Abstract
The activity of the two dominant K+ channels in the plasma membrane of Vicia faba guard cell protoplasts was examined during pressure-driven swelling. For this purpose, the K+ currents and the membrane capacitance (Cm) of guard cell protoplasts were recorded in parallel. A rise in Cm, reflecting an increase of the membrane surface area, was coupled to a proportional rise in conductance of both the K+ inward and K+ outward rectifier. The activation kinetics of the K+ channels were not affected during this process. The quantitative and temporal coupling of Cm and K+ conductance can hence be interpreted as the result of the addition of active inward and outward rectifier K+ channels to the plasma membrane during an increase in surface area.
Opening and closing of stomatal pores regulates gas exchange in plants and is mediated by the swelling and shrinking of the guard cells which surround the stomatal pore. Swelling of guard cells is achieved by accumulation of K+ salts and subsequent water influx, leading to stomatal opening. In the reverse process, the pores are closed. The main components of this process are now known in some detail. First, the pathways for passive K+ fluxes are provided by two classes of K+ channels, K+ inward and K+ outward rectifier, which account for passive K+ influx and K+ efflux, respectively. Second, the alterations in surface area, which accompany the large changes in cell volume of up to 40% (1), are the result of addition and removal, respectively of equivalent portions of plasma membrane material (2).
In eukaryotic cells ion channels are transported to the plasma membrane via exocytotic vesicles. In this way, channel density of the plasma membrane can be modulated by exocytotic insertion and endocytotic retrieval of ion channels, which in turn alters the membrane conductance. For an increasing number of cell systems, the modulation of channel density is regarded as an important mechanism for controlling the transport rate across the plasma membrane. Examples include the cAMP-induced insertion of the cystic fibrosis transmembrane conductance regulator (CFTR; ref. 3) and the promotion of delivery of epithelial Na+ channels to the apical membrane by aldosterone and insulin (4, 5). Also, hypoosmotically induced swelling of renal epithelial cells was found to result in the insertion of Na+ channels into the plasma membrane (6).
To test whether an increase in surface area of guard cell protoplasts is associated with insertion and removal of ion channels, we have used the whole-cell patch-clamp technique for parallel measurements of ionic currents (Im) and membrane capacitance (Cm). Whereas the latter parameter is proportional to the cell surface area and monitors excursions in the plasma membrane area, the former parameter provides information on the activity of the prominent K+ channels. When guard cell protoplasts were subjected to swelling by application of hydrostatic pressure via the patch pipette, we found a positive correlation between changes in surface area and changes in K+ inward and K+ outward current. These findings indicate that vesicular membrane which is inserted during swelling of guard cells carries active K+ channels of both types.
Materials and Methods
Guard cell protoplasts were prepared from Vicia faba L. cv. Bunyan as described (2). For measurements, protoplasts were bathed in 10 mM KCl/10 mM CaCl2/5 mM Mes/KOH, pH 5.6, and osmolarity was adjusted to 530 mosmol/kg with sorbitol. Patch pipettes were filled with 170 mM K+-gluconate/10 mM KCl/2 mM MgCl2/2 mM MgATP/2 mM EGTA/10 mM Hepes/KOH, pH 7.8, and osmolarity was adjusted to 560 mosmol/kg with sorbitol.
Whole-cell patch-clamp experiments were performed by using either an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany) and the PULSE acquisition program (HEKA) or a two-phase lock-in amplifier (SWAM IIC; Ljubljana, Slovenia) and an A/D converter (DigiData 1200, Axon Instruments, Foster City, CA). For reconstitution of membrane capacitance (Cm), access conductance, and the parallel combination of leak and membrane conductance (Gm) we used either the computer software PULSE (HEKA) or CAP 3 (J. Dempster, Univ. of Strathclyde, Glasgow, U.K.). This computer-aided data processing is based on the Lindau-Neher algorithm (7), which assumes linearity of the electrical parameters of the cell. However, in the case of guard cells, the activation of time- and voltage-dependent ion channels results in a nonlinearity of the membrane conductance. Thus, Cm values estimated during activation of the K+ inward and K+ outward rectifier are not precise. To overcome this problem, guard cell protoplasts were clamped to a holding voltage of −60 mV or −65 mV on which a sine wave voltage of 1 mV rms (1.6 kHz) was superimposed. In the range of voltage covered by the sinusoidal signal, the K+ inward and K+ outward rectifier are inactive, and the electrical parameters of the cell are linear.
For the calculation of the time constant of the activation of the K+ current, the current responses were fitted with a single exponential by using the computer software PULSEFIT (HEKA). For the analysis of the outward rectifying current, a constant factor b was introduced to account for the sigmoidal rise of the current response.
 |
1 |
Hydrostatic pressure was applied by means of the patch pipette, measured with a pressure monitor (PM01D, WPI, Berlin, Germany), and recorded on the computer.
Results
Increase in K+ Conductance During Surface Area Increase.
The whole-cell patch-clamp technique was applied to monitor changes in membrane capacitance (Cm), a parameter proportional to the cell surface area. Swelling of guard cell protoplasts was induced by pulses of hydrostatic pressure applied by means of the patch pipette. As demonstrated (2, 8, 9), pressure-induced increase in surface area of guard cell protoplasts is accomplished by fusion of membrane material with the plasma membrane.
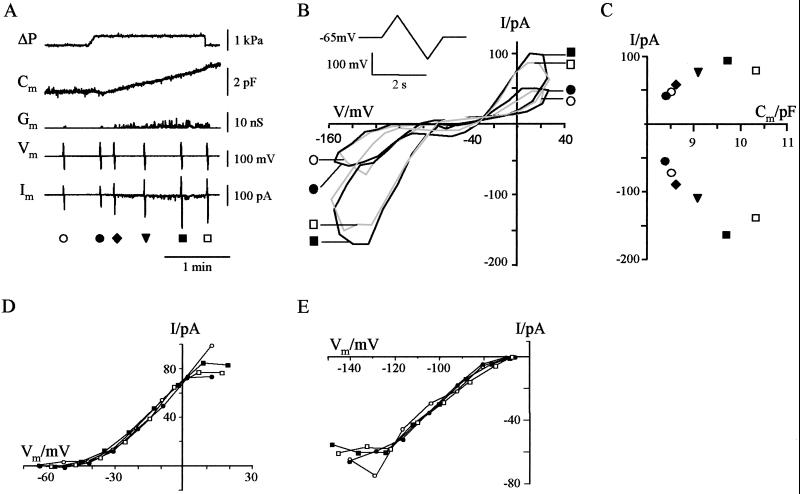
To study the ion channel composition of the plasma membrane during changes in surface area, Cm recordings were combined with measurements of membrane current. Fig. 1 shows a continuous recording of the response of a guard cell protoplast to a hydrostatic pressure pulse of 530 Pa. This rise of internal pressure resulted in a linear ongoing increase in Cm (Fig. 1A). For current measurements over a large voltage range, a short lasting saw-tooth-shaped voltage-clamp protocol was superimposed on the Cm recording. This procedure provided the essential information on the conductance and kinetic properties of ion channels (10) with a minimum of disturbance of the Cm recording. A stable reference current-voltage (I-V) scan recorded before pressure application is shown in Fig. 1B (○). The figure 8-shaped form reflects the I-V characteristics of the guard cell plasma membrane derived from the current response to square-wave voltage pulses. These characteristics include a voltage- and time-dependent K+ outward rectifier (right loop) and a K+ inward rectifier (left loop) separated by a region of low conductance. Further, I-V scans were performed during the application of pressure. One scan was taken immediately after the onset of pressure application, at a time when Cm was still close to the control value (Fig. 1B, ●). In this situation, the I-V relation was not perceivably different from the control, which means that hydrostatic pressure itself has no effect on the K+ channel conductances. Hence, the two K+ rectifiers are not mechanosensitive. The next I-V scan illustrated in Fig. 1 was taken about two min after the onset of pressure stimulation at a time when Cm had already increased by 14% over the control level. In this situation, the I-V profile had changed dramatically. Although the region of low conductance was little affected, the current in the two loops dominated by the two K+ rectifiers had nearly doubled (Fig. 1B, ▪). Finally, the pressure was released, followed by a cessation of the Cm increase. A further I-V scan recorded under the condition of zero pressure but still elevated Cm is also shown in Fig. 1B (□). The resulting currents were only slightly smaller than those recorded at the end of the pressure stimulation. This again shows that the two K+ currents are not directly mechanosensitive.
Figure 1.
K+ currents during pressure-induced increase in membrane capacitance. (A) Continuous recording of membrane capacitance (Cm), membrane conductance (Gm), holding voltage (Vm), and membrane current (Im) during changes in hydrostatic pressure (ΔP). Symbols indicate the application of saw-tooth-shaped voltage protocols (see also B Inset) which were superimposed on the holding voltage of −65 mV. (B) Current voltage loops recorded during the measurement shown in A in response to saw-tooth-shaped voltage protocols at times indicated by the symbols. (Inset) Voltage-clamp protocol. The protocol lasted 2 s, during which time the command voltage was first changed from −65 mV to +35 mV within 500 ms, then to −165 mV within 1 s and back to −65 mV within 500 ms. (C) Relation between currents recorded at 0 mV (K+ outward current) and −120 mV (K+ inward current) and the corresponding membrane capacitance. Currents were measured at the branch of the current voltage loops where the activity of the K+ outward and K+ inward rectifier was maximal (upper and lower branch of the current voltage loops, respectively). Symbols are as in A. (D) I-V relations of the K+ outward currents from Fig. 1B (upper positive current branch) normalized to current amplitude at 0 mV. (E) I-V relations of the K+ inward currents from Fig. 1B (lower negative current branch) normalized to current amplitude at −120 mV.
A plot of the current amplitudes at 0 mV and −120 mV, i.e., at voltages dominated by the K+ outward and inward rectifier, respectively, vs. the corresponding Cm values is shown in Fig. 1C. The plot demonstrates that the increase in current is correlated with the increase in Cm. In the present case, the outward and inward currents increased by 130% and 190%, respectively, for a 14% rise in Cm.
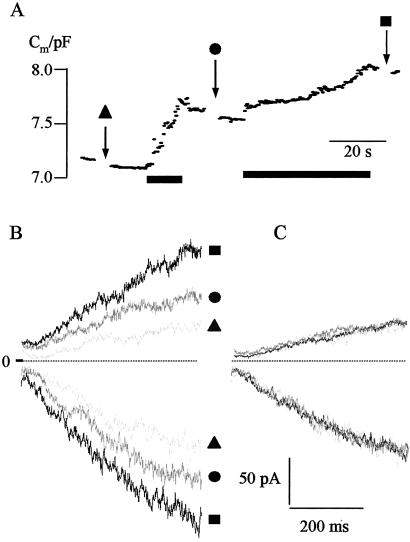
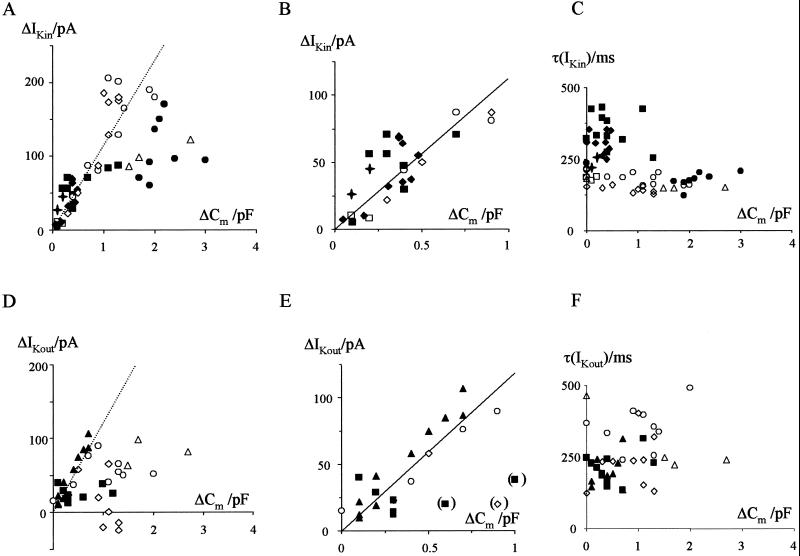
To test whether changes in current were voltage dependent, we compared the normalized currents from the I-V relationships presented in Fig. 1B. Overall, the normalized I-V relationships of the K+ outward and K+ inward rectifier superimposed well, suggesting that the current rise under pressure was not voltage dependent (Fig. 1 D and E). Small differences between the normalized current trajectories were visible only at extreme positive voltages (Fig. 1D). These deviations were not systematic, i.e., they were not correlated with the amplitude of the current. To establish further the relation between changes in Cm and current, we analyzed the current response to square-wave voltage pulses. Changes in Cm again were induced by application of pulses of hydrostatic pressure. However, currents were only recorded before the application of pressure or after the release of pressure. A representative recording is shown in Fig. 2. Stepping the membrane voltage from a holding voltage of −60 mV to −140 mV resulted in the typical time-dependent activation of the K+ inward rectifier, whereas at +60 mV, the time- and voltage-dependent K+ outward current was elicited (Fig. 2B). Again, an increase in Cm was correlated with an increase in current. Fig. 3A shows the relationship between changes in Cm and K+ inward current for eight of the nine protoplasts examined. One remaining protoplast exhibited a decrease in K+ inward current independently of changes in Cm (data not shown) and was not included in further analysis. For the other eight protoplasts, the rise in current recorded for changes in Cm below 1 pF could be described by a linear relationship with a slope of 112 pA/pF (Fig. 3B). Similar results were found for the K+ outward current. Five of the nine protoplasts examined showed a rise in K+ outward current in the course of surface area increase (Fig. 3D). For these protoplasts the correlation between K+ outward current and changes in Cm smaller than 1 pF could be fitted with a linear regression of 119 pA/pF (Fig. 3E). In the four remaining cells, the K+ outward channel activity had already been decreasing continuously before application of pressure. In the context of a well known “run-down” of the guard cell outward rectifier (11), it is likely that an inhibition of channel activity was responsible for the different behavior of these cells.
Figure 2.
Whole-cell K+ currents of a guard cell protoplast recorded before and after pressure-induced increase in surface area. (A) Membrane capacitance (Cm) of a guard cell protoplast. Increase in Cm was induced by application of hydrostatic pressure. Bars represent time of pressure application. Symbols indicate times of application of square-wave voltage pulses. (B) K+ currents recorded during the measurement shown in A at times indicated by symbols. Currents were recorded in response to square-wave voltage pulses from a holding voltage of −60 mV to test voltages of +60 mV (positive current traces) and −140 mV (negative current traces). (C) Normalized current traces from recordings shown in B.
Figure 3.
Changes in K+ current are correlated with changes in membrane capacitance and are not associated with changes in kinetics. (A) Correlation between changes in membrane capacitance (ΔCm) and K+ inward currents (ΔIKin) recorded from eight guard cell protoplasts (indicated by different symbols). Changes in current were calculated from the steady-state current amplitude recorded in response to square-wave voltage pulses from a holding voltage of −60 mV to test voltages of −140 mV. Dotted line represents regression line from B. (B) Magnified relation from A. Regression line was fitted to changes in current recorded for changes in Cm below 1 pF. (C) Correlation between the time constant of the activation of the K+ inward current [τ(IKin)] and changes in Cm. Symbols are as in A. (D) Correlation between changes in membrane capacitance (ΔCm) and K+ outward currents (ΔIKout) recorded from five guard cell protoplasts (indicated by different symbols). Changes in current were calculated from the current amplitude recorded in response to square-wave voltage pulses from a holding voltage of −60 mV to test voltages of +60 mV. Dotted line represents regression line from E. (E) Magnified relation from D. Regression line was fitted to changes in current recorded for changes in Cm below 1 pF. Symbols in brackets were not included in the fitting. (F) Correlation between the time constant of the activation of the K+ outward current [τ(IKout)] and changes in Cm. Symbols are as in D.
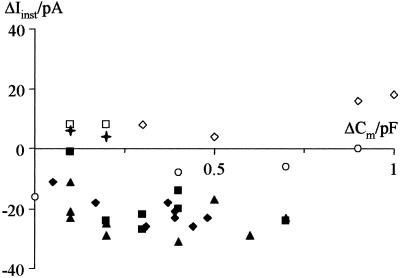
In addition to the time-dependent activation of the two K+ rectifiers, application of the square-wave voltage pulses also elicited an instantaneously activating current (Fig. 2B). This current is partially determined by the seal conductance. Application of positive hydrostatic pressure may impair the close contact between the glass of the patch pipette and the plasma membrane and thus result in an increase in seal conductance. To analyze the seal conductance during pressure-induced increase in surface area, we measured the current response 30 ms after stepping the voltage from −60 mV to +60 mV. There was no correlation between changes in this instantaneous current and changes in Cm (Fig. 4). This result demonstrates that the seal conductance was not affected by hydrostatic pressure or changes in surface area. The observed increase in membrane current can, therefore, be attributed solely to an increase in conductance of the two K+ rectifiers.
Figure 4.
Changes in instantaneous current are not correlated with changes in membrane capacitance. Relation between changes in membrane capacitance (ΔCm) and instantaneous current (ΔIinst) measured 30 ms after stepping the voltage from −60 mV to +60 mV. Symbols are as in Fig. 3.
K+ Channel Kinetics Do Not Change During Surface Area Increase.
The observed increase in current under pressure could in principle result from an elevated open probability of membrane-resident channels or from an insertion of new channels together with the inserted membrane. A combination of both is also possible.
To discriminate between kinetic modulation and increase in channel number as mechanisms underlying the rise in current, we analyzed the kinetics of the currents during elevation of Cm. The rationale for this is that the insertion of active channels should not affect the activation/deactivation kinetics of the two K+ currents, whereas any increase in current through preexisting channels must be accomplished by a modification in activation/deactivation kinetics of the time- and voltage-sensitive K+ channels.
To test whether kinetic changes occurred during the rise in surface area, we first normalized the current traces presented in Fig. 2B. The normalized K+ outward and K+ inward current superimposed well (Fig. 2C), suggesting that the rise in current occurred with no differences in kinetics. To gather additional information on the kinetic properties of channels during changes in surface area, we analyzed the time constant (τ) of the activation of the inward and outward rectifying K+ currents. There was no relation between changes in τ and changes in Cm (Fig. 3 C and F). This finding confirms that the increase in K+ current did not result from an increase in open probability of channels preexisting in the plasma membrane. Together with the fact that the increase in current amplitude is correlated to the increase in surface area, these results strongly suggest, therefore, that during surface area increase, ion channels are inserted into the plasma membrane together with new membrane material.
Discussion
Regulation of the conductance of the plasma membrane can occur via regulation of the kinetics of the channels resident in the plasma membrane and/or via control of ion channel density by insertion and retrieval of ion channels. Regulated trafficking of ion channels is increasingly considered as an important factor in the modulation of current across the membrane in a variety of animal systems. The control of ion channel density has been demonstrated to play a role in cATP-dependent activation of the CFTR chloride channels in epithelial cells (3), stimulation of Na+-transport in renal epithelial cells (5, 12), and changes in K+ current in developing neurons (13). In plant cells, namely maize coleoptiles, the phytohormone auxin has been shown to stimulate expression of K+ channels and increase in K+ channel density within the same time scale (14). This finding indirectly suggests a role for insertion of new ion channels in the regulation of membrane conductance in plant cells.
Previously, we found that changes in surface area during swelling and shrinking of guard cell protoplasts are accomplished by the addition or retrieval of vesicular membrane material to and from the plasma membrane (2, 8, 15). The present study now demonstrates a close correlation between the changes in surface area, revealed by changes in membrane capacitance, and changes in the current carried by the two K+ rectifiers. The rise in K+ current observed during pressure-stimulated increase in surface area occurred without any sign of kinetic modulation of the current. In particular, no changes in the time constant of the activation of the inward and outward rectifying K+ currents were observed in relation to swelling of the cell. Hydrostatic pressure itself had no effect on the K+ current, which excludes a mechanosensitivity of the K+ channels. The increase in current, therefore, reflects an increase in the number of active channels in the plasma membrane which may result from addition of new channels to the plasma membrane. Alternatively, the increase in current could be caused by the activation of inactive channels already present in the membrane. To discriminate between these two options it would be necessary to quantify the plasma membrane proteins on the single cell level. Such a quantification has, so far, only been carried out in mammalian systems using hemagglutinin-tagged K+ channel subunits (16). From our data, therefore, we can not exclude the possibility that the observed increase in current is caused by an activation of channels already present in the plasma membrane. However, the parallel rise of membrane capacitance and current can be best interpreted as insertion of vesicular membrane which carries active K+ channels of both types. A correlation between changes in membrane capacitance and membrane current during cell swelling also has been reported for other plant (barley aleurone protoplasts) and animal cells (renal epithelial cells). In aleurone protoplasts, pressure-driven swelling and shrinking was associated with changes in membrane conductance (17). These changes in conductance have been attributed to insertion and withdrawal of channels with the exocytotic and endocytotic membrane, respectively. However, the current was not identified further. In renal epithelial cells, hypoosmotic conditions or treatment with insulin resulted in a simultaneous increase in membrane capacitance and Na+ currents. This result could be best explained by an increase in Na+ channel density during cell swelling (5, 6).
Parallel monitoring of membrane capacitance and membrane current provides a very sensitive measure for changes in surface area and ion channel density. This technique also has been used to demonstrate cATP stimulated insertion of CFTR Cl− channels (for review see ref. 3). However, in a recent study, Chen et al. (18) have pointed out that these measurements must be interpreted with great caution, because large changes in membrane conductance during changes in Cm may lead to an artificial correlation between membrane current and Cm. In contrast to the CFTR channel, the activation of the K+ inward and outward rectifier of guard cells are voltage dependent, and under our recording conditions, the K+ channels are inactive at voltages between −80 mV and −40 mV. For measurements of Cm, we clamped the protoplasts to a holding voltage of −60 or −65 mV on which a sine wave voltage of 2.8 mV peak to peak was superimposed. Changes in K+ channel conductance thus had no effect on the conductance in the range of voltage covered by the Cm measurement (see also Material and Methods). The correlation between Cm and current revealed in our recordings, therefore, does indeed reflect a parallel increase in surface area and membrane conductance.
A correlation between changes in cell volume and K+ conductance also has been reported by Liu and Luan (19) for V. faba guard cells. They showed that swelling of guard cell protoplasts by hypoosmotic treatment resulted in an increase in K+ inward current and a decrease in K+ outward current. Results and interpretation in this study deviate in crucial points from the present work. First, these authors showed with single-channel recordings that pressure-related changes in K+ current through the inward rectifier resulted from alterations in channel activity (19). From these microscopic observations, it was extrapolated that osmotic pressure affects the conductance of the K+ inward rectifier exclusively by kinetic modulation of K+ channels probably by means of interaction of the channel protein with a dynamic actin-filament organization. However, the analysis of single-channel recordings by Liu and Luan does not allow a distinction between changes in channel activity resulting from alterations of existing channels in the plasma membrane or from changes in the number of channels in the plasma membrane. The present data now demonstrate that swelling of protoplasts affects the K+ conductance of both K+ channels by increasing the number of active channels in the plasma membrane without any significant effect on the channel kinetics. This finding suggests that insertion of channel proteins into the plasma membrane is the main cause for increase in K+ conductance during cell swelling.
Furthermore, Liu and Luan (19) also reported that osmotically driven swelling of guard cell protoplasts resulted in a reversible decrease in the conductance of the K+ outward rectifier. In contrast, we recorded an increase in the conductance of the outward rectifier with increasing surface area in the present work. The reason for the different experimental observations remains unexplained. However, one possible answer may be related to the procedure used to affect the volume of the protoplasts. In the experiments of Liu and Luan (19), an osmotic gradient was applied, whereas in the present work, an increase in hydrostatic pressure induced cell swelling. Changes in cell volume by application of an osmotic gradient are associated with influx of water resulting in a decrease in intracellular ionic strength. Even in the whole-cell configuration, where the cell is constantly dialyzed by the pipette solution, the intracellular ionic strength can differ by about 30% from the ionic strength of the pipette solution (20). Considering that K+ outward currents in Vicia faba guard cells have been shown to decrease with a decrease in intracellular K+ concentration (21), the reduction of K+ outward currents observed under osmotic imbalance (19) is most likely to be caused by decreases in intracellular potassium concentration and not by pressure-induced surface-area changes.
From the increase in current recorded in our measurements and the known properties of the guard cell K+ channels, we can estimate the number of ion channels added during swelling of guard cell protoplasts. Under the experimental conditions used in the present study, the single-channel conductance of the K+ outward and the K+ inward rectifier is about 20 pS (19). Hence, the increase in current obtained from the regression in Fig. 3 B and E for an increase in Cm of 1 pF corresponds to the addition of about 50 outward rectifier and 70 inward rectifier channels, respectively. Considering a specific capacitance of 8 mF/m2 of the guard cell plasma membrane (2), a rise in Cm of 1 pF is equivalent to an increase in surface area of about 1.25 × 10−10 m2. From measurements of unitary exo- and endocytotic events in guard cell protoplasts during pressure-driven cell expansion, we estimated that the median diameter of vesicles which account for osmotic-driven changes in surface area is around 300 nm (8). So, to provide sufficient membrane material for an increase in surface area of 1 pF, about 450 vesicles with a diameter of 300 nm have to fuse with the plasma membrane. Of these vesicles, only one of nine vesicles must contain a K+ outward rectifier channel and one of six vesicles a K+ inward rectifier channel to account for the recorded increase in current.
For changes in Cm above 1 pF, the recorded increase in current showed a larger scattering and clear deviation from the linear regression estimated for smaller alteration in Cm (Fig. 3 A and D). For a given change in Cm, the corresponding change in current was, in most experiments, lower than expected from the linear relationship. This points to a limited availability of vesicles that contain ion channels. During swelling of guard cell protoplasts, the pool of vesicles containing K+ inward and outward rectifiers may get depleted, resulting in fewer ion channels being inserted into the plasma membrane for a given increase in surface area.
Exocytotic recruitment and endocytotic retrieval of ion channels may be linked to the physiological function of cells. Such a link has been suggested for the regulation of excitability in growing neurons (13) and for cAMP regulation of CFTR-channel activity (3). Modulators of ion channel activity in guard cells typically have opposite effects on the K+ outward and K+ inward rectifier owing to their different role in the regulation of the stomatal pore. They promote either uptake (K+ inward rectifier) or release (K+ outward rectifier) of K+, resulting in opening or closing of the pore, respectively (22). In the study by Liu and Luan (19), changes in cell volume indeed had opposite effects on the two currents. From their observations, the authors proposed a model whereby the sensitivity of K+ channels to osmotic gradients acts as a feedback mechanism that accelerates stomatal movement. In contrast, the present study shows that pressure-modulated changes in surface area affected both K+ channels in a parallel manner. The observed changes in K+ channel density, therefore, can not serve as a regulatory mechanism in guard cell functioning. Instead, insertion of ion channels may prevent large fluctuations in ion channel density of the plasma membrane during surface area increase, and thus allow a separation of modulation of cell size from regulation of current density. This hypothesis would imply that the current density of the plasma membrane does not change during swelling. Before swelling, the average current density of the guard cell protoplasts was 11 ± 5 pA/pF (n = 8) for the inward current and 13 ± 11 pA/pF (n = 5) for the outward current. The slope of the linear regression in Fig. 3, which gives a current density of the added plasma membrane for changes in Cm smaller than 1 pF, is nearly an order of magnitude larger. Accordingly, the average current density of the K+ inward and K+ outward current nearly doubled upon swelling of the protoplasts. This result may imply that insertion of ion channels during increase in surface area acts as a mechanism for increasing the ion channel density of the plasma membrane without discriminating between K+ inward and outward rectifiers. However, in the physiological situation of stomatal movement the process of vesicle delivery to the plasma membrane during swelling of guard cells may be more complex and involve distinct mechanisms of sorting and fusion of vesicles. In this context, it is feasible to speculate that a more balanced insertion of K+ channels into the plasma membrane may occur, which can also serve as a mechanism for homeostasis of channel density during variations in surface area.
Acknowledgments
We thank Prof. J. Dainty for helpful comments on the manuscript. This work was supported by a grant to U.H. from the Deutsche Forschungsgemeinschaft.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cm
membrane capacitance
- Gm
membrane conductance
- I-V
current-voltage
References
- 1.Raschke K. In: Encyclopaedia of Plant Physiology. Haupt W, Feinlieb E, editors. Berlin: Springer; 1979. pp. 383–441. [Google Scholar]
- 2.Homann U. Planta. 1998;206:329–333. [Google Scholar]
- 3.Kleizen B, Braakman I, de Jonge H R. Eur J Cell Biol. 2000;79:544–556. doi: 10.1078/0171-9335-00078. [DOI] [PubMed] [Google Scholar]
- 4.Blazer-Yost B L, Liu X, Helman S I. Am J Physiol. 1998;274:C1373–C1379. doi: 10.1152/ajpcell.1998.274.5.C1373. [DOI] [PubMed] [Google Scholar]
- 5.Erlij D, de Smet P, van Dreissche W. J Physiol. 1994;481:533–542. doi: 10.1113/jphysiol.1994.sp020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jans D, Simaels J, Cucu D, Zeiske W, van Dreissche W. Pflügers Arch. 2000;439:504–512. doi: 10.1007/s004249900194. [DOI] [PubMed] [Google Scholar]
- 7.Lindau M, Neher E. Pflügers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- 8.Homann U, Thiel G. FEBS Lett. 1999;460:495–499. doi: 10.1016/s0014-5793(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 9.Bick I, Thiel G, Homann U. Eur J Cell Biol. 2001;80:521–526. doi: 10.1078/0171-9335-00189. [DOI] [PubMed] [Google Scholar]
- 10.Gradmann D, Boyd C M. Eur Biophys J. 1999;28:591–599. doi: 10.1007/s002490050241. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder J I. Philos Trans R Soc London B. 1992;338:83–89. [Google Scholar]
- 12.Niisato N, van Dreissche W, Liu M, Marunaka Y. J Membr Biol. 2000;175:63–77. doi: 10.1007/s002320001055. [DOI] [PubMed] [Google Scholar]
- 13.Wu R-L, Butler D M, Barish M E. J Neurosci. 1998;15:6261–6278. doi: 10.1523/JNEUROSCI.18-16-06261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer C S, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, et al. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubitschek U, Homann U, Thiel G. Planta. 2000;210:423–431. doi: 10.1007/PL00008151. [DOI] [PubMed] [Google Scholar]
- 16.Zerangue N, Schwappach B, Jan Y N, Jan L Y. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 17.Zorec R, Tester M. FEBS Lett. 1993;333:283–286. doi: 10.1016/0014-5793(93)80671-g. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Hwang T-C, Gillis K D. J Gen Phys. 2001;118:135–144. doi: 10.1085/jgp.118.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Luan S. Plant Cell. 1998;10:1957–1970. doi: 10.1105/tpc.10.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voets T, Droogmans G, Raskin G, Eggermont J, Nilus B. Proc Natl Acad Sci USA. 1999;96:5298–5303. doi: 10.1073/pnas.96.9.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemtiri-Chlieh F. J Membr Biol. 1996;153:105–116. doi: 10.1007/s002329900114. [DOI] [PubMed] [Google Scholar]
- 22.Blatt M. Annu Rev Cell Dev Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]