of intermediate neural plate tissue cultured in collagen in the presence of BMP4 in serum-free medium failed to produce HNK-1-positive migrating crest cells (Garcia-Castro et al., 2002). In contrast, Liu and Jessell found that BMP4 in medium containing supplements was able to induce migratory neural crest cells (Liu and Jessell, 1998). Consistent with these observations, we did not observe the production of migratory neural crest cells from intermediate neural plate explants at the concentration of BMP4 used in our assay or at a 10-fold higher concentration (data not shown). However, we did observe up-regulation in expression of many molecular markers associated with neural crest induction. It is interesting to note that changes in marker gene expression do not necessarily correlate with the production of a bona fide neural crest cell, as characterized by a migratory mesenchymal cell that expresses HNK-1 (Garcia-Castro et al., 2002). In contrast to Wnt signaling, BMP signaling can initiate appropriate gene expression but apparently cannot complete the program of neural crest generation. In support of this possibility, neural crest migration involves the down-regulation of cadherin 6B in vivo (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi 1998). Neural crest cells begin to migrate from our explants at 36 h, at which time BMP maintains elevated cadherin 6B expression, while it is down-regulated in Wnt-treated explants. One intriguing possibility is that the maintenance of cadherin 6B expression by BMP but loss with Wnt may be responsible for the difference in neural crest migration observed in these explants in the presence of these signaling molecules. In the embryo, BMP4 is not expressed in the early ectoderm at the right time to initiate gene expression, but rather comes on later, in the neural folds and neural tube, where it may act to maintain gene expression (Liem et al., 1995; Selleck et al., 1998; Garcia-Castro et al., 2002; Kleber et al., 2005). In addition, the production of neural crest cells by intermediate neural plate explants treated with BMP in the presence of some serum components appears to be a Wnt-dependent process (our unpublished data). Our in vitro gene expression observations may therefore reflect the type of response seen later on in the developing embryo, upon exposure to endogenous BMP signals.
Interestingly, at 36 h of Wnt treatment, all genes were down-regulated to baseline levels after induction with the exception of slug that was still maintained but at relatively low levels. The fact that many of these genes get turned off at the time when neural crest cells become migratory may not be surprising given that several of these markers, such as cadherin 6B, are turned off upon crest emigration (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi 1998). However, FoxD3 (and Pax3 to a lesser extent) are known to be expressed in the dorsal neural tube as well as transiently in migratory neural crest (Serbedzija and McMahon, 1997; Dottori et al., 2001; Kos et al., 2001; Mansouri et al., 2001), but were down-regulated at 36 h in the neural plate explants. This may be due to the absence in vitro of other factors that would maintain the expression of these genes in vivo. The down-regulation of gene expression at 36 h of culture corresponds to a time when neural crest cells are just beginning to emigrate from the explants, consistent with the idea that Wnt signaling may initiate the process of neural crest formation but may not be sufficient to sustain the neural crest program by up-regulating the entire range of markers for migrating crest. With this in mind, it is not surprising then that another signaling pathway, such as BMP4, would allow for maintained up-regulation of gene expression at later times in development. Clearly, this must be indirect, since our data suggest that Wnt does not turn on BMP4 in the context of this assay at the time points examined.
Wnt signaling, however, does appear to be sufficient for maintenance of slug expression in vitro, consistent with the known expression pattern of slug in premigratory and transiently in migratory neural crest. Interestingly, the response of slug to Wnt signals is the least robust out of all the candidate genes tested, and yet its expression is sustained over all times tested. One possibility is that only small increases in slug expression are sufficient to facilitate the emigration process. It is known that levels of many other transcription factors, such as β-catenin, are tightly regulated, and small changes in these levels are sufficient to alter developmental programs (Ahmed et al., 1998). Moreover, the response of slug to Wnt signals may reflect a direct regulation by Wnt signaling. Consistent with this possibility, analysis of the slug promoter has revealed several functional Lef/TCF binding sites, implicating it as a Wnt target regulated through β-catenin/Lef-TCF interactions (Vallin et al., 2001). Furthermore, Sela-Donenfeld and Kalcheim (1999) have shown that Slug mRNA and protein levels are not altered after perturbation of BMP4 expression levels in vivo, thus arguing that Slug transcriptional regulation is independent of BMP4 signaling, at least at the later stage of crest delamination (Sela-Donenfeld and Kalcheim, 1999).
The down-regulation observed for rhoB in response to Wnt signaling is striking given its known up-regulation by molecules such as BMPs, particularly during the process of neural crest delamination (Liu and Jessell, 1998). The interplay between BMP and Wnt signaling during neural crest development has been the subject of much debate. In our experiments, although we see some migratory crest cells as early as 36 h, the down-regulation we see in response to Wnt signaling was observed before crest emigration. However, rhoB expression is up-regulated at all time points in response to BMP signaling. Besides our current in vitro BMP4 data, Liu and Jessell have also shown that BMP4 up-regulates rhoB expression in chick neural plate cells, and that rhoB is expressed in the dorsal neural tube as well as transiently in early migrating neural crest cells but is down-regulated in neural crest cells during more advanced stages of migration (Liu and Jessell, 1998). Our data are consistent with two models. One possibility is that Wnt and BMP signals act sequentially such that Wnt signaling initially keeps rhoB expression low in order to preclude premature crest delamination and, later, before delamination, the Wnt signal is either inactivated and/or another signal, such as BMP4, overrides it to permit activation of rhoB and neural crest delamination. Alternatively, parallel pathways may exist, such that Wnt and BMP signaling pathways interact concomitantly to regulate the expression of rhoB in the dorsal neural tube, maintaining it at a level that is subthreshold for the delamination process. Signals and/or cues for delamination may then arise due to increased BMP signaling and/or the presence of other molecules that lead to the up-regulation of rhoB to levels sufficient for delamination and subsequent migration. Our data are still consistent with results for rhoB expression reported by Burstyn-Cohen et al., but during neural crest induction rather than delamination (Burstyn-Cohen et al., 2004). It is not surprising, then, that the response of rhoB to Wnt varies, given the different developmental contexts.
Using this in vitro assay system to recapitulate neural crest induction, we have previously shown that Wnt signals are necessary and sufficient for the induction process (Garcia-Castro et al., 2002). Furthermore, Wnt signaling is sufficient to foster neural crest migration, as HNK-1-positive migrating neural crest cells are observed upon treatment of cultured intermediate neural plate tissue with Wnt conditioned medium (Garcia-Castro et al., 2002 and present results). Thus, this in vitro system does not require additional signals besides Wnt to promote both the induction and emigration processes. However, given the in vivo evidence for the requirement of BMP for emigration (Sela-Donenfeld and Kalcheim, 1999), it is possible that Wnt signaling by-passes this need in vitro, permitting emigration to occur in our assay. Given the existing evidence and our in vitro and in vivo observations, we propose that Wnt signaling is required for the initial phase of neural crest induction, whereas signaling by other growth factors, such as BMPs, is required later to activate the expression of some of the genes found in the early migratory crest, to facilitate processes such as delamination.
Recent screens have been performed to identify Wnt target genes in a variety of different systems, and some of these screens have identified the same genes that we have examined to be direct Wnt targets (Willert et al., 2002; Buttitta et al., 2003; Prieve and Moon, 2003; Taneyhill and Pennica, 2004). Willert et al. reported Msx1 to be a direct transcriptional target of Wnt signaling in human embryonic carcinoma cells, showing activation upon Wnt treatment after 2 h in culture (Willert et al., 2002). Furthermore, Prieve and Moon identified BMP4 to be up-regulated by Wnt1 signaling in C57MG cells using a DNA microarray chip (Prieve and Moon, 2003). It has also been shown that rhoB is transcriptionally regulated by Wnt signaling in a mouse mammary epithelial cell line (Taneyhill and Pennica, 2004). Our results show that recapitulating neural crest induction in vitro clearly leads to the activation of genes implicated in neural crest induction in vivo, lending credence to our assay system. The temporal data obtained from our experiments make it possible to identify a time point (18 h) to profile genes activated early in response to Wnt signaling (or other signaling pathways) using this in vitro culture system.
This is the first report to quantitate the expression levels of genes up-regulated by Wnt and BMP4 signaling as a function of time after neural crest induction, both in vitro and in vivo. Armed with this knowledge, we hope to further elucidate the relationship between Wnts and neural crest by identifying additional, and potentially novel, targets of Wnt signaling during the induction of the neural crest.
Acknowledgments
This work was supported by NIH grant NS36585 to MBF. L. A. Taneyhill was supported in part by an NRSA fellowship from the NIH (1 F32 HD43535-01A2). Many thanks are given to Dr. Martin Garcia-Castro for technical and editorial assistance. We thank Dr. MariaElena de Bellard and Dr. Edward Coles for critical reading of the manuscript.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0210) on August 31, 2005.
References
- Ahmed, Y., Hayashi, S., Levine, A., and Wieschaus, E. (1998). Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171-1182. [DOI] [PubMed] [Google Scholar]
- Bach, A., Lallemand, Y., Nicola, M., Rames, C., Mathis, L., FAufas, M., and Robert, B. (2003). Msx1 is required for dorsal diencephalon patterning. Development 130, 4025-4036. [DOI] [PubMed] [Google Scholar]
- Bang, A., Papalopulu, N., Goulding, M., and Kintner, C. (1999). Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev. Biol. 212, 366-380. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. (1987). Perturbation of cranial neural crest migration by the HNK-1 antibody. Dev. Biol. 123, 321-331. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. (2002). Molecular analysis of neural crest formation. J. Physiol. Paris 16, 3-8. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen, T., Stanleigh, J., Sela-Donenfeld, D., and Kalcheim, C. (2004). Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 131, 5327-5339. [DOI] [PubMed] [Google Scholar]
- Buttitta, L., Tanaka, T., Chen, A., Ko, M., and Fan, C.-M. (2003). Microarray analysis of somitogenesis reveals novel targets of different WNT signaling pathways in the somitic mesoderm. Dev. Biol. 258, 91-104. [DOI] [PubMed] [Google Scholar]
- Cadigan, K., and Nusse, R. (1997). Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286-3305. [DOI] [PubMed] [Google Scholar]
- Dorsky, R., Moon, R., and Raible, D. (1998). Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370-373. [DOI] [PubMed] [Google Scholar]
- Dottori, M., Gross, M., Labosky, P., and Goulding, M. (2001). The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128, 4127-4138. [DOI] [PubMed] [Google Scholar]
- Dunn, K., Williams, B., Li, Y., and Pavan, W. (2000). Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc. Natl. Acad. Sci. USA 97, 10050-10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill, L., and Bronner-Fraser, M. (2002). Genomic analysis of neural crest induction. Development 129, 5731-5741. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro, M., Marcelle, C., and M, B.-F. (2002). Ectodermal Wnt function as a neural crest inducer. Science 297, 848-851. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell, E., and LaBonne, C. (2004). A Slug, a Fox, a pair of Sox: Transcriptional responses to neural crest inducing signals. Birth Defects Research (Part C) 72, 124-139. [DOI] [PubMed] [Google Scholar]
- Huang, X., and Saint-Jeannet, J.-P. (2004). Induction of the neural crest and the opportunities of life on the edge. Dev. Biol. 275, 1-11. [DOI] [PubMed] [Google Scholar]
- Ikeya, M., Lee, S., Johnson, J., McMahon, A., and Takada, S. (1997). Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389, 966-970. [DOI] [PubMed] [Google Scholar]
- Jin, E.-J., Erickson, C., Takada, S., and Burrus, L. (2001). Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev. Biol. 233, 22-37. [DOI] [PubMed] [Google Scholar]
- Kalcheim, C., and Burstyn-Cohen, T. (2005). Early stages of neural crest ontogeny: formation and regulation of cell delamination. Int. J. Dev. Biol. 49, 105-116. [DOI] [PubMed] [Google Scholar]
- Kleber, M., Lee, H., Wurdak, H., Buchstaller, J., Riccomagno, M., Ittner, L., Suter, U., Epstein, D., and Sommer, L. (2005). Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J. Cell Biol. 169, 309-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht, A., and Bronner-Fraser, M. (2002). Induction of the neural crest: A multigene process. Nat. Rev. Genet. 3, 453-461. [DOI] [PubMed] [Google Scholar]
- Kos, R., Reedy, M., Johnson, R., and Erickson, C. (2001). The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128, 1467-1479. [DOI] [PubMed] [Google Scholar]
- LaBonne, C., and Bronner-Fraser, M. (1998). Neural crest induction in Xenopus: Evidence for a two-signal model. Development 125, 2403-2414. [DOI] [PubMed] [Google Scholar]
- LeDouarin, N. L., and Kalcheim, C. (1999). The Neural Crest, 2nd ed., Cambridge University Press.
- Lee, H., Kleber, M., Hari, L., Brault, V., Suter, U., Taketo, M., Kemler, R., and Sommer, L. (2004). Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science 303, 1020-1023. [DOI] [PubMed] [Google Scholar]
- Leeuwen, F. V., Samos, C., and Nusse, R. (1994). Biological activity of soluble Wingless protein in cultured Drosophila imaginal disc cells. Nature 368, 342-344. [DOI] [PubMed] [Google Scholar]
- Liem, K. F., Tremml, G., Roelink, H., and Jessel, T. (1995). Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82, 969-979. [DOI] [PubMed] [Google Scholar]
- Liu, J.-P., and Jessell, T. (1998). A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125, 5055-5067. [DOI] [PubMed] [Google Scholar]
- Mansouri, A., Pla, P., Larue, L., and Gruss, P. (2001). Pax3 acts cell autonomously in the neural tube and somites by controlling cell surface properties. Development 128, 1995-2005. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq, A., Wang, E., and Harland, R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167-178. [DOI] [PubMed] [Google Scholar]
- Muroyama, Y., Fujihara, M., Ikeya, M., Kondoh, H., and Takada, S. (2002). Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S., and Takeichi, M. (1995). Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121, 1321-1332. [DOI] [PubMed] [Google Scholar]
- Nakagawa, S., and Takeichi, M. (1998). Neural crest emigration from the neural tube depens on regulated cadherin expression. Development 125, 2963-2971. [DOI] [PubMed] [Google Scholar]
- Nieto, M.A., Sargent, M., Wilkinson, D., and Cooke, J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835-839. [DOI] [PubMed] [Google Scholar]
- Pohl, B., and Knochel, W. (2001). Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech. Dev. 103, 93-106. [DOI] [PubMed] [Google Scholar]
- Prieve, M., and Moon, R. (2003). Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev. Bio. 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet, J.-P., He, X., Varmus, H., and Dawid, I. (1997). Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. USA 94, 13713-13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T., Sasai, N., and Sasai, Y. (2005). Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 132, 2355-2363. [DOI] [PubMed] [Google Scholar]
- Sechrist, J., Nieto, M., Zamanian, R., and Bronner-Fraser, M. (1995). Regulative response of the cranial neural tube after neural fold ablation: spatiotemporal nature of neural crest regeneration and up-regulation of Slug. Development 121, 4103-4115. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld, D., and Kalcheim, C. (1999). Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development 126, 4749-4762. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld, D., and Kalcheim, C. (2000). Inhibition of noggin expression in the dorsal neural tube by somitogenesis: a mechanism for coordinating the timing of neural crest emigration. Development 127, 4845-4854. [DOI] [PubMed] [Google Scholar]
- Selleck, M., Garcia-Castro, M., Artinger, K., and Bronner-Fraser, M. (1998). Effects of Shh and noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development 125, 4919-4930. [DOI] [PubMed] [Google Scholar]
- Selleck, M., and Bronner-Fraser, M. (1995). Origins of avian neural crest: the role of neural plate-epidermal interactions. Development 121, 417-428. [DOI] [PubMed] [Google Scholar]
- Serbedzija, G., and McMahon, A. (1997). Analysis of neural crest cell migration in Splotch mice using neural-crest specific LacZ reporter. Dev. Biol. 185, 139-147. [DOI] [PubMed] [Google Scholar]
- Shibamoto, S., Higano, K., Takada, R., Ito, F., Takeichi, M., and Takada, S. (1998). Cytoskeletal reorganization by soluble Wnt3a protein signalling. Genes Cells 3, 659-670. [DOI] [PubMed] [Google Scholar]
- Taneyhill, L., and Pennica, D. (2004). Identification of Wnt responsive genes using a murine mammary epithelial cell line model system. BMC Dev. Biol. 4, 6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer, J., Wang, C., and Niswander, L. (2002). BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459-2472. [DOI] [PubMed] [Google Scholar]
- Vallin, J., Thuret, R., Giacomello, E., Faraldo, M. M., Thiery, J., and Broders, F. (2001). Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/b-catenin signaling. J. Biol. Chem. 276, 30350-30358. [DOI] [PubMed] [Google Scholar]
- Willert, J., Epping, M., Pollack, J., Brown, P., and Nusse, R. (2002). A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. 2, 8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]