Abstract
Several branched-chain volatile compounds are involved in the flavor of Swiss cheese. These compounds are probably produced by enzymatic conversion of branched-chain amino acids, but the flora and the pathways involved remain hypothetical. Our aim was to determine the ability of Propionibacterium freudenreichii, which is one of the main components of the secondary flora of Swiss cheese, to produce flavor compounds during leucine catabolism. Cell extracts and resting cells of two strains were incubated in the presence of l-leucine, α-ketoglutaric acid, and cofactors, and the metabolites produced were determined by high-performance liquid chromatography and gas chromatography. The first step of leucine catabolism was a transamination that produced α-ketoisocaproic acid, which was enzymatically converted to isovaleric acid. Both reactions were faster at pH 8.0 than at acidic pHs. Cell extracts catalyzed only the transamination step under our experimental conditions. Small amounts of 3-methylbutanol were also produced by resting cells, but neither 3-methylbutanal norα-hydroxyisocaproic acid was detected. l-Isoleucine and l-valine were also converted to the corresponding acids and alcohols. Isovaleric acid was produced by both strains during growth in a complex medium, even under conditions simulating Swiss cheese conditions (2.1% NaCl, pH 5.4, 24°C). Our results show that P. frendenreichii could play a significant role in the formation of isovaleric acid during ripening.
The development of cheese flavor during ripening results from the enzymatic breakdown of curd components into sapid and aroma compounds (11). Catabolism of branched-chain (BC), aromatic, and S-containing amino acids has recently received attention because these amino acids are precursors of various volatile compounds, such as acids, aldehydes, alcohols, esters, and thiols, which can contribute to the development of flavor or off-flavor in cheese depending on their levels and the type of cheese (7, 23). In Swiss cheese, the following BC compounds have been identified as flavor impact compounds: 3-methylbutanal, 2-methylbutanal, 3-methylbutanoic acid (isovaleric acid), and the ethyl ester of 3-methylbutanoic acid. These compounds are probably produced from enzymatic conversion of BC amino acids (BCAAs), particularly leucine, but the flora and the pathways involved in their formation in Swiss cheese remain unclear. Therefore, a better understanding of the origin of these compounds is needed in order to control and accelerate the formation of cheese flavor.
Dairy propionic acid bacteria (PAB) constitute one of the major floras that grow during the ripening of Swiss type cheeses and are commonly used as secondary starters (26). They are involved in formation of the characteristic flavor and openings of Swiss cheese via fermentation of lactate into acetic acid, propionic, acid and CO2 (18). While the presence of PAB has been associated with the presence of isovaleric acid in controlled-flora Swiss cheese (28), the ability of these organisms to generate flavor compounds from amino acid catabolism remains unclear. Resting cells of PAB incubated in the presence of single amino acids or an amino acid mixture were shown to degrade significant amounts of aspartic acid, alanine, serine, glycine, and arginine, but very little or no degradation of BCAAs or aromatic amino acids was found (16). PAB are, however, capable of producing isovaleric acid and other BC acids when they are grown in complex media (4).
The aims of the present study were to determine the ability of Propionibacterium freudenreichii to degrade BCAAs and to determine the pathway of leucine catabolism. Our results showed that the first step of leucine catabolism by resting cells was transamination that produced α-ketoisocaproic acid, which was then enzymatically converted to isovaleric acid.
MATERIALS AND METHODS
Chemicals.
Amino acids, keto acids, hydroxy acids, BC acids, BC aldehydes and alcohols, pyridoxal 5"-phosphate (PLP), thiamine pyrophosphate chloride (TPP), NAD+, NADH, vitamins, and salts were obtained from Sigma-Aldrich (St. Quentin Fallavier, France), oxalic acid was obtained from Prolabo (Paris, France), pyruvic acid was obtained from Merck (Darmstadt, Germany), 3-methylbutanol was obtained from Fluka Chemical (Ronkonkoma, N.Y.), and l-[4,5-3H]leucine (60 Ci mmol−1) was obtained from Isotopchim (Peyruis, France).
Strains and growth media.
P. freudenreichii subsp. shermanii TL 34 (a strain reisolated from ATCC 9614) from the TL collection (Laboratoire de Recherches de Technologie Laitière, INRA, Rennes, France) and ITGP23 from the collection of the Institut Technique Français du Fromage (Rennes, France) were used. Cells were routinely grown in yeast extract-sodium lactate medium (pH 6.8) (YEL medium) (20) that was sterilized at 115°C for 20 min and incubated semianaerobically (static cultures without modification of the atmosphere). For some experiments, cells were grown in a modified YEL medium (YEL-S medium) under conditions that simulated Swiss cheese conditions (the pH of YEL medium was reduced to 5.4 and 21 g of NaCl (21 g liter−1 was added). YEL-S medium was sterilized by filtration (pore size, 0.2 μm; Nalgene top filter), inoculated (1%, vol/vol) with a culture grown in the same medium, and incubated at 24°C anaerobically (in anaerobic jars with Anaerocult A [Merck, Nogent-sur-Marne, France]) (32). The effect of α-ketoglutaric acid (α-KG) was studied by supplementing YEL and YEL-S media with 20 mM α-KG before inoculation. These media were sterilized by filtration and incubated under the same conditions as the corresponding media without α-KG.
Growth was monitored by measuring the optical density at 650 nm (OD650). Cultures with OD650 of >0.8 were diluted with sterile medium in order to retain good linearity between absorbance and cell mass. One test tube was used for each measurement. PAB were enumerated on YELA medium (YEL medium containing 15 g of agar per liter) that was incubated at 30°C anaerobically for 6 days. Cell dry weights were determined by drying preparations under a vacuum (5 h at 60°C) with a SpeedVac concentrator (Savant Instrument, Holbrook, N.Y.).
Resting cells and CE preparation.
Cells grown in YEL medium to the late log phase were harvested by centrifugation (8,500 × g, 10 min, 4°C) and washed twice with sterile distilled water. Cell extracts (CE) were prepared by subjecting cells that were resuspended in cold distilled water (OD650, ∼20) to two treatments with a French press (SLM Instruments, Urbana, Ill.) at 4°C and 138 MPa for 10 min, followed by centrifugation at 30,000 × g for 20 min to eliminate unbroken cells and cell walls. The extracts were then sterilized by filtration through a 0.45-μm-pore-size membrane before use.
Leucine catabolism assays.
Cells or CE were suspended in 60 mM phosphate buffer (pH 5.6, 6.8, or 8.0) or in 60 mM Tris HCl (pH 8), as indicated below, containing 5 mM l-leucine, 10 mM α-KG, 50 μM PLP, and 50 μM TPP and were incubated for ∼48 h at 30°C. Blank test preparations without cells or CE and control test preparations without leucine were also included. In one experiment, l-leucine was replaced by l-valine or l-isoleucine. Experiments were also carried out by using α-ketoisocaproic acid or α-hydroxyisocaproic acid as the substrate in 50 mM phosphate buffer (pH 8.0) containing 2.0 mM substrate, 0.2 mM TPP, 1 mM MgCl2 · 6H2O, and 1.8 mM coenzyme A (CoA) in 1.56 mM cysteine HCl (29), with or without 3 mM NAD+ or 3 mM NADH.
In all experiments, the contents of one test tube were used for each analysis. Cell lysis was evaluated during incubation in two ways, by determining the changes in the OD650 of the cell suspension and by measuring the cell protein content in the supernatant after centrifugation of cells (7,500 × g, 15 min, 4°C) by using the micromethod of Bradford and bovine serum albumin as the standard (2). An aliquot of the supernatant was acidified with 1 volume of 300 mM oxalic acid per 9 volumes of sample, (final pH, ∼3.0) and centrifuged (7,500 × g, 15 min, 4°C), and the supernatant was used for subsequent analysis of the products of amino acid catabolism.
HPLC analysis.
The levels of α-ketoisocaproic acid (2-oxo-4-methylpentanoic acid) and α-hydroxyisocaproic acid (2-hydroxy-4-methylpentanoic acid), as well as the levels of the corresponding products formed from valine (α-ketoisovaleric acid and α-hydroxyisovaleric acid) and from isoleucine (α-keto-β-methylvaleric acid and α-hydroxy-β-methylvaleric acid),α-KG, and lactic acid, were determined by high-performance liquid chromatography (HPLC) by using an Aminex A-6 ion-exchange column (Bio-Rad, Hercules, Calif.) at 55°C with 0.01 N H2SO4 at a flow rate of 1.0 ml min−1 as the eluent. Both UV (210 nm) and refractometric detectors were used. In some experiments, l-[4,5-3H]leucine (0.08 μM) was used as a tracer in addition to 2 mM unlabeled l-leucine. Radioactive products were separated by HPLC by using an IC-Pack column (Waters) and were detected with a radio-HPLC detector (Packard Instrument Co., Meriden, Conn.), as described by Yvon et al. (45). Glutamic acid was analyzed enzymatically by using Boehringer kits (R-Biopharm, Darmstadt, Germany). In some experiments, leucine and glutamic acid were analyzed with an amino acid analyzer (AlphaPlus series 2; Pharmacia, Uppsala, Sweden).
GC and GC-MS analyses.
Volatile acids were analyzed with a Varian gas chromatograph (GC) (model 3800; Varian, Walnut Creek, Calif.) equipped with a flame ionization detector and a capillary column (25 m by 0.53 mm; film thickness, 0.5 μm) coated with modified polyethylene glycol (BP21; SGE, Ringwood, Victoria, Australia) under the following conditions: on-column injection of 0.5 μl at 60°C; injector programmed so that the temperature increased at a rate of 20°C min−1 to 200°C after 0.2 min; detector temperature, 200°C; carrier gas, hydrogen at a pressure of 3 lb/in2; initial temperature, 50°C, followed by an increase at a rate of 25°C min−1 to 100°C after 0.5 min and then by an increase at a rate of 6°C min−1 to 135°C and at a rate of 20°C min−1 to 180°C. These conditions did not separate 2-methylbutyric acid from 3-methylbutyric acid, which together are referred to in this paper as isovaleric acid. Compounds were quantified by using regression curves obtained with standard compounds (linearity range, 0 to 5 mM; detection threshold, 5 μM; coefficient of variation, less than 5%).
Low levels of neutral volatile compounds were detected by headspace GC-mass spectrometry (MS) as previously described (38). Briefly, compounds were trapped with a Vocarb 3000 trap (Supelco, Bellefonte, Pa.), thermally desorbed at 250°C, and cryofocused at −100°C before they were injected and separated with an HP5 capillary column (Hewlett-Packard). They were detected with an HP7972A quadrupole MS (Hewlett-Packard) after ionization by electronic impact and were identified by comparing their spectra and retention times with those of reference compounds. They were quantified by performing regression analyses of the peak areas of total ion chromatograms and the concentrations of reference compounds (linearity range, 0 to 50 μM; detection threshold, 0.1 μM; coefficient of variation, less than 20%).
RESULTS
Leucine conversion by CE of P. freudenreichii.
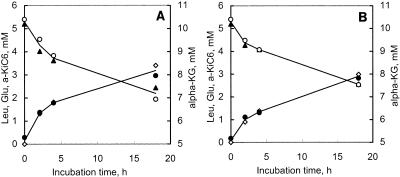
CE of TL 34 and ITGP23 cultures were incubated in phosphate buffer (pH 6.8) at a cell protein concentration of 0.33 mg ml−1. Under these conditions, leucine was converted to 3.0 and 3.4 mM α-ketoisocaproic acid by strains TL 34 and ITGP23, respectively, after 18 h of incubation (Fig. 1). Leucine was not degraded in the presence of heated (12 min, 100°C) CE (data not shown). Leucine conversion took place only if α-KG was present and was accompanied by equimolar α-KG conversion and glutamic acid formation, demonstrating that the first step of the conversion was a transamination. The results of duplicate assays performed with two ITGP23 CE samples, prepared from two different cultures, agreed within 10% (data not shown). TL 34 CE produced 60% more α-ketoisocaproic acid than ITGP23 CE produced after 2 h of incubation. Small amounts of isovaleric acid (<0.05 mM) and traces of two other compounds, 2-methylpropanal (2.0 μM) and 3-methylbutanal (0.2 μM), were detected, showing that α-ketoisocaproic acid was not converted by CE under these conditions.
FIG. 1.
Conversion of l-leucine by CE of P. freudenreichii TL 34 (A) and ITGP23 (B) in 60 mM phosphate (pH 6.8) containing 5 mM l-leucine, 10 mM α-KG, 50 μM PLP, and 50 μM TPP at 30°C. Symbols: ▴, leucine; ◊, α-ketoisocaproic acid (K-iC6); •, glutamic acid; ○, α-KG.
CE of both strains also did not convert α-ketoisocaproic acid to isovaleric acid when they were incubated under conditions that favored decarboxylase activity (60 mM phosphate buffer [pH 5.8], 50 μM TPP). Under conditions designed to evaluate α-keto acid dehydrogenase activity (50 mM phosphate buffer [pH 8.0], 0.2 mM TPP, 1 mM MgCl2, 3 mM NAD+, CoA [29]), CE catalyzed the formation of only a small amount of isovaleric acid (0.07 mM after 24 h of incubation).
When 10 mM phenylpyruvic acid was used as an acceptor of amino groups, the amounts of α-ketoisocaproic acid produced were 16 and 24% of the amounts produced with α-KG for strains TL 34 and ITGP23, respectively, whereas the amounts produced when pyruvic acid was used were only 4% for both strains.
Conversion of leucine by resting cells of P. freudenreichii.
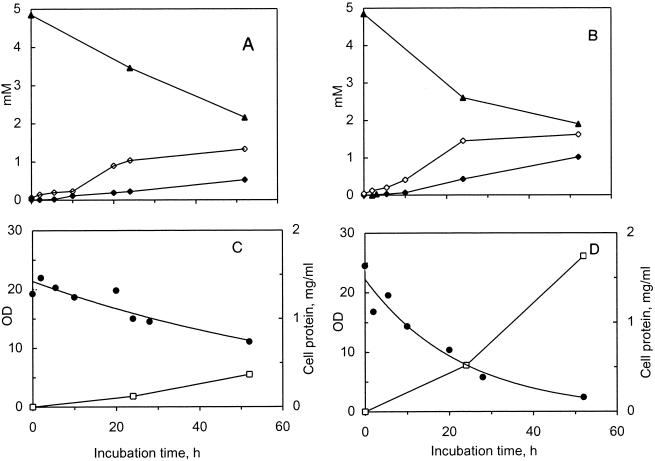
Resting cells of strains TL 34 and ITGP23 were incubated in phosphate buffer (pH 6.8) at initial OD650 of 20 and 24 (∼7 and 8 g [dry weight] liter−1, respectively; i.e.,∼3 × 1010 CFU/ml) in the presence of 5 mM BCAA. Under these conditions, 2.7 and 3.0 mM leucine were consumed after 52 h of incubation by strains TL 34 and ITGP23, respectively, and α-ketoisocaproic acid and isovaleric acid were produced (Fig. 2). Addition of a carbon substrate (10 mM sodium lactate) did not change the pattern of products (Table 1). Omission of PLP or TPP from the incubation mixture only slightly decreased the amounts of products formed, indicating that sufficient levels of cofactors were present in the cells (data not shown). The two strains exhibited similar patterns of degradation of the other BCAAs, valine and isoleucine. Their products of transamination, α-ketoisovaleric acid and α-keto-β-methylvaleric acid, were formed and then converted to isobutyric and 2-methylbutyric acids, respectively (Table 1). The proportion of BC acids was 24% of the products on average after 24 h of incubation and increased during further incubation. Strain ITGP23 consumed and produced 1.5 to 2.1 times as much BC compounds as strain TL 34 consumed and produced after 24 h of incubation (Table 1).
FIG. 2.
Conversion of l-leucine by resting cells of P. freudenreichii TL 34 (A and C) and ITGP23 (B and D) in 60 mM phosphate (pH 6.8) containing 5 mM l-leucine, 10 mM α-KG, 50 μM PLP, and 50 μM TPP at 30°C. Symbols: ▴, leucine; ◊, α-ketoisocaproic acid; ⧫, isovaleric acid. Cell lysis was monitored by determining the decrease in the OD650 (OD) (•) and the amount of proteins released (□).
TABLE 1.
Catabolism of BCAAs by resting cells of two strains of P. freudenreichii subsp. shermanii incubated for 24 h in 60 mM phosphate (pH 6.8) with different compounds added or omitted
| Strain | Incubation mixturea | Concn (mM)
|
||||
|---|---|---|---|---|---|---|
| Amino acid consumed | α-KG consumed | Glutamic acid | BC α-keto acid | BC acid | ||
| TL 34 | Basal medium | 1.4 | 3.9 | 3.7 | 1.0 | 0.22 |
| Basal medium without Leu | 1.8 | 1.0 | <0.1 | <0.02 | ||
| Basal medium without α-KG | <0.1 | 1.4 | <0.1 | <0.02 | ||
| Basal medium + 10 mM sodium lactate | 1.2 | 3.8 | 4.0 | 0.8 | 0.18 | |
| Control (basal medium without cells) | <0.1 | <0.1 | <0.03 | <0.1 | <0.02 | |
| Ile + α-KG + PLP + TPP | 2.4 | 3.9 | 2.1 | 0.7 | 0.46 | |
| Val + α-KG + PLP + TPP | 1.3 | 4.4 | 3.7 | 0.8 | 0.24 | |
| ITGP23 | Basal medium | 2.4 | 2.3 | 1.4 | 1.4 | 0.42 |
| Basal medium without Leu | 0.5 | 0.4 | <0.1 | <0.02 | ||
| Basal medium without α-KG | 0.4 | 0.8 | <0.1 | <0.02 | ||
| Basal medium + 10 mM sodium lactate | 2.1 | 2.0 | 3.1 | 1.6 | 0.47 | |
| Control (basal medium without cells) | <0.1 | <0.1 | <0.03 | <0.1 | <0.02 | |
| Ile + α-KG + PLP + TPP | 3.8 | 2.7 | 2.3 | 1.5 | 0.44 | |
| Val + α-KG + PLP + TPP | 2.8 | 1.7 | 1.6 | 1.8 | 0.40 | |
Basal medium contained Leu, α-KG, PLP, and TPP.
BC neutral compounds were tentatively detected by headspace GC-MS; traces (∼20 μM) of 3-methylbutanol and 2-methylbutanol were found with resting cells incubated in the presence of leucine and isoleucine, respectively, whereas 3-methylbutanal and 2-methylbutanal were not detected (<0.1 μM). BC hydroxy acids (detection limit, around 80 μM) were not detected by the HPLC method used throughout incubation.
Since it is known that resting cell experiments are difficult to control, the reproducibility of the results was assessed by performing six separate experiments with resting cells of strain TL 34 incubated under comparable conditions (OD650, 5 to 20; 2 to 5 mM leucine; pH 6.8; incubation for ∼48 h at 30°C). In all cases, α-ketoisocaproic acid and isovaleric acid were the main products of leucine catabolism. On average, the amount of these products together obtained was 2.4 ± 1.35 μM h−1 per OD650 unit (i.e., 2.3 mM in 48 h at an OD650 of 20), and 42% ± 15% was isovaleric acid. The variations could have resulted, at least in part, from small differences in the time of cell harvest, which resulted in variations in the metabolic activities of the cells and in the extents of lysis.
The stoichiometry of substrates and products in resting cell experiments differed from what was expected a priori and was observed in CE experiments. The results were validated in separate experiments with strain TL 34. On average, the ratio of BC compounds was 0.87 ± 0.22 mol per mol of BCAA consumed. The ratio of amount of leucine consumed to amount of α-KG consumed was 0.5 ± 0.11. This result was consistent with the observed disappearance of α-KG in the absence of added amino acid (Table 1). Some of the α-KG could have been used for transamination of other amino acids in the cells or for reactions other than transamination, but no end products were identified under these conditions by the methods used. The ratio of amount of glutamic acid produced to amount of α-KG consumed was 0.6 ± 0.29. Glutamic acid could have been partially converted, for example by decarboxylation (1). Some differences between the expected ratio and the observed ratio could also have been due to a transport phenomenon.
Both strains lysed during resting cell experiments (Fig. 2). ITGP23 cells lysed faster and to a greater extent than TL 34 cells, and 1.74 and 0.37 mg of proteins ml−1, respectively, were released. At the same time, the cell viabilities dropped to<1 and 7% of the initial values for strains ITGP23 and TL 34, respectively. Therefore, the formation of BC compounds in these experiments resulted from both the activity of resting cells and the activity of the enzymes released into the incubation mixture. When the results obtained in CE and resting cell experiments for both strains were considered together, it appeared that cell lysis could enhance leucine conversion under our experimental conditions.
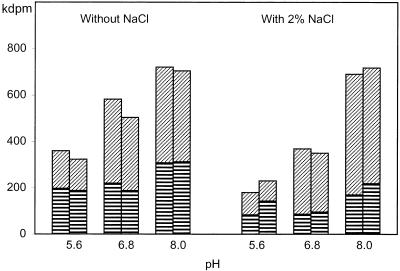
The effects of pH and NaCl on conversion of leucine were investigated with resting cells of P. freudenreichii TL 34 (OD650, ∼17) incubated at pH 5.6, 6.8, or 8.0 with or without 2% NaCl. The origin of the compounds formed from leucine was confirmed by adding [3H]leucine as a tracer. Isovaleric acid production and, by implication, transamination increased markedly as the pH increased and were inhibited by NaCl except at pH 8.0 (Fig. 3). Under the salt concentration and pH conditions of cheese (pH 5.6, 2% NaCl), the levels of leucine degradation and isovaleric acid production were still 30 and 18%, respectively, of the levels observed under the best conditions (pH 8.0, 2% NaCl). Whatever the conditions, the level of lysis remained low in this experiment; the amount of protein released was <0.1 mg ml−1 (i.e., <4% of the total cell protein) after 43 h of incubation. Therefore, the effects of pH and NaCl on the kinetics of leucine conversion were not due to or were only partially due to differences in cell lysis in this experiment. Other labeled BC compounds (aldehydes, alcohols, and α-hydroxy acids; detection limit, around 0.05 mM) were not detected. Small amounts of neutral volatile compounds were, however, detected by headspace GC-MS. The production of these compounds was greater under acidic or neutral conditions; the level of 3-methylbutanol at pH 5.6 or 6.8 (∼8 μM) was 10 times greater than the level at pH 8, and trace amounts of 2-methylpropanal (∼0.5 μM) were also detected (data not shown). Nevertheless, these compounds represented less than 1.5% of the total products formed from leucine in all cases.
FIG. 3.
Effects of pH and NaCl on conversion of [3H] leucine by resting cells of P. freudenreichii TL 34. The cells were incubated for 43 h at 30°C in 60 mM phosphate at three pH values in presence or absence of 2% NaCl. ▤, α-ketoisocaproic acid; ▨, isovaleric acid. The results are expressed in 103 disintegrations per minute. The results of duplicate experiments are shown and agreed within 14% on average. The following conditions were used: 2 mM l-leucine, 10 mM α-KG, 50 μM PLP, and 50 μM TPP.
Conversion of α-ketoisocaproic acid by resting cells of P. freudenreichii.
In order to determine the possible pathways of leucine catabolism, resting cells were also incubated in the presence of the corresponding α-keto orα-hydroxy acid. α-Hydroxyisocaproic acid was not metabolized by cells even when NAD+ was present in the reaction mixture (data not shown). α-Ketoisocaproic acid was converted to isovaleric acid (Table 2). This reaction was almost completely inhibited by sodium arsenite (Table 2). Since arsenite is a known inhibitor of the E2 component of the pyruvate dehydrogenase complex but not of pyruvate decarboxylase (41), this result supports the hypothesis that a BC keto acid dehydrogenase complex is involved in α-keto acid conversion, leading to the formation of the isovaleryl-CoA ester. The acyl-CoA would then be hydrolyzed by an acyl-CoA hydrolase, forming isovaleric acid. Addition of either NAD+ or NADH enhanced isovaleric acid formation, suggesting that the amounts of these cofactors were limited in the cells and that NADH was probably oxidized to NAD+.
TABLE 2.
Catabolism of α-ketoisocaproic acid by resting cells of P. freudenreichii subsp. shermanii TL 34 incubated for 48 h in 50 mM phosphate (pH 8.0) containing 2 mM α-ketoisocaproic acid with different compounds added or omitted
| Incubation mixturea | Concn (mM)
|
|
|---|---|---|
| α-Ketoisocaproic acid consumed | Isovaleric acid | |
| Basal medium | 1.5b | 0.72 |
| Basal medium without α-ketoisocaproic acid | 0.02 | |
| Basal medium + 10 mM arsenite | <0.1 | 0.02 |
| Basal medium + 5 mM NAD+ | 1.4 | 0.99 |
| Basal medium + 5 mM NAD+ + 10 mM arsenite | <0.1 | 0.02 |
| Basal medium + 5 mM NADH | 1.4 | 1.20 |
| Control (basal medium without cells) | <0.1 | <0.02 |
Basal medium contained α-ketoisocaproic acid, TPP, and CoA.
The values are means based on the results of duplicate assays (two tubes incubated on the same day). The values for replicates did not differ by more than 20%.
Production of isovaleric acid during growth.
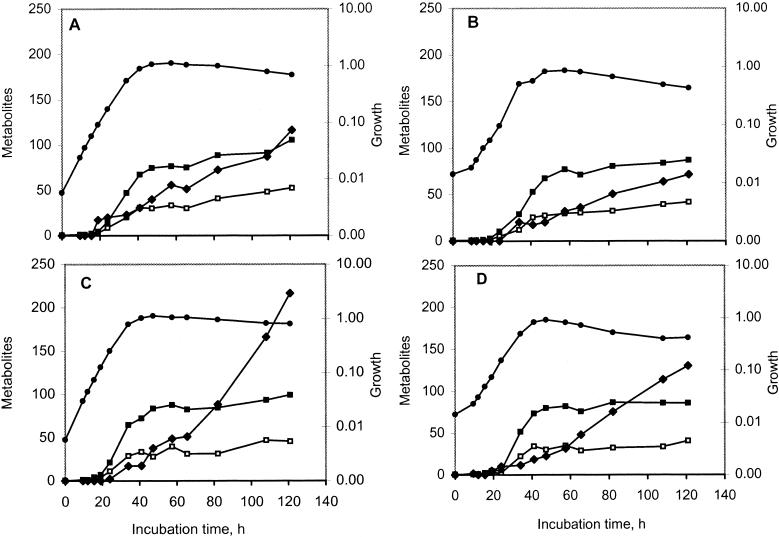
Both strains produced isovaleric acid during growth in complex media under optimal conditions (YEL medium) and under conditions that simulated Swiss cheese conditions (YEL-S medium). Whatever the conditions, strain TL 34 produced 1.7 to 2.0 times as much isovaleric acid as strain ITGP23 produced. The kinetics of growth and volatile fatty acid production for both strains grown in YEL medium are shown in Fig. 4. The end of the growth phase was concurrent with the exhaustion of lactic acid, which occurred after 48 h of incubation in YEL medium and after 120 h of incubation in YEL-S medium (data not shown). At these times, the amounts of isovaleric acid produced were 2.6 and 3.1 times higher in YEL-S medium than in YEL medium for strains TL 34 and ITGP23, respectively (Table 3).
FIG. 4.
Growth of P. freudenreichii TL 34 (A and C) and ITGP23 (B and D) in YEL medium with (C and D) or without (A and B) 20 mM α-KG. Symbols: •, dry weight (grams per liter); □, concentration of acetic acid (millimolar); ▪, concentration of propionic acid (millimolar); ⧫, concentration of isovaleric acid (micromolar).
TABLE 3.
Growth of and isovaleric acid production by two strains of P. freudenreichii in two culture media with or without 20 mM α-KG added
| Medium | Strain | α-KG | Growth
|
Isovaleric acid concn (μM)a
|
||
|---|---|---|---|---|---|---|
| Generation time (h) | Maximal dry wt (g/liter) | Maximal growthb | End of incubationc | |||
| YEL | TL 34 | − | 4.8 ± 0.06 | 1.2 | 40 | 116 ± 4.4 |
| + | 4.3 ± 0.06 | 1.2 | 38 | 216 ± 4.5 | ||
| ITGP23 | − | 8.8 ± 0.80 | 1.1 | 20 | 72 ± 5.4 | |
| + | 7.4 ± 0.51 | 1.1 | 22 | 130 ± 1.9 | ||
| YEL-Sd | TL 34 | − | 13.2 ± 0.65 | 0.9 | 102 ± 7 | 150 ± 29.2 |
| + | >100 | NDe | ND | ND | ||
| ITGP23 | − | 15.3 ± 0.71 | 0.8 | 61 ± 1 | 129 ± 27.0 | |
| + | >100 | ND | ND | ND | ||
The isovaleric acid concentration is the sum of the 2- and 3-methylbutyric acids concentrations. The values are means ± standard deviations based on the results obtained for different culture tubes incubated at the same time (n = 3 or 4).
Maximal growth in YEL and YEL-S media occurred at 48 h and 6 days, respectively.
The end of incubation in YEL and YEL-S media occurred at 120 h and 15 days, respectively.
YEL-S medium was YEL medium that was modified by lowering the pH to 5.4 (compared to pH 6.8 in YEL medium) and adding 2.1% NaCl; YEL-S medium was incubated at 24°C, whereas YEL medium was incubated at 30°C.
ND, not determined.
During the stationary phase, both strains lysed and the isovaleric acid concentration still increased (Fig. 4 and Table 3). Strain ITGP23 lysed to a greater extent than strain TL 34 (54 to 58 and 29 to 40% decreases in OD650, respectively), as observed with resting cells. The amounts of isovaleric acid produced per hour and per gram of dry cells under cheese conditions were 26% (strain TL 34) and 67% (strain ITGP23) of the amounts produced under optimal conditions, as observed with resting cells under similar conditions.
Addition of α-KG to YEL medium slightly decreased the generation times for both strains and resulted in increases of more than 80% in the amounts of isovaleric acid produced during the stationary phase (Fig. 4). α-KG was consumed during the growth phase (10.8 and 7.1 mM for strains TL 34 and ITGP23, respectively) and during the stationary phase (1.9 and 4.1 mM). Addition of α-KG to YEL-S medium, in which both strains grew at a low rate, almost totally inhibited growth (Table 3), probably because of the cumulative inhibitory effects of α-KG, the low pH, and the high osmotic pressure due to the NaCl content (360 mM).
DISCUSSION
This study is the first report on the ability of P. freudenreichii to catabolize BCAAs. Previous studies of amino acid catabolism by resting cells of PAB showed that there was very little or no degradation of BCAAs. However, these studies were carried out in the absence of an added keto acid acceptor (3, 16). We showed that P. freudenreichii is capable of degrading l-leucine to α-ketoisocaproic acid, but only in the presence of α-KG, and is capable of converting the α-ketoisocaproic acid produced to isovaleric acid. BCAA-derived α-keto acids and acids were also produced from isoleucine and valine as expected, since these two steps are common to the three BCAAs (21).
Transamination is the first step of BCAA catabolism, as demonstrated by the fact that a keto acid is required to accept the amino group from leucine degradation. Transamination was enhanced at pH 8.0, as observed previously in lactic acid bacteria (12, 44, 45). In previous studies, alanine dehydrogenase activity was observed (3), and as a consequence, the pyruvic acid formed could have been used as an amino group acceptor for the transamination of other amino acids. Our results show, however, that compared to α-KG, pyruvic acid is an inefficient amino group acceptor for BCAA transamination by PAB, as also observed for Lactococcus lactis (44). As in BCAA catabolism by L. lactis, the first step of BCAA catabolism by PAB, transamination, could be limited in cheese by the concentration of a suitable amino group acceptor (31, 43). α-KG and several other α-keto acids (pyruvic acid, p-hydroxyphenylpyruvic acid) have been found in cheese (34), but their concentration in Swiss cheese has not been investigated. The transamination step is more efficiently catalyzed by CE than by resting cells, probably because the transport of substrates and products limits the conversion. This step could therefore be favored by cell lysis, but the activity and the stability of aminotransferase in cheese have not been determined. Growth experiments have shown that PAB are capable of producing an amino group acceptor since isovaleric acid is produced in the absence of added α-KG. The presence of BCAA aminotransferase activity in PAB is expected for strains prototrophic for amino acids because aminotransferases are involved in the last step of BCAA synthesis. The observations of Schwartz (35) indicated that “Propionibacterium shermanii” was capable of growing with NH4+ as a sole nitrogen source under strictly anaerobic conditions. Transaminase activities were also found in CE of Propionibacterium jensenii (15).
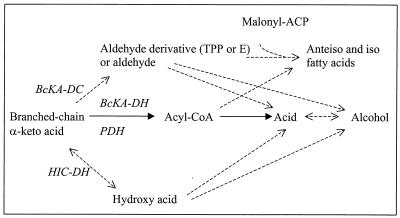
Isovaleric acid is the major end product of leucine catabolism, whatever the conditions of incubation. This conversion is slow, since isovaleric acid could not be detected before a preparation had been incubated for 10 h. This reaction was inhibited by arsenite and required the presence of cells, indicating that it is an enzymic reaction (Table 2). BC acids can be produced from BC α-keto acids by two pathways. The first pathway is oxidative decarboxylation via a BC α-keto acid dehydrogenase complex (EC 1.2.4.4), yielding BC acyl-CoA, which can then be converted to the acid. The second pathway is nonoxidative decarboxylation by pyruvate decarboxylase (EC 4.1.1.1) or a pyruvate decarboxylase-like enzyme to obtain aldehyde, which can be converted to the acid. Both of these pathways are enhanced at pH ∼8.0 (19, 27). The drastic reduction in activity caused by the addition of arsenite and the failure to detect aldehydes support the hypothesis that an α-keto acid dehydrogenase is involved in the second step of leucine conversion by P. freudenreichii (Fig. 5). A multienzyme complex has been found in numerous organisms, including Bacillus subtilis (19), Pseudomonas putida (36), and Saccharomyces cerevisiae (8). Some components of this complex, particularly the E3 component, are found in the pyruvate dehydrogenase complex. Pyruvate dehydrogenase activity has been found in PAB, but it is not known whether this complex is active with other α-keto acids (1). No conclusion can be drawn about the effect of lysis on isovaleric acid production, since the strain that exhibited the higher level of lytic activity produced smaller amounts of isovaleric acid during growth but larger amounts during resting cell experiments than the less lytic strain produced. Formation of BC acids by cheese-related bacteria has not been extensively studied, but it does not appear to be common in lactococci (7). L. lactis, however, is able to produce low levels of isovaleric acid (less than 3% of the metabolites produced from leucine) (43). Isovaleric and isobutyric acids were produced by resting cells of a Lactobacillus strain that was isolated from cheddar cheese and incubated in the presence of BCAAs (25). Such nonstarter lactobacilli can be found in Swiss cheese too. Recently, it was shown that thermophilic lactobacilli, especially Lactobacillus helveticus, are also capable of producing isovaleric acid from leucine in resting cell experiments (S. Helinck, D. Moreau, and M. Yvon, NIZO Dairy Conf. Food Microbes, poster abstr. P37, 2001).
FIG. 5.
Hypothetical pathways for catabolism of BC keto acids. ACP, acyl carrier protein; E, enzyme; BcKA-DC, BC keto acid decarboxylase; BcKA-DH, BC keto acid dehydrogenase complex; PDH, pyruvate dehydrogenase complex; HIC-DH, hydroxyisocaproic acid dehydrogenase. The solid lines indicate the proposed pathway for BC acid production by P. freudenreichii; the dashed lines indicate other pathways, partially adapted from reference 27.
The physiological role of production of BC acids in PAB remains to be determined. In clostridia, the ability to convert BCAAs to the corresponding acids is related to the presence of BC fatty acids in lipids (10). In P. freudenreichii, the cellular fatty acids contain high proportions of BC fatty acids, including 34 to 64% anteiso-C15 (13, 24). Moreover, the proportion of anteiso-C15 and iso-C15 in the cellular fatty acids of P. freudenreichii was increased by addition of isoleucine or leucine to the growth medium (24). Therefore, the production of volatile BC acids in PAB may be related to the biosynthesis of cellular BC fatty acids. The synthesis of these compounds in B. subtilis requires the synthesis of primers, which can be either a BC acyl-CoA or an aldehyde derivative (Fig. 5) (27). The greater production of isovaleric acid by PAB in YEL-S medium at 24°C than in YEL medium at 30°C (Table 3) could be related to an increase in the synthesis of BC fatty acids at a low temperature, as observed in various species, including PAB (13, 14, 17).
Other compounds produced during leucine catabolism were only minor end products (less than 5% of the total products formed under the most favorable conditions). The concentrations of BC alcohols were low (<20 μM). These compounds can be produced from reduction of BC aldehydes or acids (9). No BC aldehydes were detected in the present study. These compounds can be produced by several food-related microorganisms, including L. lactis, Lactobacillus casei, and Carnobacterium sp. (7, 22, 39, 42). BCAA-derived α-hydroxy acids were neither detected (<0.08 mM) nor degraded under our experimental conditions; however, they are produced by Propionibacterium avidum, a cutaneous species of PAB (6). The formation of these compounds could be related to the ratio of NAD+ to NADH in cells (7). BC α-hydroxy acids are the major compounds resulting from BCAA catabolism in several species of lactic acid bacteria. However, it is thought that these compounds do not contribute to cheese flavor (7).
The catabolism of BCAAs by P. freudenreichii differs markedly from the catabolism of BCAAs by lactococci based on the high concentrations of acids in the end products. The enzymes involved in this pathway have not been characterized yet. The concentration of isovaleric acid produced by PAB under Swiss cheese-ripening conditions in YEL-S medium was about 200-fold higher than the threshold value in water (0.7 μM) (5) and was in the range of the values found in Swiss cheese (0.1 to 0.5 mM) (30, 33, 40). Therefore, PAB could play a role in the formation of flavor compounds derived from BCAA catabolism during Swiss cheese ripening, as recently suggested (37).
Acknowledgments
We thank M. Piot for performing the amino acid analyses and M.C. Montel for advice and help in some experiments with [3H]leucine carried out at the Station de Recherches sur la Viande, INRA Theix, Saint Genès-Champanelle, France.
Part of this work was supported by grants from the Ministère de l'Education Nationale, de la Recherche et de la Technologie, Paris, France.
REFERENCES
- 1.Beck, S., and B. Schink. 1995. Acetate oxidation through a modified citric acid cycle in Propionibacterium freudenreichii. Arch. Microbiol. 163:182-187. [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of portein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brendehaug, J., and T. Langsrud. 1985. Amino acid metabolism in propionibacteria: resting cells experiments with four strains. J. Dairy Sci. 68:281-289. [Google Scholar]
- 4.Britz, T. J., and P. L. Steyn. 1979. Volatile fatty acid production by the dairy and clinical propionibacteria and related coryneforms. Phytophylactica 11:111-115. [Google Scholar]
- 5.Buttery, R. G., J. G. Turnbaugh, and L. C. Ling. 1988. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 36:1006-1009. [Google Scholar]
- 6.Carlier, J. P., and N. Sellier. 1989. Gas chromatographic-mass spectral studies after methylation of metabolites produced by some anaerobic bacteria in spent media. J. Chromatogr. 493:257-273. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, J. E., E. G. Dudley, J. A. Pederson, and J. L. Steele. 1999. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Leeuwenhoek 76:217-246. [PubMed] [Google Scholar]
- 8.Dickinson, J. R., and I. W. Dawes. 1992. The catabolism of branched-chain amino acids occurs via 2-oxoacid dehydrogenase in Saccharomyces cerevisiae. J. Gen. Microbiol. 138:2029-2033. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson, J. R., M. M. Lanterman, D. J. Danner, B. M. Pearson, P. Sanz, S. J. Harrison, and M. J. Hewlins. 1997. A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 272:26871-26878. [DOI] [PubMed] [Google Scholar]
- 10.Elsden, S. R., M. G. Hilton, K. R. Parsley, and R. Self. 1980. The lipid fatty acids of proteolytic clostridia. J. Gen. Microbiol. 118:115-123. [Google Scholar]
- 11.Fox, P. F., B. A. Law, P. L. H. McSweeney, and J. Wallace. 1993. Biochemistry of cheese ripening, p. 389-438. In P. F. Fox (ed.), Cheese: chemistry, physics and microbiology. Elsevier Applied Science Publishers, London, United Kingdom.
- 12.Gao, S., D. H. Oh, J. R. Broadbent, M. E. Johnson, B. C. Weimer, and J. L. Steele. 1997. Aromatic amino acid catabolism by lactococci. Lait 77:371-381. [Google Scholar]
- 13.Hofherr, L. A., E. G. Hammond, B. A. Glatz, and P. F. Ross. 1983. Relation of growth temperature to fatty acid composition of Propionibacterium strains. J. Dairy Sci. 66:1622-1629. [Google Scholar]
- 14.Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinney, R. W., and C. H. Werkman. 1958. Transamination in Propionibacterium jensenii. Iowa State Coll. J. Sci. 32:455-461. [Google Scholar]
- 16.Kiuru, V. J. T. 1949. Über die Propionsäuregärung in bezug auf Emmentaler Käse. Ph.D. thesis. University of Helsinki, Finland.
- 17.Klein, W., M. H. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langsrud, T., and G. W. Reinbold. 1973. Flavor development and microbiology of Swiss cheese—a review. III. Ripening and flavor production. J. Milk Food Technol. 36:593-609. [Google Scholar]
- 19.Lowe, P. N., J. A. Hodgson, and R. N. Perham. 1983. Dual role of a single multienzyme complex in the oxidative decarboxylation of pyruvate and branched-chain 2-oxo acids in Bacillus subtilis. Biochem. J. 215:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik, A. C., G. W. Reinbold, and E. R. Vedamuthu. 1968. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 14:1185-1191. [DOI] [PubMed] [Google Scholar]
- 21.Massey, L. K., J. R. Sokatch, and R. S. Conrad. 1976. Branched-chain amino acid catabolism in bacteria. Bacteriol. Rev. 40:42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masson, F., L. Hinrichsen, R. Talon, and M. C. Montel. 1999. Factors influencing leucine catabolism by a strain of Staphylococcus carnosus. Int. J. Food Microbiol. 49:173-178. [DOI] [PubMed] [Google Scholar]
- 23.McSweeney, P. L. H., H. E. Nursten, and G. Urbach. 1997. Flavours and off-flavours in milk and dairy products, p. 403-468. In P. F. Fox (ed.), Advanced dairy chemistry. Lactose, water, salts and vitamins. Chapman & Hall, London, United Kingdom.
- 24.Moss, C. W., V. R. Dowell, Jr., D. Farshtchi, L. J. Raines, and W. B. Cherry. 1969. Cultural characteristics and fatty acid composition of propionibacteria. J. Bacteriol. 97:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae, T., and J. A. Elliot. 1965. Production of volatile fatty acids by some lactic acid bacteria. II. Selective formation of volatile fatty acids by degradation of amino acids. J. Dairy Sci. 48:293-299. [DOI] [PubMed] [Google Scholar]
- 26.Noël, Y., P. Boyaval, A. Thierry, V. Gagnaire, and R. Grappin. 1999. Eye formation and Swiss-type cheeses, p. 222-250. In B. A. Law (ed.), Technology of cheesemaking. Sheffield Academic Press Ltd, Sheffield, United Kingdom.
- 27.Oku, H., and T. Kaneda. 1988. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. A decarboxylase is essential for branched-chain fatty acid synthetase. J. Biol. Chem. 263:18386-18396. [PubMed] [Google Scholar]
- 28.Paulsen, P. V., J. Kowalewska, E. G. Hammond, and B. A. Glatz. 1980. Role of microflora in production of free fatty acids and flavor in Swiss cheese. J. Dairy Sci. 63:912-918. [Google Scholar]
- 29.Perham, R. N., and P. N. Lowe. 1988. Isolation and properties of the branched-chain 2-keto acid and pyruvate dehydrogenase multienzyme complex from Bacillus subtilis. Methods Enzymol. 166:330-341. [DOI] [PubMed] [Google Scholar]
- 30.Preininger, M., R. Warmke, and W. Grosch. 1996. Identification of the character impact flavour compounds of Swiss cheese by sensory studies of models. Z. Lebensm. Unters. Forsch. 202:30-34. [Google Scholar]
- 31.Rijnen, L., P. Courtin, J. C. Gripon, and M. Yvon. 2000. Expression of a heterologous glutamate dehydrogenase gene in Lactococcus lactis highly improves the conversion of amino acids to aroma compounds. Appl. Environ. Microbiol. 66:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolin, D. B., F. Girard, J. D. De Certaines, and P. Boyaval. 1995. 13C-NMR study of lactate metabolism in Propionibacterium freudenreichii subsp. shermanii. Appl. Microbiol. Biotechnol. 44:210-217. [Google Scholar]
- 33.Rychlik, M., R. Warmke, and W. Grosch. 1997. Ripening of Emmental cheese wrapped in foil with and without addition of Lactobacillus casei subsp. casei. III. Analysis of character impact flavour compounds. Lebensm. Wiss. Technol. 30:471-478. [Google Scholar]
- 34.Schormüller, J. 1968. The chemistry and biochemistry of cheese ripening. Adv. Food Res. 16:231-334. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz, A. C. 1968. Wachstum von Propionibacterium shermanii mit einzelnen Aminosäuren als Stickstoffquelle. Z. Allg. Mikrobiol. 8:275-279. [PubMed] [Google Scholar]
- 36.Sokatch, J. R. 1988. Purification of branched-chain keto acid dehydrogenase and lipoamide dehydrogenase-valine from Pseudomonas putida. Methods Enzymol. 166:342-350. [DOI] [PubMed] [Google Scholar]
- 37.Thierry, A., and M. B. Maillard. 2002. Production of cheese flavour compounds derived from amino acid catabolism by Propionibacterium freudenreichii: a review. Lait 82:17-32.
- 38.Thierry, A., M. B. Maillard, and J. L. Le Quéré. 1999. Dynamic headspace analysis of Emmental aqueous phase as a method to quantify changes in volatile flavour compounds during ripening. Int. Dairy J. 9:453-463. [Google Scholar]
- 39.Tucker, J. S., and M. E. Morgan. 1967. Decarboxylation of α-keto acids by Streptococcus lactis var. maltigenes. Appl. Microbiol. 15:694-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vangtal, A., and E. G. Hammond. 1986. Correlation of the flavor characteristics of Swiss-type cheeses with chemical parameters. J. Dairy Sci. 69:2982-2993. [Google Scholar]
- 41.Webb, J. L. 1966. Arsenicals, p. 595. In Enzyme and metabolic inhibitors. Academic Press, New York, N.Y.
- 42.Weerkamp, A. H., N. Klijn, R. Neeter, and G. Smit. 1996. Properties of mesophilic lactic acid bacteria from raw milk and naturally fermented raw milk products. Neth. Milk Dairy J. 50:319-332. [Google Scholar]
- 43.Yvon, M., S. Berthelot, and J. C. Gripon. 1998. Adding alpha-ketoglutarate to semi-hard cheese curd highly enhances the conversion of amino acids to aroma compounds. Int. Dairy J. 8:889-898. [Google Scholar]
- 44.Yvon, M., E. Chambellon, A. Bolotin, and F. Roudot-Algaron. 2000. Characterization and role of the branched-chain aminotransferase (BcaT) isolated from Lactococcus lactis subsp. cremoris NCDO 763. Appl. Environ. Microbiol. 66:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yvon, M., S. Thirouin, L. Rijnen, D. Fromentier, and J. C. Gripon. 1997. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl. Environ. Microbiol. 63:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]