Abstract
The influence of transport, catching, and processing on contamination of broiler chickens with Salmonella and Campylobacter was investigated. Transport crates were reused with high frequency and were often still contaminated with Salmonella and Campylobacter when they arrived at the farm despite the fact that they were washed at the factory, and thus they were a potential route of infection. These organisms contaminated the feathers of previously Campylobacter- and Salmonella-negative birds going to the processing plant and were isolated from processed carcasses, albeit at a low frequency. The Campylobacter types which were the predominant organisms on the live birds when they arrived at the processing plant were not necessarily the types that were most frequently isolated from processed carcasses. This finding may reflect cross-contamination that occurred during processing or differences in the tolerance of the strains to the hostile environments that the bacteria experienced. The process of catching and putting the birds in crates significantly increased the chance of contamination with Campylobacter (P < 0.001).
Human infections with Campylobacter spp. continue to be of international importance, and in England and Wales the number of confirmed cases continues to exceed 50,000 per annum (4). This is in line with the rate of Campylobacter infection in the United States, where the Centers for Disease Control and Prevention has estimated that the overall rate of infection is 1,000 cases per 100,000 people (28). Recent work on infectious intestinal disease in England and Wales (30) has indicated that cases are underreported and that the actual number of Campylobacter infections in these countries is likely to be about eight times the published number.
Contaminated poultry meat is considered an important vehicle of human infection with Campylobacter (1, 14, 22, 23), and because of the difficulties in controlling the spread of the bacteria in the kitchen (6, 7) and abattoir (17), control on the farm may be more effective. Identification of control measures requires a good understanding of the epidemiology of Campylobacter spp. in poultry meat production. There have been a number of studies of this important topic in recent years, and the environment (16), drinking water (28), and even vertical transmission (10, 24) have been suggested as possible sources of flock colonization. Improved farm hygiene measures, such as boot dipping (15) and boot changing (31), which presumably prevent the introduction of Campylobacter spp. from the external environment into a broiler chicken house, can either delay or prevent colonization. Transport vehicles and crates may be an additional source of contamination between batches of birds and farms (19). Such contamination may be particularly important for the introduction of Campylobacter into previously uninfected flocks during transport or during flock thinning (i.e., the removal of birds to reduce stock density) (12). Newell et al. (21) found that on one occasion carcasses from a Campylobacter-negative flock were contaminated with a Campylobacter subtype that was isolated from crates prior to loading of the birds. This was a limited study in which the researchers examined only the crates prior to loading of the birds during the transport of one flock. In our study we examined not only the levels of contamination of crates arriving at farms but also the effects of different crate-washing regimens. The influence of catching and transport on the levels of contamination of Campylobacter- and Salmonella-negative broiler chickens was determined, and the effect of processing on the distribution of Campylobacter subtypes on carcasses was examined.
(Some of the results were presented at the 1997 PHLS Annual Scientific Conference and the 99th General Meeting of the American Society for Microbiology, 1999.)
MATERIALS AND METHODS
Audit of crate washing.
To evaluate the problem, samples of washed crates and samples of crate wash water were collected at a poultry processing plant during five visits. The crate-washing system consisted of a long soak tunnel (20 s of exposure with agitation and detergent [Alcask; Holchem Laboratories, Preston, United Kingdom]) linked to a short tunnel washer (10 s of exposure), followed by a pressure rinse with a quaternary ammonium compound (QAC) (Holquat; Holchem Laboratories). During all visits a float was observed to be poorly calibrated, which led to overflow of the wash water and rapid dilution of the detergent. On each occasion, three 125-ml crate wash water samples were taken and three washed crates were swabbed with sterile cotton wool (∼10 g) soaked in maximum recovery diluent (CM733; Oxoid Ltd., Basingstoke, United Kingdom). These samples were examined for the presence of Salmonella and Campylobacter, and Campylobacter was also enumerated by using the most-probable-number (MPN) technique described below.
Crate and crate wash water samples were obtained during two additional visits when care was taken to ensure that the concentration of detergent used during the washing procedure was the concentration recommended by the manufacturer. Each time three samples of crate wash water and swabs from three crates were collected at regular intervals during the wash procedure, both before and after addition of the detergent. During the first visit detergent was added twice, at 0.25 and 1.5 h after the wash procedure was begun. During the second visit detergent was added three times, after 1.7, 4.3, and 4.7 h. Samples were cultured to determine the presence of Salmonella and Campylobacter, and Campylobacter was enumerated by the MPN technique described below. The pH of each of the crate wash water samples was determined with a reference pH meter (pHM93; Radiometer, Copenhagen, Denmark), and the detergent concentration was determined with an alkaline test kit (SKS 00800; Holchem Laboratories Ltd.). The temperature of the crate wash water was measured in situ during the first visit by using an HMP35 humidity and temperature probe (Vaisala, Helsinki, Finland).
The effects of different disinfectants (which were used instead of the QAC spray normally used in the washing procedure) on the presence of Salmonella and Campylobacter on crates were assessed during one final visit to the processing plant. Swab samples were obtained from five unwashed crates, six crates that were washed but not sprayed with QAC, and four crates that were washed and sprayed with QAC as described above. An additional 15 crates were soaked in detergent before 5 of the crates were dipped into QAC (10%, vol/vol), 5 of the crates were dipped into peracetic acid (0.25%, vol/vol; Holchem Laboratories), and 5 of the crates were dipped into hypochlorite (100 ppm; Hays Chemicals, Leeds, United Kingdom). A swab sample of each crate was taken immediately after each treatment. Crate swab samples were enriched for Campylobacter and Salmonella as described below.
Influence of catching, transport, and processing.
The effects of catching, transport, and processing on the Campylobacter and Salmonella status of birds and carcasses from uncolonized flocks was investigated. Flocks I, II, and III were reared on the same intensive broiler farm but at different times (December 1997 to April 1998). Each flock comprised ∼17,200 birds, and the birds were killed when they were 42 days old. Dogs, horses, and cows were also present on this farm. In order to ascertain that the flocks were Campylobacter and Salmonella negative prior to transport, litter and cloacal samples were taken regularly and examined for the presence of Campylobacter and Salmonella. On the day of depopulation, the day on which birds were removed from the farm and transported to the processing plant, cloacal swabs (flock II) and litter samples (10 samples for flock II, 3 samples for flock III) were collected and examined. Swab samples were also taken from the feathers of the birds (as described above for crate samples) immediately before catching (flocks II and III) or after catching (flock I) and again after placement in transport crates (flocks II and III). Transport crates were swabbed prior to loading of birds and after emptying (flock I only). All birds sampled at the farm were marked and reexamined at the poultry processing plant. The time that the birds spent in the crates, either on the lorry or waiting before slaughter, was approximately 2 h, and there were approximately 23 birds in each crate. A minimum of five carcasses from each flock were sampled before the scald tank, and five carcasses from flock I and five carcasses from flock III were sampled after processing. Carcasses were rinsed by shaking them vigorously in 500 ml of buffered peptone water (BPW) (CM509; Oxoid Ltd.) for 30 s. Ten ceca from flock I, 37 ceca from flock II, and 40 ceca from flock III were collected and examined to confirm that the birds from these flocks were not colonized by Campylobacter or Salmonella at the time of slaughter. All samples were examined by using the enrichment methods described below. Campylobacter in chicken rinses from flock II was also enumerated by the MPN technique (see below). Each feather or crate swab was homogenized in a stomacher for 30 s with 250 ml of BPW prior to enrichment for Campylobacter spp.
Culture, MPN analysis, and identification of presumptive Campylobacter.
To obtain discrete colonies, feces samples were streaked onto modified charcoal cefoperazone deoxycholate agar (mCCDA) (CM739, SR155; Oxoid Ltd.) plates and incubated microaerobically at 37°C for 48 h. Aliquots (500 μl) of each carcass rinse were spread plated directly onto two mCCDA plates and incubated microaerobically at 37°C for 48 h before colonies were enumerated. Microaerobic conditions were generated by the gas replacement method (a partial vacuum [500 mm of Hg] in a 10-liter jar was replaced by a mixture of CO2, H2, and N2 so that the gas concentrations in the jar were approximately 5% O2, 5% CO2, 5% H2, and 85% nitrogen [5]).
To enrich for Campylobacter spp., litter (25 g), crate and feather swab homogenates (25 ml), and carcass rinses (25 ml) were incubated in 225 ml of modified Exeter broth, which contained 25 g of nutrient broth (Mast DM180; Mast Diagnostics, Bootle, Merseyside, United Kingdom) per liter, Campylobacter growth supplement (Mast SV61; 250 mg of sodium metabisulfate per liter, 250 mg of sodium pyruvate per liter, 250 mg of ferrous sulfate per liter), Campylobacter selective supplement (Mast SV59; 10 mg of trimethoprim per liter, 5 mg of rifampin per liter, 2,500 IU of polymyxin B per liter, 15 mg of cefoperazone per liter, 2 mg of amphotericin B per liter; Mast SV59), and 1% lysed defibrinated horse blood (10 ml liter; E & O Laboratories, Bonnybridge, Scotland). Feces samples (1 g) and cloacal swabs were each enriched in 25 ml of modified Exeter broth. Crate wash water was analyzed by adding 25 ml to an equal volume of double-strength modified Exeter broth (i.e., modified Exeter broth with the concentrations of all ingredients, including supplements, doubled). The broth preparations were incubated at 37°C for 48 h before 10-μl portions were plated onto mCCDA plates and incubated microaerobically at 37°C for 48 h. Campylobacter in crate swab homogenates, crate wash water, and carcass rinses was enumerated by an MPN technique (3). Aliquots of the homogenates were each cultured in 25 ml of modified Exeter broth and incubated as described above. Ten microliters of each preparation was then plated onto mCCDA and incubated microaerobically at 37°C for 48 h. The identities of presumptive Campylobacter isolates were confirmed by using the following parameters: growth on blood agar in aerobic and microaerobic atmospheres at 37°C after 48 h, oxidase activity, and cell morphology as determined by phase-contrast microscopy.
Culture and identification of Salmonella.
The presence of Salmonella in feces (1 g) and cloacal swab samples was assessed by culturing samples in 9-ml portions of selenite cystine broth (CM699, L121; Oxoid Ltd.) for 24 h at 37°C before 10-μl aliquots were spread onto xylose lysine deoxycholate agar plates (CM469; Oxoid Ltd.), which were incubated at 37°C for 24 h. Initially, Salmonella was identified based on colony morphology, and identities were confirmed by using standard biochemical and serological techniques (29).
To detect the presence of Salmonella spp., litter samples (25 g in 225 ml of BPW), BPW-carcass rinses (220 ml), and BPW-swab homogenates (220 ml) were incubated for 24 h at 37°C. One hundred microliters of each preparation was inoculated into 10 ml of Rappaport Vassiliadis soya peptone (CM866; Oxoid Ltd.) broth and incubated at 41.5°C for 24 h before it was plated onto xylose lysine deoxycholate agar plates as described above.
To isolate Salmonella from crate wash water, 25 ml was added to an equal volume of double-strength BPW (40 g liter−1). After incubation at 37°C for 18 to 24 h, the sample was cultured in Rappaport Vassiliadis soya peptone broth as described above.
Data analysis.
A censored normal regression model (2) for the natural logarithms of the combined MPNs was used to determine the effect of pH and exposure time on the MPNs of Campylobacter in the crate wash water. The pH of the crate wash water was used as a proxy for the amount of detergent present.
Logistic regression analysis (13) was performed for the number of birds contaminated with Campylobacter and Salmonella before and after the birds were handled by the catchers and after they were put in the crates to determine if these procedures significantly increased the levels of contamination by the bacteria. Logistic regression analysis was also used to determine if there was a significant difference between the number of birds contaminated with Salmonella and Campylobacter before processing and the number contaminated after processing.
Storage of Campylobacter and Salmonella isolates.
Ten Campylobacter colonies and one Salmonella colony from each positive sample were streaked onto blood agar (CM331; Oxoid Ltd.) containing 5% defibrinated horse blood (E&O Laboratories) for purification. Isolates were then stored on cryobeads (CRYO/M MAST Diagnostics) at −40°C.
Species identification and typing for Campylobacter isolates.
A total of 279 Campylobacter isolates were identified to the species level by using previously described methods (5). The Penner serogroups of 26 of the isolates were determined by using standard procedures (25).
(i) flaA subtyping.
Subtypes of 257 isolates were determined by flaA PCR-restriction fragment length polymorphism (RFLP) analysis by using an annealing temperature of 45°C, forward primer pg 50, and reverse primer RAA19 (18). Amplicons were restricted with PstI and EcoRI in a double digest to obtain between two and five fragments. Unique PCR-RFLP profiles were assigned arbitrary letters to distinguish subtypes.
(ii) PFGE.
SmaI-restricted total genomic DNA was analyzed by pulsed-field gel electrophoresis (PFGE). Fragments were separated by electrophoresis at 14°C for 22 h at 200 V with pulse times ramped linearly from 10 to 35 s and by electrophoresis at 14°C for 23 h at 200 V with pulse times ramped linearly from 5 to 20 s for Campylobacter jejuni and Campylobacter coli, respectively (11). Arbitrary numbers were assigned to unique profiles.
RESULTS
Audit of crate washing.
The results of the analyses of crate swabs and the wash water were different. Campylobacter was isolated from crates cleaned under normal conditions on four of five visits to the processing plant but was isolated from wash water only on two visits (Table 1). In contrast, Salmonella was isolated from crates on two occasions and from the wash water on four of five visits.
TABLE 1.
MPNs of Campylobacter isolated from transport crate swabs and crate wash water under normal conditionsa
| Visit | No. of Campylobacter per crate (MPN)
|
No. of Campylobacter in wash water (MPN ml−1)
|
||||
|---|---|---|---|---|---|---|
| Swab A | Swab B | Swab C | Sample A | Sample B | Sample C | |
| 1 | >3,600b | >3,600 | >3,600 | 54 | <0.2c | <0.2 |
| 2 | 40 | 160 | 80 | <0.2 | <0.2 | <0.2 |
| 3 | 400 | <40 | <40 | <0.2 | <0.2 | <0.2 |
| 4 | <40 | <40 | <40 | <0.2 | <0.2 | <0.2 |
| 5 | <40 | >3,600 | >3,600 | >180 | >180 | >180 |
Three samples of each type were taken during each visit; enumeration was by the MPN method (3).
For the crate samples the upper limit of detection was 3,600 MPN and the lower limit of detection was 40 MPN.
For the wash water samples the lower limit of detection was 0.2 MPN ml−1 and the upper limit of detection was 180 MPN ml−1.
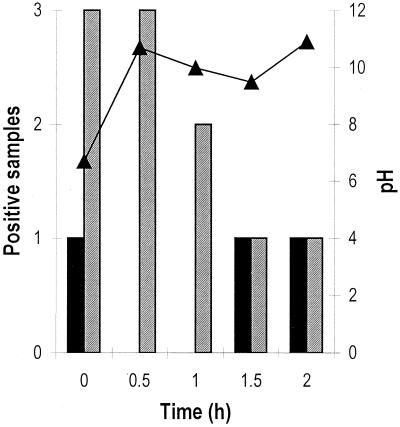
When crates were washed using the recommended concentration of detergent (0.5 to 1% [vol/vol] at 40 to 80°C), Campylobacter was isolated from a lower proportion of crates, but only after a second addition of detergent (Fig. 1). Campylobacter was isolated from all six unwashed crates, and during this visit the temperature of the crate wash water was 30°C. The proportion of crate wash water samples that tested positive for Campylobacter by enrichment, however, was not affected by the addition of detergent despite a considerable increase in the pH of the wash water, both after the first addition and after the second addition (Fig. 1). The detergent concentration increased from 0 to 0.7% (vol/vol) after the first addition of detergent and from 0.2 to 0.8% (vol/vol) 10 min after the second addition of detergent (at 0.25 and 1.5 h, respectively) and decreased to 0.4% 0.5 h after the first addition. All of the crate and crate wash water samples collected before and after the addition of detergent were positive for Salmonella on this occasion.
FIG. 1.
Effect of addition of detergent at 0.25 and 1.5 h on pH (▴) and the number of Campylobacter-positive crate wash water samples (solid bars) and crates (shaded bars). Three samples of each type were analyzed.
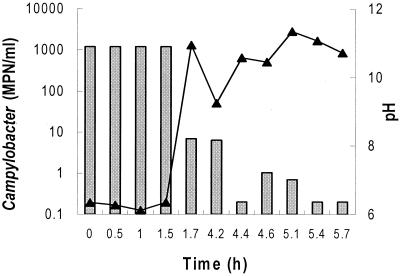
During a subsequent visit Campylobacter numbers before and after the addition of detergent were determined and decreased from >1,100 to <0.3 MPN per ml of crate wash water (the limit of detection) over a period of 4 h (Fig. 2). This decrease was found to be significantly associated both with an increase in the pH (P < 0.001), a proxy for the amount of detergent, and with exposure time (P < 0.001). Despite the significant increase in the pH of the crate wash water, all crates examined were Campylobacter positive. The detergent concentration increased from 0.6 to 0.9% after the third addition. Together, these results suggested that detergent could very effectively reduce the number of Campylobacter cells suspended in crate wash water but could not eradicate Campylobacter attached to crates. Crates and crate wash water were Salmonella negative prior to addition of the detergent.
FIG. 2.
Effect of addition of detergent at 1.7, 4.3, and 4.7 h on pH (▴) and level of Campylobacter in crate wash water (bars) over a 5.7-h period.
Replacement of QAC by different disinfectants did not consistently result in eradication of Campylobacter, although dipping in QAC and/or hypochlorite appeared to result in a lower proportion of Campylobacter-positive crates (Table 2). All five of the unwashed crates examined and all six of the washed crates examined (washed with detergent only) were Campylobacter positive (Table 2), while Salmonella was not isolated from any of the crates.
TABLE 2.
Presence of Campylobacter on unwashed and treated crates
| Crate treatment | No. of Campylobacter-positive crates/no. of crates examinedb | |
|---|---|---|
| Washa | Disinfection | |
| No | None | 5/5 |
| Yes | None | 6/6 |
| Yes | Sprayed with 10% QACc | 4/4 |
| Yes | Immersion in 10% QAC | 2/5 |
| Yes | Immersion in 0.25% peracetic acid | 5/5 |
| Yes | Immersion in 100 ppm of hypochlorite | 3/5 |
The crates were immersed in a soak tunnel containing detergent.
The crate swab samples were examined by the enrichment method.
The QAC used was Holquat.
Influence of catching, transport, and processing on the presence of Campylobacter subtypes and Salmonella spp. on birds.
The status of the flocks before arrival of the catchers was assessed by using 145 samples taken from 132 different birds. Campylobacter and Salmonella were not isolated from cecal samples (flocks I, II, and III), cloacal swabs (flock II), or litter samples (flocks II and III) taken on the day of depopulation, demonstrating that the birds in these flocks were probably not colonized by Campylobacter or Salmonella prior to catching (Table 3). Surface swabs taken from birds before the catchers arrived (flocks II and III) were also Campylobacter negative, but Salmonella (serotype 9g) was present in 3 of the 10 surface swab samples obtained from flock III birds.
TABLE 3.
C. jejuni subtypes isolated from samples taken during catching, transport, and processing of birds from three broiler flocks reared at the same farm but at different times
| Flock | Sample | No. of Campylobacter-positive samples/no. of samples examined | Serotypea | flaA typeb | PFGE typec |
|---|---|---|---|---|---|
| I | Birds, before catchers | 0/10d | |||
| Crates, before birds | 1/1 | 1 (1)e | A (5) | 1 (1) | |
| UT (1)f | B (4) | 2 (1) | |||
| Crates, after birds | 1/1 | 1 (3) | A (9) | 1 (1) | |
| Birds, after catching | 3/10 | 1 (3) | A (24) | 1 (3) | |
| Carcasses, before scald tank | 1/5 | UT (1) | UT (6) | NTg | |
| Carcasses, after processing | 2/5 | 1 (1) | A (18) | 1 (2) | |
| II | Birds, before catchers | 0/72h | |||
| Litter samples | 0/10 | ||||
| Crates, before birds | 3/3 | 4 (1) | C (29) | NT | |
| Birds in crates | 15/15 | 4 (7) | C (66) | 3 (4) | |
| UT (3) | E (5) | NT | |||
| Carcasses, before scald tank | 1/16i | NT | NT (5) | NT | |
| III | Birds, before catchers | 0/50j | |||
| Litter samples | 0/3 | ||||
| Crates, before birds | 1/3 | NT | D (1) | NT | |
| Birds in crates | 1/15 | 11 (1) | UT (2) | 4 (1) | |
| NT | F (6) | NT | |||
| Carcasses, before scald tank | 5/10 | 55 (1), UT (1) | UT (40) | NT | |
| 5 (1) | G (7) | NT | |||
| Carcasses, after processing | 3/5 | UT (1) | UT (30) | NT |
HS type (Penner serotyping scheme).
Unique flaA PCR-RFLP profiles were assigned arbitrary letters to distinguish types.
Unique SmaI-restricted profiles were assigned arbitrary numbers to distinguish types.
Ten ceca representing 10 birds.
The numbers in parentheses are numbers of isolates.
UT, untypeable.
NT, not tested.
Thirty cloacal swabs, 37 cecal samples, and five feather swabs representing 72 different birds.
Seven carcasses were Campylobacter positive. All isolates from one carcass were C. coli; one carcass was contaminated with C. coli and C. jejuni; and the isolates from five carcasses were not identified to the species level.
Ten feather swabs and 40 cecal samples representing 50 different birds.
Five of the seven crates sampled at the farm prior to depopulation of the flocks were contaminated with Campylobacter (Table 3). Prior to depopulation of flock III, Salmonella (serotypes 6,7b and 6,7c) was isolated from one of three crates.
The results obtained after the birds were caught are as follows. Three of 10 feather surface swab samples taken from flock I birds after professional catchers caught the birds but before the birds were placed in the crates were contaminated with both Salmonella (serotype 9g) and Campylobacter (C. jejuni serotype 1, flaA type A, PFGE type 1 [i.e., subtype 1-A-1]). This Campylobacter subtype was also isolated from the crates before and after the chickens were placed in them and from carcasses after processing (Table 3).
Surface swab samples were taken from flock II and III birds just after the birds were loaded into transport crates by the catchers in order to determine the levels of contamination due to catching and putting the birds in crates. One of the 15 crated birds from flock II was contaminated with Salmonella (serotype 4i), and all of these birds were contaminated with Campylobacter, particularly with one subtype (subtype 4-C-3). A subtype 4-C-NT isolate (where NT indicates not tested) was detected in the crates before the chickens were placed in them (Table 3). In contrast, Campylobacter was isolated from only one of the crated birds from flock III. One of the 15 crated birds from flock III was also Salmonella positive (serotype 9g). Analysis of Campylobacter-positive and -negative samples showed that catching and placing birds in crates significantly increased the chance that the birds were contaminated with Campylobacter (P < 0.001). The risk of contamination with Salmonella after the birds were caught by catchers was also significantly greater (P = 0.003).
At least five carcasses from each flock were removed from the factory line prior to the scald tank, just after stunning and bleeding. Only one of the five carcasses of flock I birds examined was contaminated with Campylobacter at this point, and 4 of the 10 isolates examined were C. coli (data not shown). Two of the carcasses were contaminated with Salmonella (serotype 3,10e). Seven carcasses of flock II birds were positive for Campylobacter; however, C. jejuni was isolated from only one of two carcasses whose isolates were identified to the species level. One carcass was Salmonella positive (serotype 4i). When flock III was studied, C. jejuni was present in 5 of the 10 carcasses examined, while C. coli was not isolated. Two C. jejuni subtypes 55-UT-NT and 5-G-NT (where UT indicates untypeable), and one nontypeable strain (subtype UT-UT-NT) were isolated at this point. Salmonella (serotype 4i) was isolated from only one of the carcasses.
At the postprocessing stage two of five carcasses of flock I birds yielded a C. jejuni subtype (subtype 1-A-1) which was also present on the crates before the chickens were placed in them and on the birds just after catching. Salmonella (serotype 9g) was isolated from two of the five carcasses. Three of the five carcasses of flock III birds examined were Campylobacter positive, and none was Salmonella positive. All 30 Campylobacter isolates from these birds were C. jejuni, and none of them was typeable by flaA subtyping. MPN enumeration of Campylobacter on carcasses of flock III birds before and after processing revealed that the level of contamination decreased from 75 ± 81 MPN per carcass (median ± standard error; range, 15 to 450 MPN per carcass) to 20 ± 9 MPN per carcass (range, 14 to 45 MPN per carcass). The numbers of carcasses which were Salmonella positive before and after processing were not significantly different (P = 0.59), nor were the numbers of carcasses which were Campylobacter positive before and after processing (P = 0.66).
In summary, the majority (90%) of the Campylobacter isolates obtained in this study were identified to the species level, and the flaA types of 83% of the isolates were determined. In addition, the serotypes of 8% of the isolates were determined, and the PFGE types of 5% of the isolates were determined. When the data were collated, a number of C. jejuni strains were recognized.
DISCUSSION
Our findings are directly relevant to current hazard analysis critical control point systems. The number of contaminated crates at the farms and the results of washing experiments performed under normal conditions (a soak tunnel with agitation and Alcask, followed by a pressure rinse with a QAC) demonstrated that the cleaning process had little (if any) effect on the Campylobacter and Salmonella status of the transport crates. Organic matter was regularly detected on the crates after they were washed. The status of the crate wash water did not necessarily reflect crate contamination, which has significance for quality control procedures. Organisms present on the crates were likely to be embedded in organic matter and therefore more protected than if they were freely suspended (8). Thorough cleaning, including the removal of fecal material, needs to occur before adequate disinfection can take place. Inadequate cleaning is not confined to one factory, and Mead et al. (19) reported similar findings.
The effects of a detergent and various disinfectants on the levels of contamination on crates and in crate wash water were examined. When the agents were used at recommended working concentrations, the levels of Campylobacter in the wash water and the number of contaminated crates were reduced. We did not recover Salmonella from unwashed crates and were unable to assess the abilities of the different detergents and disinfectants to reduce the levels of this organism. The temperature of the crate wash water was suboptimal (30°C) and should have been between 40 and 80°C (manufacturer's recommendations). This may be another area for improvement, although heating large volumes of water throughout the working day is expensive unless it can be incorporated into a factory water recycling system. Although Campylobacter is generally considered sensitive to disinfectants, none of the treatments eliminated Campylobacter from the crates. Wang et al. (32) found that 1.25 mg of hypochlorite per liter killed three strains of Campylobacter suspended in Sorenson buffer (103 to 104 CFU/ml−1) within 1 min. It is likely that the effectiveness of the chlorine compounds was reduced due to the presence of organic matter (9), indicating that this method may not be effective on an industrial scale and highlighting how problematic it can be to extrapolate data from the laboratory to the factory.
Although frequent addition of detergent and dipping in concentrated disinfectant reduced the level of contamination, more time and investment are needed to improve the washing procedure and crate design if Salmonella and Campylobacter on crates are to be completely eradicated. Contaminated crates can lead to contamination of birds during transport to the processing plant, but the use of contaminated crates during flock thinning is perhaps of more concern. Campylobacter spp. could be introduced into previously Campylobacter-negative flocks, leading to much higher levels of contamination; it is at this point that the use of Campylobacter-negative crates is critical. It has been suggested that biodegradable crates or crate liners could be used. Crate liners would provide a way to remove the bulk of the organic material from crates and would provide a physical barrier between the birds and the crates, decreasing the need for improved cleaning mechanisms, but they could increase costs. Brushes could also be included in the soak tank in order to provide a mechanical means to remove the organic material from the crates; in addition, faster turnover of water would reduce the level of organic material present, but additional detergent would be needed.
Three Campylobacter- and Salmonella-negative flocks were studied during catching, transportation, and processing in order to determine a possible link between contaminated transport crates and carcass contamination. One strength of this study was that several typing methods were used for Campylobacter isolates. The use of several methods has been shown previously to improve strain characterization (26).
Although Salmonella was not isolated from the feces or ceca of birds in this study, three surface swab samples taken from flock III birds prior to catching were Salmonella positive. These results may have been due to contamination from the environment at the time of depopulation or, less likely, to undetected infection within the flock. Catchers may have contributed to contamination of flock I birds which were contaminated with C. jejuni subtype 1-A-1 and Salmonella after they were caught. The catchers opened the crate drawers and may have contaminated their hands before they handled the birds; the Campylobacter subtype (subtype 1-A-1) was isolated from the crates before the birds were caught. Although Salmonella was not isolated from the crates sampled before birds were loaded, it is possible that Salmonella was present at levels below the limit of detection or was present on other crates which were not sampled but which contaminated the birds.
All 15 flock II birds sampled became contaminated with C. jejuni subtype 4-C-3 after they were placed in crates. C. jejuni subtype 4-C-NT was already present in the crates and may have been the subtype which contaminated the birds. There was no evidence that the crates were a source of Salmonella contamination of carcasses. Although one crate that was used during depopulation of flock III was contaminated with Salmonella, the serotype was different from that isolated from the birds. For both flock II and flock III 1 of the 15 crated birds sampled was contaminated with Salmonella, but there was not a significant difference (P = 0.78) between the birds sampled in the crates and the birds sampled before catching.
The birds were in the crates for about 2 h. No Salmonella or Campylobacter was isolated from the ceca, indicating that this was probably not sufficient time for intestinal colonization. During processing, the level of Campylobacter on carcasses of flock III birds decreased. Similar results were obtained by Mead et al. (20), who observed 10- to 1,000-fold reductions in the levels of Campylobacter on skin samples after processing. The decreases in levels of contamination may have been due to the susceptibility of the strains to the stresses of processing, or the organisms may have simply been removed. The majority of contamination on poultry carcasses is believed to be due to intestinal leakage (27). No Campylobacter was isolated from the ceca of the birds, so this was not a source in this study. One of the prescald carcasses of flock I birds and one of the prescald carcasses of flock III birds were contaminated with Salmonella serotypes 3,10e and 4i, respectively, but these serotypes were not isolated from the carcasses after processing. Salmonella serotype 9g was isolated from the processed carcasses of flock 1 birds; this serotype had been isolated previously from live birds at the farm. Serotype 9g could have persisted on the carcasses during processing, or it is possible that carcasses were recontaminated with this serotype during processing from contaminated equipment or the hands of workers.
Prescald carcasses of flock III birds became contaminated with two C. jejuni subtypes (subtypes 55-UT-NT and 5-G-NT) and one Campylobacter isolate which was nontypeable by serotyping and flaA typing. These isolates had not been isolated previously at the farm or from transport crates, and contamination probably occurred at the factory, possibly from the hands of workers (already contaminated from a previously Campylobacter-positive flock) as the birds were placed on the line or possibly from the stun water. A nontypeable strain was also isolated from carcasses after processing; it is possible that this strain is the same strain which contaminated carcasses after processing and was able to persist during processing. Campylobacter subtype 1-A-1 was isolated from live birds at the farm (flock I) and also from some of the processed carcasses. This organism was able to persist throughout processing, surviving not only the temperatures associated with the different areas of the processing plant (which ranged from 6 ± 5°C in the chilling room to 54 ± 1°C in the scald tank) but also the drying conditions and oxygen levels associated with being on the outside of the carcasses. A C. coli strain isolated from a flock I carcass prescald tank was not isolated from the processed carcasses, possibly due to the initial low level of contamination (only one carcass was contaminated) or possibly because this strain was less robust than the strains of C. jejuni and did not survive processing.
Transportation involving contaminated crates resulted in carcasses that were contaminated with Campylobacter, albeit at low frequencies. The use of contaminated crates and catchers during a stressful event such as flock thinning is of great concern as Campylobacter and Salmonella may be introduced into the remainder of the flock. This is obviously a potential control point.
Acknowledgments
We thank Janet Gibson and Elena Lorenz for their advice during typing of the Campylobacter spp. and David Wareing and Andrew Walbridge for serotyping and PFGE typing some of the isolates. We also thank Neville Verlander of the Statistics Unit, PHLS Communicable Disease Surveillance Centre, for carrying out the statistical analysis.
We gratefully acknowledge the Department of Health for financial support (project codes DH227B and 227B).
REFERENCES
- 1.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. W. Swerdlow. 1999. Campylobacter jejuni—an emerging food borne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amemiya, T. 1973. Regression analysis when the dependent variable is truncated normal. Econometrica 41:997-1016. [Google Scholar]
- 3.Anonymous. 1995. Enumeration of micro-organisms, p. 95-120. In D. Roberts, W. Hooper, and M. Greenwood (ed.), Practical food microbiology. Public Health Laboratory Service, London, United Kingdom.
- 4.Anonymous. 2000. Common gastrointestinal infections, England and Wales: laboratory reports, week 49-52/00 and 01/00. Commun. Dis. Rep. Wkly. 10:11. [PubMed]
- 5.Bolton, F. J., D. R. A. Wareing, M. B. Skirrow, and D. N. Hutchinson. 1992. Identification and biotyping of campylobacters. Soc. Appl. Bacteriol. Tech. ser. 29:151-161. [Google Scholar]
- 6.Cogan, T. A., S. F. Bloomfield, and T. J. Humphrey. 1999. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcasses in the domestic kitchen. Lett. Appl. Microbiol. 29:354-358. [DOI] [PubMed] [Google Scholar]
- 7.deWit, J. C., G. Broekhuizen, and E. H. Kampelmacher. 1979. Cross-contamination during the preparation of frozen chickens in the kitchen. Hygiene 83:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhir, V. K., and E. R. Dodd. 1995. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 61:1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Assaad, F. J., L. E. Stewart, E. T. Mallinson, L. E. Carr, F. Joseph, and G. Berney. 1995. Prediction of residual chlorine in a poultry crate disinfection system. Am. Soc. Agric. Eng. 38:179-185. [Google Scholar]
- 10.Genigeorgis, C. A., M. Hassuneh, and P. Collins. 1986. Campylobacter jejuni infection on poultry farms and its effect on poultry meat contamination during slaughtering. J. Food Prot. 49:895-903. [DOI] [PubMed] [Google Scholar]
- 11.Gibson, J. R., K. Sutherland, and R. J. Owen. 1994. Inhibition of DNAse activity in PFGE analysis of DNA from Campylobacter jejuni. Lett. Appl. Microbiol. 19:357-358. [DOI] [PubMed] [Google Scholar]
- 12.Hald, B., A. Wedderkopp, and M. Madsen. 2000. Thermophilic Campylobacter spp. in Danish broiler production: a cross sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol. 29:123-131. [DOI] [PubMed] [Google Scholar]
- 13.Hirji, K. F., C. R. Mehta, and N. R. Patel. 1987. Computing distributions for exact logistic regression. J. Am. Stat. Assoc. 82:1110-1117. [Google Scholar]
- 14.Hood, A. M., A. D. Pearson, and S. Shanker. 1988. The extent of surface contamination of retailed chickens with Campylobacter jejuni serogroups. Epidemiol. Infect. 100:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey, T. J., A. Henley, and D. G. Lanning. 1993. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 110:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs-Reitsma, W. F., A. W. van de Giessen, N. M. Bolder, and R. W. A. W. Mulder. 1995. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 114:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, F. T., R. C. Axtell, D. V. Rives, S. E. Scheideler, F. R. Tarver, R. L. Walker, and M. J. Wineland. 1991. A survey of Campylobacter jejuni contamination in modern broiler production and processing systems. J. Food Prot. 54:259-262. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz, E., A. Lastovica, and R. J. Owen. 1998. Subtyping of Campylobacter jejuni Penner serotypes 9,38 and 63 from human infections, animals and water by pulsed field gel electrophoresis and flagellin gene analysis. Lett. Appl. Microbiol. 26:179-182. [DOI] [PubMed] [Google Scholar]
- 19.Mead, G. C., W. R. Hudson, and M. H. Hinton. 1994. Use of a marker organism in poultry processing to identify sites of cross-contamination and evaluate possible control measures. Br. Poult. Sci. 35:345-354. [DOI] [PubMed] [Google Scholar]
- 20.Mead, G. C., W. R. Hudson, and M. H. Hinton. 1995. Effect of changes in processing to improve hygiene control on contamination of poultry carcasses with Campylobacter. Epidemiol. Infect. 115:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.On, S. L. W., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oosterom, J., C. H. Uyl, J. R. J. Banffer, and J. Huisman. 1984. Epidemiological investigations on Campylobacter jejuni in households with a primary infection. J. Hyg. Camb. 92:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson, A. D., M. H. Greenwood, R. Kevin, A. Feltham, T. D. Healing, J. Donaldson, D. M. Jones, and R. R. Colwell. 1996. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl. Environ. Microbiol. 62:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penner, J. L., and J. N. Hennessy. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J. Clin. Microbiol. 12:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen, L., and S. L. W. On. 2000. Efficacy of flagellin gene typing for epidemiological studies of Campylobacter jejuni in poultry estimated by comparison with macrorestriction profiling. Lett. Appl. Microbiol. 31:14-19. [DOI] [PubMed] [Google Scholar]
- 27.Rivoal, K., M. Denis, G. Salvat, P. Colin, and G. Ermel. 1999. Molecular characterization of the diversity of Campylobacter spp. isolates collected from a poultry slaughterhouse: analysis of cross-contamination. Lett. Appl. Microbiol. 29:370-374. [DOI] [PubMed] [Google Scholar]
- 28.Solomon, E. B., and D. G. Hoover. 1999. Campylobacter jejuni: a bacterial paradox. J. Food Saf. 19:121-136. [Google Scholar]
- 29.Threlfall, E. J., and J. A. Frost. 1990. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological application. J. Appl. Bacteriol. 68:5-16. [DOI] [PubMed] [Google Scholar]
- 30.Tompkins, L. S., M. J. Hudson, H. R. Smith, R. P. Eglin, J. G. Wheeler, M. M. Brett, R. J. Owen, J. S. Brazier, P. Cumberland, V. King, P. E. Cook, and H. J. M. Aarts. 1999. A study of infectious intestinal diseases in England: microbiological findings in cases and controls. Commun. Dis. Public Health 2:108-113. [PubMed] [Google Scholar]
- 31.van de Giessen, A. W., B. P. M. Bloemberg, W. Ritmeerster, and J. J. H. C. Tilburg. 1996. Epidemiological study on risk factors and risk reducing measures for Campylobacter infections in Dutch broiler flocks. Epidemiol. Infect. 117:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, W. L., B. W. Powers, N. W. Luechtefield, and M. J. Blaser. 1983. Effects of disinfectants on Campylobacter jejuni. Appl. Environ. Microbiol. 45:1202-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]