Abstract
We analyzed the phylogenetic composition of bacterioplankton assemblages in 11 Arctic Ocean samples collected over three seasons (winter-spring 1995, summer 1996, and summer-fall 1997) by sequencing cloned fragments of 16S rRNA genes. The sequencing effort was directed by denaturing gradient gel electrophoresis (DGGE) screening of samples and the clone libraries. Sequences of 88 clones fell into seven major lineages of the domain Bacteria: α (36%)-, γ (32%)-, δ (14%)-, and ɛ (1%)-Proteobacteria; Cytophaga-Flexibacter-Bacteroides spp. (9%); Verrucomicrobium spp. (6%); and green nonsulfur bacteria (2%). A total of 34% of the cloned sequences (excluding clones in the SAR11 and Roseobacter groups) had sequence similarities that were <94% compared to previously reported sequences, indicating the presence of novel sequences. DGGE fingerprints of the selected samples showed that most of the bands were common to all samples in all three seasons. However, additional bands representing sequences related to Cytophaga and Polaribacter species were found in samples collected during the summer and fall. Of the clones in a library generated from one sample collected in spring of 1995, 50% were the same and were most closely affiliated (99% similarity) with Alteromonas macleodii, while 50% of the clones in another sample were most closely affiliated (90 to 96% similarity) with Oceanospirillum sp. The majority of the cloned sequences were most closely related to uncultured, environmental sequences. Prominent among these were members of the SAR11 group. Differences between mixed-layer and halocline samples were apparent in DGGE fingerprints and clone libraries. Sequences related to α-Proteobacteria (dominated by SAR11) were abundant (52%) in samples from the mixed layer, while sequences related to γ-proteobacteria were more abundant (44%) in halocline samples. Two bands corresponding to sequences related to SAR307 (common in deep water) and the high-G+C gram-positive bacteria were characteristic of the halocline samples.
Studies of the biogeography of marine bacteria have become feasible as a result of the application of molecular biological techniques to environmental sciences (13). For example, Fuhrman et al. (20) and Mullins et al. (35) compared the phylogenetic diversity of populations from similar latitudes in the Atlantic and Pacific by using 16S rRNA gene sequences. These studies and studies like them (reviewed in reference 21) have concluded that broad classes of bacterioplankton tend to be cosmopolitan, a conclusion supported by incidental observations made as additional sequences are obtained from samples collected at other locations. However, these studies primarily address distributions in temperate and tropical waters where barriers to genetic exchange have been minimal.
Paleo-oceanographic evidence suggests that the Earth's polar oceans are relatively new features of the global marine environment and that, unlike temperate and tropical oceans, they evolved separately. A deepwater connection between the Arctic Ocean basin and the Atlantic Ocean is thought to have opened ca. 50 million years before present (MYBP) as the Mid-Atlantic Ridge propagated into the basin, creating Fram Strait. Before that, the Arctic Ocean basin was an isolated, land-locked sea (65). The Antarctic Circumpolar Current came into being when Antarctica and Australia separated ca. 25 MYBP (50). This feature isolated surface waters inshore (south) of the Polar Front from the southern reaches of the Atlantic, Pacific, and Indian Oceans. At about this time, polar oceans and oceanic deep water cooled rapidly, resulting in cold polar oceans separated by warm tropical gyres.
The present day Arctic Ocean is perennially ice covered, is surrounded by continents, and receives ca. 10% of the freshwater flowing into the world ocean. This runoff contributes organic matter and nutrients to the Arctic Ocean, and a significant portion of the dissolved organic matter in mixed-layer waters, which is elevated relative to temperate oceans, is derived from riverine inputs (40, 60). Organic carbon and nitrogen concentrations are highest in the surface mixed layer and decrease below 100 m. A recent budgeting exercise (59, 60) identified three major sources of dissolved organic carbon to the central Arctic Ocean: in situ production (56%); river runoff (25%); and Pacific Ocean water, which transports organic material from the productive Bering and Chukchi Shelf regions (19%) into the central Arctic Ocean. The relative contribution of primary production from the Eurasian shelf is not known but is probably significant because sea ice is thinner, the open water season is longer, and a large portion of these extensive, shallow continental margins are ice-free during the summer.
Polar cooling and the resulting formation of sea ice impart a unique signature to the water mass structure of the Arctic Ocean. The sea ice itself is a unique habitat that has received recent attention from microbiologists (7, 24-26, 53). Freshwater inflow, brine exclusion during sea ice formation, the annual cycle of ice formation and melting, and the effective absence of wind mixing result in a very stable, highly stratified water column consisting of three major water masses (1, 2). The upper 60 m consists of relatively well-mixed, low-salinity (30‰) water with a seasonal, shallow halocline. The intermediate layer (between 75 and 150 m) contains high-salinity (>34‰), cold (−2 to −4°C) water separated from the surface layer by an extremely stable halocline. Relatively warm (2 to 4°C) Atlantic Ocean water is found below this intermediate layer. Adding to this habitat diversity is the large Coriolis parameter of the High Arctic, which results in a complex pattern of horizontal circulation and dynamic eddy fields (1).
One key question concerning polar prokaryotes is whether the composition of bacterial communities that evolved in perennially cold oceans has diverged substantially from those in temperate and tropical waters. For example, Synechococcus and Prochlorococcus cyanobacteria are ubiquitous and important members of plankton communities in temperate and tropical oceans, yet they are not found in polar oceans. Similarly, gas vacuolate bacteria are important in sea ice communities, but they have not been reported in temperate or tropical seas (56). Does this apply to the distributions of other prokaryotes, for example, the ubiquitous (at least in temperate and tropical waters) α-proteobacterial group SAR11? The SAR11 group was first discovered in samples from a subtropical gyre (9) and subsequently demonstrated to be associated with the surface layer of the ocean, characteristics similar to those of Synechococcus and Prochlorococcus spp.
A related question concerns the similarities or differences of bacterioplankton in the polar oceans. Although both oceans contain cold water and sea ice, the Southern Ocean is quite different from the Arctic Ocean. It surrounds a continent (Antarctica) with narrow, deep continental shelves and is bounded by an oceanic front. The Southern Ocean receives virtually no freshwater inflow, no terrigenous organic matter, and negligible terrestrially derived micronutrients (e.g., Fe [28, 31, 57]). Warm tropical gyres separate the surface waters of these two basins and always have (50), whereas the deepwater connection, if it exists for biota, is via global thermohaline circulation with a time scale of the order of 1,000 years (50, 61). The separate evolution and current isolation of cold polar oceans in the Arctic and Antarctic suggest that polar marine prokaryotes may have evolved independently, as have many other polar species, including some sea ice bacteria (24, 27). Little is known about the similarities or differences of the microbial communities found in the two polar oceans, yet the question of whether bacterioplankton species in polar oceans are the same or different is of interest from the standpoint of biogeography, biogeochemistry, and genetic exchange.
To date, there is little information on the phylogenetic composition of bacterial assemblages in either of the polar oceans with which to address these questions. Although data are emerging for the Southern Ocean (6, 32, 33, 37, 38; J. T. Hollibaugh, N. Bano, and H. W. Ducklow, unpublished data), there have been no comparable studies of the Arctic Ocean. Ferrari and Hollibaugh (17) used banding patterns expressed in denaturing gradient gel electrophoresis (DGGE) analyses of samples to compare the composition of Bacteria assemblages in the Arctic Ocean; however, that study did not provide sequence information that could be used for phylogenetic analysis. Yager et al. (63) showed that the composition and physiological properties of bacterial assemblages from the Chukchi Sea changed in response to an algal bloom. This study provided a few 16S ribosomal DNA (rDNA) sequences from continental shelf samples; however, neither study provided an analysis of the phylogenetic composition of Arctic Ocean bacterial assemblages with enough resolution to draw biogeographic conclusions. So far, such studies have been restricted to sea ice samples where polar endemism has been demonstrated (24, 25, 53).
The study reported here had three goals. First, we sought to provide phylogenetic characterization of Arctic Ocean bacterioplankton that could be used to test hypotheses about Bacteria biogeography. Second, we wanted to establish the relationship between DGGE bands and nucleotide sequences for Arctic Ocean samples to constrain DGGE analysis of a larger sample set. Finally, we wanted to begin to evaluate the spatial and seasonal variation in the distributions of prominent Arctic Ocean phylotypes, recognizing that an analysis with higher resolution than is feasible by the cloning and sequencing approach used here would follow from examination of the larger sample set by DGGE.
We used the PCR to amplify a portion of the 16S rRNA gene. These mixed-template amplicons were then resolved by DGGE to assess the phylogenetic complexity of Arctic Ocean bacterioplankton assemblages. Phylogenetic affiliations of sequences represented by bands in DGGE were established by (i) cloning genes encoding 16S rRNA from the original sample, (ii) screening the clone libraries by comparing the migration of DGGE bands from cloned sequences with DGGE bands in gels of the samples used to generate the clone libraries, and (iii) sequencing cloned genes and comparing them with sequences of fragments extracted from DGGE bands and with the database (GenBank).
We found that the Arctic bacterioplankton assemblage was composed of a mixture of uniquely polar and cosmopolitan phylotypes. All clones from our clone library fell into α-, δ-, γ-, and ɛ-Proteobacteria; Cytophaga-Flexibacter-Bacteroides (CFB); Verrucomicrobium spp.; and green nonsulfur bacteria (GNSB) groups, with the majority of clones being in the α- and γ-Proteobacteria groups. None of the clones grouped with β-Proteobacteria. Although some of the phylotypes were similar to isolate sequences, the majority were most closely related to uncultured, environmental sequences. Prominent among these were members of the SAR11 group, which were ubiquitous and common in samples from the surface layer but were rare or absent from halocline samples.
MATERIALS AND METHODS
Sampling.
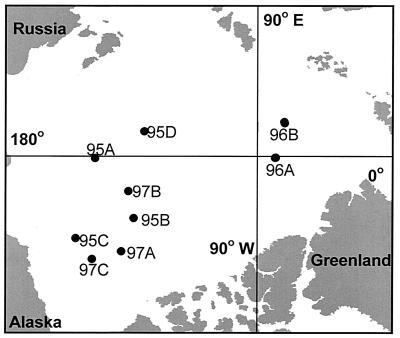
The samples used in this study were collected from the Central Arctic Ocean during the SCICEX 95 (26 March to 8 May 1995), SCICEX 96 (13 September to 28 October 1996), and SCICEX 97 (21 August to 15 October 1997) cruises aboard the U.S. Navy nuclear submarines Cavalla, Pogy, and Archerfish (see references 5 and 17 for details of sample collection and processing). We report here the results of analyses of a subset of these samples: four from the SCICEX 95 cruise (95A, 95B, 95C, and 95D), four from the SCICEX 96 cruise (96A, 96B, 96AD, and 96BD), and three from the SCICEX 97 cruise (97A, 97B, and 97C). All of these samples were from a depth of 55 m (except samples 96AD and 96BD, which were from 131 m). These samples were selected after examining the banding patterns of all samples collected on the SCICEX cruises (ca. 200 samples) by DGGE (data not shown). Samples were selected to represent common DGGE banding patterns; to contrast assemblages from the mixed layer (55 m) with those in the underlying, permanent halocline (131 m); and to capture spatial, seasonal, and interannual variability. The locations of the stations we selected for detailed phylogenetic analysis are shown in Fig. 1. Table 1 gives the SCICEX station codes, station location, depth, and bacterioplankton abundance.
FIG. 1.
Location of Arctic Ocean stations where the samples used to generate clone libraries were obtained.
TABLE 1.
Station identifiers and location and bacterioplankton abundance
| Sample code | Station code | Depth (m) | Latitude (N) | Longitude (E) | Bacteria (109•liter−1) |
|---|---|---|---|---|---|
| 95A | S.3.3 | 55 | 75°47" | 180°38" | 0.13 |
| 95B | 2.1.16.B | 55 | 77°56" | 206°32" | 0.10 |
| 95C | 1.4.18 | 55 | 72°34" | 204°13" | 0.11 |
| 95D | 1.19.10 | 55 | 79°53" | 167°34" | 0.09 |
| 96A | S99C9B1 | 40 | 88°30" | 355°15" | 0.15 |
| 96AD | S99C9B2 | 131 | 88°28" | 356°11" | 0.051 |
| 96B | S113C9B1 | 55 | 86°14" | 52°49" | 0.13 |
| 96BD | S113C9B2 | 131 | 86°17" | 51°45" | 0.077 |
| 97A | 5.4.1 | 55 | 75°29" | 214°52" | 0.39 |
| 97B | 4.8.1 | 55 | 78°19" | 195°05" | 0.26 |
| 97C | 1.24.1 | 55 | 72°59" | 211°45" | 0.24 |
DNA extraction and PCR amplification.
The procedures used to extract and purify DNA from our samples were as described elsewhere (5, 17). The primers used in PCR amplifications were synthesized either by Operon Technologies (Oakland, Calif.) or the University of Georgia Molecular Genetics Instrumentation Facility (MGIF). DNA was amplified with primers at positions 340 to 356f (primer 356f; forward, 5"-CCTACGGGAGGCAGCAG-3") and positions 517 to 533 (primer 517r; reverse, 5"-ATTACCGCGGCTGCTGG-3") of the Escherichia coli gene (10). A 40-bp GC clamp (5"-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCC-3" [39]) was added to the 5′ end of the 356f primer. Fluorescein was attached to the 5′ end of primer 517r.
PCR mixtures were prepared in a total volume of 100 μl containing 1× PCR buffer (50 mM Tris-HCl, 100 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol, 50% glycerol, 1% Triton X-100; pH 8.0), 2.5 mM MgCl2, a 200 μM concentration of each deoxyribonucleotide triphosphate (dATP, dCTP, dGTP, and dTTP), a 0.5 μM concentration of each primer, and 20 to 100 ng of template DNA. PCR conditions were similar to those used elsewhere (16). Reactions containing genomic DNA from Clostridium perfringens and Bacillus thuringiensis (Sigma Chemical Co.) were used as standards and positive controls; extracts from filters through which no seawater had passed served as negative controls. The concentration of the resulting PCR product was estimated by the Hoechst dye assay (42), and then the mixed-template product was resolved by DGGE.
DGGE.
DGGE was performed by using a CBS Scientific DGGE system (Del Mar, Calif.) essentially as described earlier (5). For each sample, 500 ng of PCR product was loaded on a 6.5% polyacrylamide gel containing a 40 to 65% gradient of denaturant (urea and formamide). Gels were run for 15 h at a constant voltage of 75 V in 1× TAE buffer (40 mM Tris-20 mM sodium acetate-1 mM EDTA, with the pH adjusted to 7.4 with acetic acid) at a constant temperature of 60°C. Gels were scanned by using an FMBIO II (Hitachi) gel scanner set to measure fluorescein fluorescence. We refer to the resulting pattern of bands in a lane as the fingerprint for that sample. To obtain sequence information from bands of interest, the bands were excised from the gel and DNA was eluted from them into 100 μl of water by incubation at 60°C for 2 h. The eluted DNA was amplified as described above by using the 356f and 517r primers, and the PCR product was purified by using Wizard PCR Preps (Promega) and then sequenced on an automated sequencer (MGIF) with 356f, 517r, or both primers.
Clone libraries.
Small subunit rRNA genes in eight samples (95C, 95D, 96A, 96B, 96AD, 96BD, 97B, and 97C) were amplified with primers 9f (forward, 5"-GAGTTTGATCCTGGCTCAG-3") and 1525r (reverse, 5"-AGAAAGGAGGTGATCCAGCC-3"). The other three samples (95A, 95B, and 97A) were amplified with primers 9f and 1492r (reverse, 5"-GGTTACCTTGTTACGACTT-3"). All PCRs were performed as described above under the following conditions. (i) For the 9f and 1525r primers, there was an initial denaturation of the template DNA at 95°C for 10 min and then a pause at 82°C to add Taq DNA polymerase (2.5 U; Promega). This was followed by 30 cycles of denaturation (30 s at 94°C), annealing (1 min at 57°C), and extension (1.25 min at 72°C), with a final extension at 72°C for 30 min. For the 9f and 1492r primers, there was an initial denaturation at 95°C for 5 min and a pause at 82°C to add Taq DNA polymerase (2.5 U; Promega). This was followed by 20 cycles of denaturation (45 s at 94°C), annealing (45 s at 48°C), and extension (1 min at 72°C), with a final extension at 72°C for 45 min. Reactions were run in triplicate and then combined and purified by using Wizard PCR Preps (Promega). PCR product (50 ng) was ligated into pGEM-T Easy Vector (Promega) and transformed into competent E. coli JM109 cells. The transformed cells were plated on Luria-Bertani (LB) plates containing 100 μg of ampicillin ml−1, 80 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) ml−1, and 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) as recommended by the manufacturer and incubated overnight at 37°C.
A total of 184 white colonies from the 11 clone libraries were chosen at random, plated on ampicillin-supplemented LB plates, and then incubated overnight. Clones were screened by PCR-DGGE by using the 356f-517r primer pair. This allowed us to identify groups of clones containing (presumably) the same inserts. One (or, occasionally, two to four) representative of each group was then chosen for sequencing.
Phylogenetic analysis.
All sequences were obtained from an automatic sequencer operated by MGIF. We sequenced 105 clones by using plasmid primer Sp6, yielding readable sequences of ∼650 bp. Sixty-nine of these clones were also sequenced with plasmid primer T7 and an internal primer (356f) to obtain nearly complete sequences of the 16S rRNA gene. Primer sequences were not included in phylogenetic analyses. Sequences were checked for chimeras by using the Ribosomal Database Project's CHECK-CHIMERA program. Chimeras were also detected by generating phylogenetic trees with different regions of the sequence. Sequences were aligned by using the Wisconsin package (version 10.0; Genetics Computer Group, Inc.) and compared to known sequences by using the basic local alignment search tool (BLAST) (4). Sequences were then assigned to major groups (α-Proteobacteria, γ-Proteobacteria, and everything else) by using BLAST similarities. Phylogenetic analysis of these groups used nearly full-length sequences (66 of the 88 sequences representing nearly all of the partial sequences found in the libraries) from positions (E. coli numbering [10]) 71 to 1366 (α-Proteobacteria), 58 to 1466 (γ-Proteobacteria), or 68 to 1404 (all other sequences). Phylogenetic trees were inferred, and bootstrap analysis (100 replicates) was performed with the PHYLIP package (15) by using evolutionary distances (i.e., Jukes-Cantor distances) and the neighbor-joining method.
Nucleotide sequence accession numbers.
Clone sequences have been deposited in GenBank under the accession numbers given in Table 2.
TABLE 2.
Cloned sequences with phylogenetic affiliation, nearest neighbor from GenBank, percent similarity based on aligned base pairs, band position in DGGE (Fig. 2), and total clones migrated with each band
| Clone (accession no.) | Sequence length (bp) | No. of clonesa | Groupb | Closest relative (accession no.) | % Similarity | Band position |
|---|---|---|---|---|---|---|
| 96BD-6 (AF355053) | 1,456 | 2/24 | GNSB | Clone SAR307 (U20798) | 97 | B |
| 96A-18 (AF353233) | 1,442 | 1/24 | α, SAR116 | Clone OM38 (U70679) | 98 | D |
| 97A-12 (AF355044) | 1,446 | 1/16 | δ | Nitrospina gracilis (L35504) | 91 | C |
| 95A-16 (AF353227) | 1,397 | 1/20 | α, SAR11 | Clone SAR407 (U75253) | 98 | F |
| 95C-6 (AF355042) | 1,473 | 2/10 | δ | Nitrospina gracilis (L35504) | 89 | E |
| 96A-3 (AF353218) | 652 | 1/24 | α, SAR11 | Clone SAR407 (U75253) | 98 | F |
| 96B-5 (AF353219) | 641 | 1/23 | α, SAR11 | Clone SAR407 (U75253) | 98 | F |
| 96BD-18c (AF355034) | 1,100 | 1/24 | α, SAR11 | Clone SAR407 (U75253) | 98 | F |
| 96AD-8 (AF353212) | 1,430 | 1/20 | α, SAR11 | Clone SAR203 (U75255) | 97 | G |
| 95A-12 (AF353230) | 967 | 2/20 | α, SAR11 | Clone PLY43 (U13159) | 99 | H |
| 95B-11 (AF353220) | 650 | 1/17 | α, SAR11 | Clone PLY43 (U13159) | 99 | H |
| 95C-1 (AF353221) | 627 | 3/10 | α, SAR11 | Clone OM188 (U70687) | 99 | H |
| 96A-20 (AF353208) | 1,430 | 3/24 | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 96B-3 (AF353209) | 1,430 | 5/23 | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 96B-15 (AF353213) | 668 | Dup | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 97A-2 (AF353231) | 994 | 5/16 | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 97A-8 (AF353232) | 721 | Dup | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 97B-2 (AF353216) | 645 | 3/10 | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 97B-10 (AF353217) | 609 | Dup | α, SAR11 | Clone OM188 (U70687) | 100 | H |
| 97C-1 (AF353210) | 1,430 | 1/10 | α, SAR11 | Clone SAR407 (U75253) | 98 | H |
| 96A-8 (AF353234) | 1,421 | Comigrated with 96A-20 | α, Roseo | Clone NAC1-19 (AF245628) | 97 | H |
| 96A-1 (AF353235) | 1,420 | 8/24 | α, Roseo | Clone NAC11-3 (AF245632) | 99 | I |
| 96B-6 (AF353224) | 1,429 | 3/34 | α, SAR11 | Clone SAR407 (U75253) | 96 | I |
| 97B-5 (AF353225) | 1,429 | 2/10 | α, SAR11 | Clone SAR407 (U75253) | 94 | I |
| 95B-1 (AF353214) | 1,396 | 3/17 | α, SAR11 | Clone ZD0410 (AJ400351) | 99 | I |
| 95C-4 (AF353222) | 619 | Dup | α, SAR11 | Clone ZD0410 (AJ400351) | 96 | I |
| 95C-7 (AF353215) | 1,440 | 2/10 | α, SAR11 | Clone ZD0410 (AJ400351) | 98 | I |
| 95D-8 (AF353223) | 1,441 | 1/12 | α, SAR11 | Clone ZD0410 (AJ400351) | 97 | I |
| 97A-1 (AF353228) | 1,407 | 1/16 | α, SAR11 | Clone ZD0410 (AJ400351) | 95 | I |
| 96BD-3 (AF355041) | 1,472 | 1/24 | δ | Clone NAC60-12 (AF245652) | 98 | I |
| 96B-16 (AF354595) | 1,500 | 1/23 | γ | Fundibacter jadensis (AJ001150) | 88 | I |
| 96AD-16 (AF354601) | 1,491 | 2/20 | γ | Pseudoalteromonas atlantica (AF173963) | 99 | J |
| 96AD-23 (AF354602) | 629 | Dup | γ | Pseudoalteromonas atlantica (AF173963) | 99 | J |
| 95A-13 (AF355038) | 1,439 | 3/20 | δ | Clone NAC60-12 (AF245652) | 98 | K |
| 95C-5 (AF355039) | 1,472 | 1/10 | δ | Clone NAC60-12 (AF245652) | 98 | K |
| 96AD-7 (AF355040) | 1,472 | 1/20 | δ | Clone NAC60-12 (AF245652) | 99 | K |
| 96A-7 (AF353226) | 1,439 | 1/24 | α, SAR11 | Clone OM155 (U70686) | 94 | L |
| 96B-22d (AF353229) | 1,330 | 1/23 | α, SAR11 | Clone OM155 (U70686) | 95 | L |
| 96B-1 (AF353242) | 1,493 | 2/23 | γ | -e | M | |
| 97A-11 (AF355037) | 1,462 | 1/16 | γ | - | M | |
| 95B-17 (AF353241) | 1,461 | 1/17 | γ | Oceanospirillum sp. (AJ302699) | 90 | M |
| 95A-1 (AF354599) | 650 | Dup | γ | Alteromonas macleodii (Y18228) | 99 | N |
| 95A-15 (AF354598) | 1,455 | 10/20 | γ | Alteromonas macleodii (Y18228) | 99 | N |
| 95A-4 (AF354600) | 650 | Dup | γ | Alteromonas macleodii (Y18228) | 99 | N |
| 95B-13 (AF353237) | 1,461 | Dup | γ | Oceanospirillum sp. (AJ302699) | 90 | O |
| 95B-19 (AF353240) | 644 | Dup | γ | Oceanospirillum sp. (AJ302699) | 96 | O |
| 95B-7 (AF353238) | 1,461 | 7/17 | γ | Oceanospirillum sp. (AJ302699) | 90 | O |
| 95B-8 (AF353239) | 1,455 | Dup | γ | Oceanospirillum sp. (AJ302699) | 96 | O |
| 96AD-24 (AF354604) | 1,497 | 1/20 | γ | Alcanivorax borkumensis (Y12579) | 96 | P |
| 96BD-24 (AF354603) | 1,497 | 1/24 | γ | Alcanivorax borkumensis (Y12579) | 96 | P |
| 96A-14 (AF354611) | 1,489 | 1/24 | γ | Clone ZD0424 (AJ400355) | 97 | P |
| 97A-6 (AF354612) | 1,456 | 1/16 | γ | Clone ZD0424 (AJ400355) | 97 | P |
| 95B-15 (AF354609) | 1,464 | 1/17 | γ | Fundibacter jadensis (AJ001150) | 98 | P |
| 96B-17 (AF354597) | 1,491 | 1/23 | γ | Pseudoalteromonas atlantica (AF173963) | 98 | P |
| 96B-9 (AF354596) | 1,492 | 1/23 | γ | Pseudomonas fluorescens (AF094726) | 91 | Q |
| 96AD-3 (AF354607) | 1,502 | 1/20 | γ | Thiomicrospira sp. (AJ237758) | 90 | R |
| 96BD-1 (AF354605) | 1,494 | 2/24 | γ | Alcanivorax borkumensis (Y12579) | 94 | S1 |
| 96A-12 (AF354610) | 1,498 | 3/24 | γ | Fundibacter jadensis (AJ001150) | 98 | S1 |
| 95D-6 (AF354615) | 617 | 1/12 | γ | Clone OM241 (U70702) | 93 | S2 |
| 96B-24 (AF354616) | 1,485 | 1/23 | Cyto | Clone ZD0403 (AJ400347) | 91 | S3 |
| 97A-17 (AF354617) | 1,444 | 1/16 | Cyto | Strain agg58 (L10946) | 85 | T |
| 96BD-19 (AF354606) | 1,495 | 1/24 | γ | Clone ZD0405 (AJ400348) | 99 | U1 |
| 97A-18 (AF354613) | 1,457 | 1/16 | γ, SAR86 | Clone OCS5 (AF001651) | 99 | U2 |
| 96BD-2c (AY08221) | 1,269 | 1/24 | Verr | Clone DEV005 (AJ401105) | 88 | V1 |
| 97A-13 (AF354618) | 1,444 | 1/16 | Cyto | Clone OM271 (U70708) | 93 | V2 |
| 96B-11 (AF354621) | 1,477 | 1/23 | CFB | Polaribacter irgensii (M61002) | 97 | V2 |
| 97A-14 (AF355051) | 1,452 | 1/16 | Cyto | Clone ZD0403 (AJ400347) | 91 | V3 |
| 97A-15 (AF354620) | 1,440 | 2/16 | CFB | Polaribacter irgensii (M61002) | 95 | W |
| 97A-5 (AF355052) | 650 | Dup | CFB | Polaribacter irgensii (M61002) | 97 | W |
| 95A-3 (AF355043) | 1,457 | 1/20 | δ | Clone BD4-10 (AB015560) | 87 | - |
| 95D-9 (AY08220) | 1,492 | 1/12 | Verr | Clone DEV022 (AJ401118) | 94 | - |
| 95A-2 (AF355046) | 1,485 | 1/20 | δ, SAR406 | Clone OCS307 (U41450) | 89 | - |
| 97B-4 (AY08219) | 1,523 | 1/10 | Verr | Clone OPB35 (AF027005) | 83 | - |
| 96B-13 (AF355050) | 1,465 | 1/23 | ɛ | Clone PVB-55 (U15105) | 95 | - |
| 96A-24 (AF355048) | 650 | 1/24 | δ, SAR406 | Clone SAR406 (U34043) | 80 | - |
| 96B-7 (AF355047) | 1,518 | 1/23 | δ, SAR406 | Clone SAR406 (U34043) | 90 | - |
| 96BD-15 (AF355049) | 638 | 1/24 | δ, SAR406 | Clone SAR406 (U34043) | 80 | - |
| 96B-10 (AF353211) | 1,430 | 2/23 | α, SAR11 | Clone SAR407 (U75253) | 98 | - |
| 95B-10 (AY08222) | 1,444 | 1/17 | Verr | Clone VadinHB65 (U81755) | 83 | - |
| 95B-14 (AF355055) | 1,444 | 1/17 | Verr | Clone VadinHB65 (U81755) | 83 | - |
| 95A-18 (AF355054) | 1,421 | 1/20 | GNSB | Clone WS52f (AF186417) | 80 | - |
| 97C-5 (AF354614) | 1,495 | 1/10 | γ | Clone ZD0408 (AJ400349) | 98 | - |
| 96BD-22d (AF355045) | 1,279 | 1/24 | δ | Nitrospina gracilis (L35504) | 92 | - |
| 95B-2 (AF355036) | 1,419 | 1/17 | α | Olavius loisae symbiont (AF104473) | 89 | - |
| 97A-7 (AF353236) | 1,426 | 1/16 | α | Olavius loisae symbiont (AF104473) | 88 | - |
| 96AD4 (AF354619) | 1,481 | 2/20 | Cyto | Psychroserpens burtonensis (U62913) | 93 | - |
| 95B-9c (AF355035) | 1,364 | 1/17 | α | Strain BD1-17 (AB015526) | 88 | - |
| 96AD-9 (AF354608) | 1,499 | 1/20 | γ | Thiomicrospira sp. (AJ237758) | 96 | - |
Number of clones in a library out of the total number screened in that library that gave DGGE bands migrating to the position indicated in the column marked “Band position.” We usually only sequenced one of these clones, but in some instances we sequenced additional clones. When these contained the same sequence as the first clone sequenced, they are listed as “Dup” in the column headed “No. of clones”; if they contained a different sequence, they were listed as “comigrated with (Clone ID).”
α, δ, γ, and ɛ, α-, δ-, γ-, and ɛ-Proteobacteria, respectively; Roseo, Roseobacter spp.; Cyto, Cytophaga spp.; Verr, Verrucomicrobium spp.
Chimeric sequences and portion of sequences were deleted.
Partial inserts.
-, Band position was not identified.
RESULTS
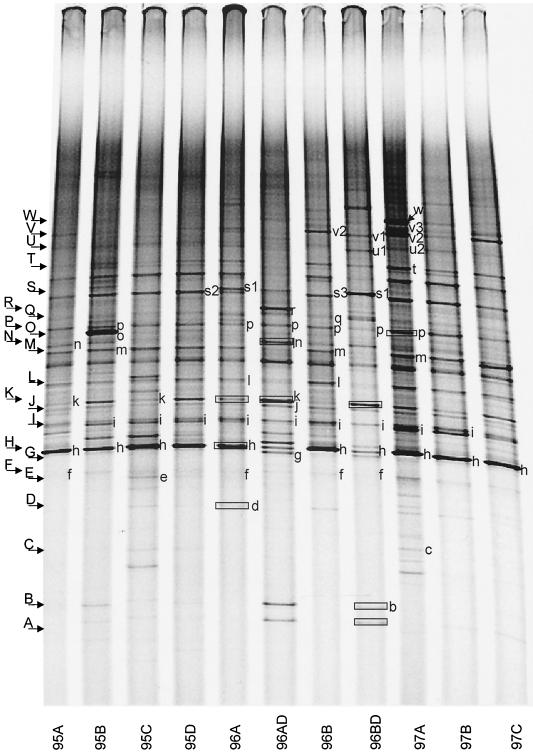
DGGE fingerprints of the selected samples (Fig. 2) showed that most bands were common to all samples in all three seasons. However, one sample (96B) from SCICEX 96 and two samples (97A and 97C) from SCICEX 97 contained additional bands. DGGE fingerprints also revealed differences between mixed-layer and halocline samples in the composition of the Bacteria assemblage. A more detailed analysis of the DGGE fingerprints of all of our Arctic samples will be presented elsewhere.
FIG. 2.
DGGE profiles of samples that were used to generate clone libraries. Bands indicated by small letters were assigned to cloned sequences by comparing mobility of PCR-DGGE fragments from clones with bands in the sample. The phylogenetic affiliations of these bands are given in Table 4. DNA from bands enclosed in boxes was sequenced by eluting the fragment and then sequencing it as described in the text.
Of the 184 clones screened, 36 contained no inserts (Table 3). Clones with inserts (i.e., the remaining 148) were grouped according to their DGGE mobility as shown in Fig. 2. Where it could be determined, the association between cloned inserts from the library of a given sample and bands in the DGGE fingerprint for the sample is indicated by small letters in Fig. 2. One or more cloned inserts representing each DGGE mobility group was sequenced from one or more of the libraries (total of 105 clones). Six of these sequences were ambiguous and were discarded. Fourteen clones were positively identified as containing chimeric sequences. Three chimeric sequences were included in the subsequent analysis after deleting portions of the sequence. The 88 remaining sequences were compared to GenBank sequences by using BLAST.
TABLE 3.
Summary of total clones analyzed from each library
| Library | Clones
|
|||
|---|---|---|---|---|
| No. analyzed | No. without inserts | No. sequenced | No. chimeric | |
| 95A | 20 | 1 | 9 | 0 |
| 95B | 17 | 0 | 12 | 1 |
| 95C | 10 | 0 | 7 | 1 |
| 95D | 10 | 7 | 3 | 0 |
| 96A | 24 | 1 | 13 | 4 |
| 96AD | 20 | 9 | 10 | 0 |
| 96B | 23 | 1 | 15 | 0 |
| 96BD | 24 | 8 | 14 | 7 |
| 97A | 16 | 0 | 13 | 0 |
| 97B | 10 | 1 | 7 | 1 |
| 97C | 10 | 8 | 2 | 0 |
| Total | 184 | 36 | 105 | 14 |
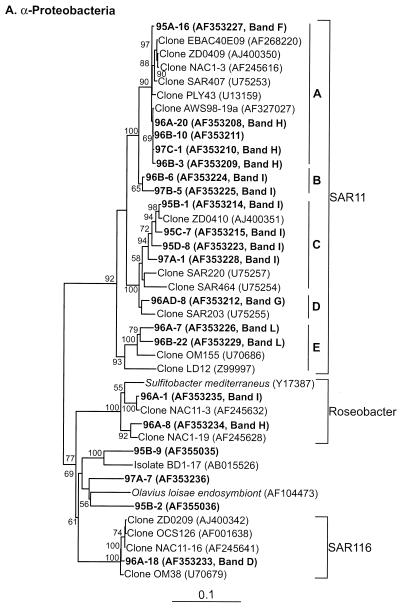
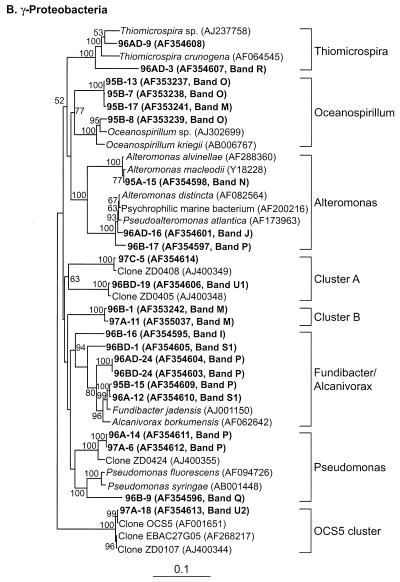
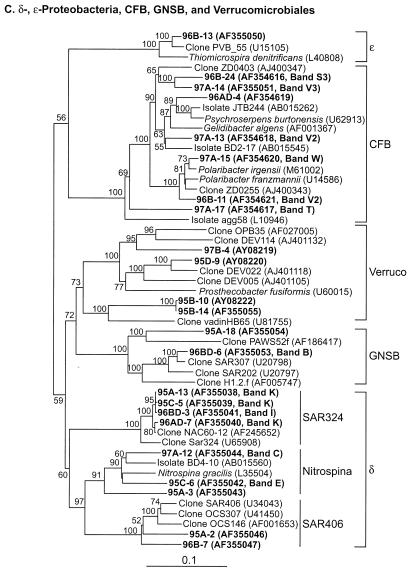
Table 2 presents the phylogenetic affiliations and similarity values of the most closely related GenBank sequences for all of the sequences obtained in this study. All of our sequences fell into seven major lineages of the domain Bacteria: α-, γ-, δ-, and ɛ-Proteobacteria; the CFB group; Verrucomicrobium spp.; and GNSB. The phylogenetic relationships between our sequences and the database sequences are shown in Fig. 4.
FIG. 4.
Neighbor-joining trees showing phylogenetic relationships of 16S rDNA sequences cloned from Arctic Ocean samples to closely related sequences from GenBank. Clones from this study are indicated in boldface type. Bootstrap values of >50% (of 100 iterations) are shown. The trees are unrooted, with Halobacterium salinarum as the out group. Scale bars indicate Jukes-Cantor distances. (A) α-Proteobacteria; (B) γ-Proteobacteria; (C) all others.
Table 4 identifies the sequence(s) corresponding to DGGE bands in Fig. 2. We were able to assign cloned inserts to 30 to 50% of the distinct bands in Fig. 2. One exception was band A, which was identified by sequencing the fragment eluted from the band. Another exception is the prominent band between bands L and M on Fig. 2. Although this band was present in all of the samples we analyzed, none of the clones we screened contained inserts that yielded fragments with the same DGGE mobility. We were not able to identify it from DNA eluted from the band because the sequencing electropherograms we obtained were ambiguous, presumably because the band contained more than one sequence.
TABLE 4.
Phylogenetic affinity of sequences co-migrating with DGGE bands in Fig. 2a
| Band | Sample | Accession no. of cloned sequence | Closest sequenceb (accession no.) | Groupc | % Similarity | No. of clones |
|---|---|---|---|---|---|---|
| A | 96BD | 125 BP (not submitted) | Sva0996 (AJ241005) | High G+C, gram positive | 96 | 0 |
| B | 96BD | AF355053 | Clone SAR307 (U20798) | GNSB | 97 | 2 |
| C | 97A | AF355044 | Nitrospina gracilis (L35504) | δ | 91 | 1 |
| D | 96A | AF353233 | Clone OM38 (U70679) | α, SAR116 | 98 | 1 |
| E | 95C | AF355042 | Nitrospina gracilis (L35504) | δ | 89 | 2 |
| F | 95A | AF353227 | Clone SAR407 (U75253) | α, SAR11 | 98 | 4 |
| G | 96AD | AF353212 | Clone SAR203 (U75255) | α, SAR11 | 97 | 1 |
| H | 96A | AF353208 | Clone SAR407 (U75253) | α, SAR11 | 98 | 23 |
| H | 96A | AF353234 | Clone NAC1-19 (AF245628) | α, Roseo | 97 | 1 |
| I | 96B | AF354595 | Fundibacter jadensis (AJ001150) | γ | 88 | 1 |
| I | 96BD | AF355041 | Clone NAC60-12 (AF245652) | δ | 98 | 1 |
| I | 96B | AF353224 | Clone SAR407 (U75253) | α, SAR11 | 96 | 5 |
| I | 96A | AF353235 | Clone NAC11-3 (AF245632) | α, Roseo | 99 | 8 |
| I | 95B | AF353214 | Clone ZD0410 (AJ400351) | α, SAR11 | 99 | 7 |
| J | 96AD | AF354601 | Pseudoalteromonas atlantica (AF173963) | γ | 99 | 2 |
| K | 96AD | AF355040 | Clone NAC60-12 (AF245652) | δ | 99 | 5 |
| L | 96B | AF353229 | Clone OM155 (U70686) | α, SAR11 | 95 | 2 |
| M | 96B | AF353242 | No match | γ | 3 | |
| M | 95B | AF353241 | Oceanospirillum sp. (AJ302699) | γ | 90 | 1 |
| N | 95A | AF354598 | Alteromonas macleodii (Y18228) | γ | 99 | 10 |
| O | 95B | AF353239 | Oceanospirillum sp. (AJ302699) | γ | 96 | 7 |
| P | 96AD | AF354604 | Alcanivorax borkumensis (Y12579) | γ | 96 | 2 |
| P | 96B | AF354597 | Pseudoalteromonas atlantica (AF173963) | γ | 98 | 1 |
| P | 96A | AF354611 | Clone ZD0424 (AJ400355) | γ | 97 | 2 |
| P | 95B | AF354609 | Fundibacter jadensis (AJ001150) | γ | 98 | 1 |
| Q | 96B | AF354596 | Pseudomonas fluorescens (AF094726) | γ | 91 | 1 |
| R | 96AD | AF354607 | Thiomicrospira sp. (AJ237758) | γ | 90 | 1 |
| S1 | 96A | AF354610 | Fundibacter jadensis (AJ001150) | γ | 98 | 3 |
| S1 | 96BD | AF354605 | Alcanivorax borkumensis (Y12579) | γ | 94 | 2 |
| S2 | 95D | AF354615 | Clone OM241 (U70702) | γ | 93 | 1 |
| S3 | 96B | AF354616 | Clone ZD0403 (AJ400347) | Cyto | 91 | 1 |
| T | 97A | AF354617 | Strain agg58 (L10946) | Cyto | 85 | 1 |
| U1 | 96BD | AF354606 | Clone ZD0405 (AJ400348) | γ | 99 | 1 |
| U2 | 97A | AF354613 | Clone OCS5 (AF001651) | γ, SAR86 | 99 | 1 |
| V1 | 96BD | AY08221 | Clone DEV005 (AJ401105) | Verr | 88 | 1 |
| V2 | 96B | AF354621 | Polaribacter irgensii (M61002) | CFB | 97 | 1 |
| V2 | 97A | AF354618 | Clone OM271 (U70708) | Cyto | 93 | 1 |
| V3 | 97A | AF355051 | Clone ZD0403 (AJ400347) | Cyto | 91 | 1 |
| W | 97A | AF354620 | Polaribacter irgensii (M61002) | CFB | 95 | 2 |
With the exception of band A, which was identified by sequencing DNA contained in the band, identifications were made by comparing the mobility of fragments amplified from cloned inserts with the mobility of fragments amplified from samples. Where more than one clone contained an insert of a given phylotype that matched a band, the accession number given is for the clone with the longest sequence with the greatest BLAST similarity value to the database sequence and the number of clones matching that phylotype is given. Twenty-one clones contained sequences that could not be assigned to bands in Fig. 2.
As determined by BLAST.
Abbreviations are as defined in Table 2, footnote b.
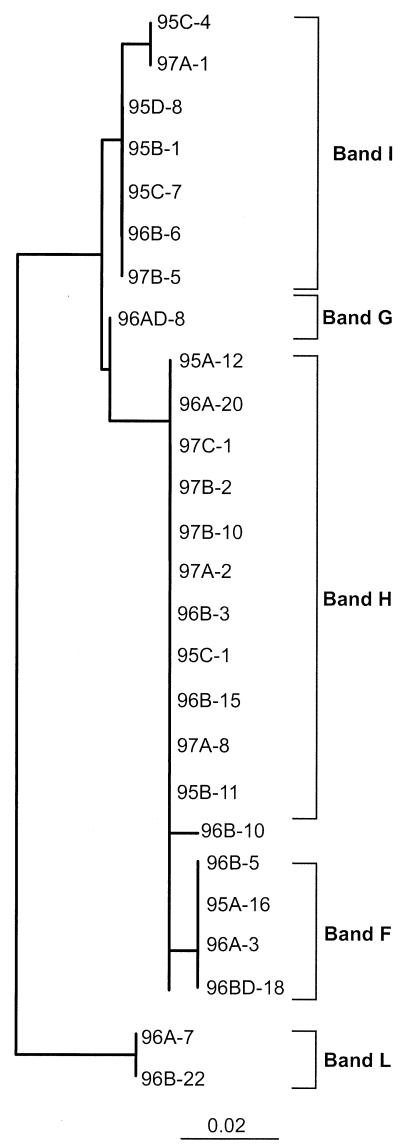
Table 4 and Fig. 2 reveal two key constraints on the analysis of community composition by DGGE. First, as demonstrated by sequencing cloned inserts that gave DGGE bands migrating with band I, a given DGGE band can represent more than one, often quite different, sequence. We found this ambiguity in 4 (bands H, I, M, and P) of the 30 bands we analyzed with up to five (band I) different sequences represented by the same band. However, one of the cloned sequences attributed to band I contains a 1-bp cloning error that affected its DGGE mobility; without this error it would run with band K. Second, as indicated by the SAR11 sequences whose closest phylogenetic affinity by BLAST was clone SAR407 (U75253), very closely related sequences can appear in two or more quite distinct bands. In the case of the SAR11 sequences, this is due to small but consistent differences in the region of the sequence amplified by our DGGE primers (Fig. 3).
FIG.3.
Neighbor-joining tree showing the correspondence between DGGE bands and phylogenetic relationships of SAR11 group sequences from the Arctic Ocean. Trees were constructed by using only the portion of cloned sequences interrogated by the primers used in our DGGE analysis (356f to 517r, including primer regions). The trees are unrooted, with E. coli as the out group. The bar indicates a Jukes-Cantor distance of 0.02.
α-Proteobacteria.
A total of 32 sequences (36% of all sequences we obtained) grouped with the α-Proteobacteria, dominated (26 of 32) by SAR11 sequences (Fig. 4A, Table 2). Sequences within the SAR11 cluster fell into five subclusters. All but two of the cloned SAR11 sequences produced fragments that migrated with four DGGE bands (bands F, H, I, and L; Fig. 2 and Table 4) that were found in all mixed-layer samples. Each of these DGGE bands represents a different SAR11 ribotype with distinct sequence differences that are consistent over several clones (Fig. 3). Cluster A represents sequences previously reported primarily from surface water samples (18); we only encountered them in mixed-layer samples. All sequences in subcluster A were quite similar (with similarity values ranging from 98 to 99%) to one another and to the sequences of several other clones obtained from a number of marine environments (18, 23, 63). This ribotype is commonly encountered in clone libraries.
The two sequences in subcluster B and the members of subcluster C yielded fragments that migrated with band I because their sequences are identical in the region interrogated by the DGGE primers we used (Fig. 3). Sequences that grouped with cluster C were all from the mixed layer and were related (95 to 99% similarity) to the sequence from clone ZD0410 found in a sample taken after an algal bloom in the North Sea (GenBank description). Sequences from subcluster D were found only in halocline samples and grouped with a sequence cloned from a deepwater sample, SAR203 (18). Two cloned sequences (96A-7 and 96B-22, 96% similar) fell into subcluster E and were similar (94 to 95%) to the sequence from clone OM155 retrieved from a sample collected on the continental shelf off Cape Hatteras, N.C. (45).
Two sequences (both from SCICEX 96) clustered with the Roseobacter group (Fig. 4A). They were related (97 to 99% similarity) to sequences in clones recovered from surface waters of the North Atlantic during a spring algal bloom (23). Clones with inserts related to SAR11 and Roseobacter sequences could not be differentiated on DGGE because they comigrated in bands H and I, despite significant differences between the sequences in the region interrogated by our DGGE primers. Clones containing Roseobacter sequences that migrated with these bands were found less frequently than SAR11 clones (2 versus 20). Even though one of the Roseobacter clones came from sample 96A, the direct sequence of the fragment in band H from that sample was identical to the sequence from clone 96A-20, a SAR11 insert, further indicating that band H dominantly represented SAR11 sequences. One clone (96A-18) was recovered that contained an insert related to the SAR116 cluster (migrated with band D, a faint band present in most of the mixed-layer samples). The sequence from this clone contained a region that was 100% identical to the direct sequence of band D (125 bp). Three additional clones (97A-7, 95B-2, and 95B-9) contained inserts that were not closely related (88 to 89% similarity) to any of the database sequences (Fig. 4A, Table 2).
γ-Proteobacteria.
Twenty-eight sequences (32%) clustered with the γ-Proteobacteria (Fig. 4B, Table 2). These belonged to five genera (Thiomicrospira, Oceanospirillum, Alteromonas, Alcanivorax-Fundibacter, and Pseudomonas) and three clusters containing only environmental sequences.
Two clones (96AD-3 and 96AD-9) from one of the halocline samples clustered with the Thiomicrospira group. Sequences from clones 96AD-9 (band R) and 96AD-3 (a corresponding DGGE band has not been identified) were 96 and 90% similar, respectively, to a Thiomicrospira sp. isolated from a shallow-water hydrothermal vent in the Aegean Sea (8). Comparision of DGGE fingerprints revealed that band R was an intense band present only in one halocline sample. Sequences that fell into the Oceanospirillum group were found in only one of the mixed-layer samples (95B). DGGE screening grouped 8 of 16 clones in the library from this sample with bands O and M, representing Oceanospirillum sequences. Seven of these sequences migrated with DGGE band O, a very intense band found only in that sample (Fig. 2). The inserts in four of these clones were sequenced. Two of these sequences (clones 95B-8 and 95B-19) were most similar (96%) to Oceanospirillum sp., a newly described marine bacterium that is reported to be an obligate hydrocarbon degrader (GenBank description). The other two clones (95B-7 and 95B-13) contained inserts with low similarity (90%) to Oceanospirillum sp. or any other database sequence (Table 2, Fig. 4B).
Cloned sequences that clustered with the Alteromonas group were very similar (98 to 99%) to previously reported sequences from isolates (Fig. 4B, Table 2). Sequences of two clones (96B-17, mixed layer; 96AD-16, halocline) were 98 to 99% similar to Pseudoalteromonas atlantica and to several other psychrophilic marine bacteria isolated from a wide variety of environments, including sea ice isolates from the Antarctic (7, 14, 53). Fifty percent of the clones in library 95A contained inserts that migrated with band N. Three of these (95A-1, 95A-4, and 95A-15) were sequenced; all three were 99% similar to each other and to Alteromonas macleodii and several other Alteromonas sp. This suggested that Alteromonas sp. dominated in this sample. Band N was not intense in the fingerprint for this sample shown in Fig. 2. However, this band was intense and dominated the fingerprints of previous analyses of this sample (17; J. T. Hollibaugh, unpublished data). Band N was also found in other samples, including both of the halocline samples, but none of the clones recovered from any other sample contained inserts with the same sequence or with the same mobility in PCR-DGGE. The direct sequence of band N excised from a halocline sample (96AD) was 100% identical (130 bp) to the sequence of clone 95A-15 and confirmed that Alteromonas sp. was also present in the halocline sample.
Three cloned sequences (96AD-24, 96BD-1, and 96BD-24) from halocline samples were related to Alcanivorax borkumensis (94 to 96% similarity), a hydrocarbon-degrading, surfactant-producing bacterium (64). Two cloned sequences (96A-12 and 95B-15) from the mixed layer were 98% similar to Fundibacter jadensis, an intertidal sediment isolate (11).
Three clones contained sequences that clustered with the Pseudomonas group, but they were not closely related (<91% similarity) to sequences from Pseudomonas isolates. Two of these, cloned from mixed-layer samples (96A-14 and 97A-6, 99% similar), formed a separate lineage within the Pseudomonas cluster (Fig. 3B) and were most similar (97%) to an uncultured bacterium (ZD0424) from the North Sea (GenBank description). Both of these clones produced PCR-DGGE fragments that migrated with band P, which was present in all samples (Fig. 2). However, other clones that yielded PCR-DGGE fragments migrating with band P contained inserts with full sequences related to different genera. The direct sequence of band P from sample 97A was 100% identical to the corresponding region of the insert from clone 97A-6, confirming the identity of band P in that sample. The sequence contained in a third clone (96B-9) was distantly related (91% similar) to Pseudomonas fluorescens.
Two cloned sequences (96B-1 and 97A-11) had no close relatives in GenBank and formed a separate cluster (cluster B, Fig. 4B). Sequences in these clones were most closely related (94% similar) to a partial sequence (600 bp) of clone OM182 recovered from the continental shelf off Cape Hatteras, N.C. (45). Two other clones (97C-5 and 96BD-19) were grouped into cluster A, which contained only environmental clones from the North Sea (GenBank description). One clone (97A-18) corresponding to band U2 (Fig. 2) contained a sequence that clustered with SAR86. The sequence in this clone was 99% similar to the sequence from clone OCS5 (14, 45). DGGE showed that band U2 was more intense in this sample than in others, where it was either faint or not present.
δ-Proteobacteria.
The sequences of 12 clones (14%) fell into three subclusters of the δ-Proteobacteria (Table 2, Fig. 4C). Two of these clusters (SAR406 and SAR324) contained only environmental sequences, while the third cluster (Nitrospina) contained cultured organisms. Four of the cloned sequences (95A-2, 96A-24, 96B-7, and 96BD-15) were distantly related (80 to 90%) to sequences from clones SAR406 or OCS307 (Fig. 3C). The sequence from clone 96B-7 was most closely related (97% similarity over 973 bp) to the sequence from clone Car168, retrieved from a sample taken at the redox interface of the Carioco Basin (GenBank description). Bands corresponding to these clones were not detected in the fingerprints of samples from which they were recovered. Four cloned sequences (95A-13, 95C-5, 96AD-7, and 96BD-3) fell into the SAR324 cluster and were 98 to 99% similar to an environmental sequence, clone NAC60-12, obtained from the surface water of the North Atlantic during a spring algal bloom (23). All of these clones (except 96BD-3) produced PCR-DGGE fragments that migrated with band K, a band found in all of our samples. Direct sequences (130 bp) of band K obtained from samples 96BD, 96AD, and 96A were identical to the corresponding region of NAC60-12, confirming the tentative assignment based on the mobility of these sequences in PCR-DGGE. Four cloned sequences (95C-6, 95A-3, 96BD-22, and 97A-12) were distantly related (similarity 87 to 92%) to Nitrospina gracilis, isolated from the Atlantic Ocean (54), and to strain BD4-10, obtained from deep-sea sediment (30).
ɛ-Proteobacteria.
Only one clone, 96A-13, contained an insert that clustered with the ɛ-Proteobacteria (Fig. 4C, Table 2). The sequence of this insert was most closely related (95% similarity) to clone PVB_55, recovered from a clone library generated from a microbial mat sample collected at an active hydrothermal vent system (GenBank description).
CFB group.
Eight clones (9%) contained inserts that clustered with the CFB group (Fig. 4C, Table 2), including five from one mixed-layer sample (97A). Sequences from three of these clones (97A-15, 97A-5, and 96B-11) were 95 to 97% similar to the gas vacuolate bacterium Polaribacter irgensii and to several other Polaribacter strains isolated from Arctic and Antarctic water and sea ice samples (7, 53). Clones that grouped with Cytophaga were less similar (85 to 93%) to previously reported sequences (Fig. 4C, Table 2). None of the clones from SCICEX 95 contained inserts that clustered with the CFB group and most clones related to the CFB group were from one of the SCICEX 97 mixed-layer samples. Figure 2 shows that bands T, V2, V3, and W, representing CFB group sequences, were dominant in the DGGE fingerprints from sample 97A, more so than in other samples.
Verrucomicrobium spp.
Five clones (6%) contained sequences that clustered with the Verrucomicrobium group (Fig. 4C, Table 2). None of these were closely related to cultured organisms, and only 1 sequence (95D-9) was related to a previously reported sequence.
GNSB.
Two clones contained sequences that grouped with the GNSB group. One of these sequences (95A-18) had no close matches in GenBank. The most similar sequence was from an uncultured sponge symbiont (Fig. 4C, BLAST similarity, 80%). The other cloned sequence (96BD-6) migrated with DGGE band B (characteristic of halocline samples) and was 98% similar to environmental sequence SAR307, obtained from a water sample collected at 250 m in the Sargasso Sea (22). The direct sequence of band B (125 bp) aligned perfectly with the sequence of clone 96BD-6 in the appropriate region, verifying the tentative assignment, based on melting point in PCR-DGGE, of this clone to band B.
None of the clones recovered migrated with band A (characteristic of halocline samples, Fig. 2). However, the direct sequence (125 bp) of band A from sample 96BD was 96% similar to a high-G+C, gram-positive bacterium, Sva0996, isolated from permanently cold marine sediments (46).
Variation of composition with depth.
We limited our analysis of spatial and temporal variation of cloned sequences to groups that were encountered at least three times, recognizing that an even larger sample size is desirable. Two mixed-layer and two halocline samples were used to examine the vertical distribution of phytotypes. DGGE fingerprints showed that band A (related to high-G+C, gram-positive bacteria) and band B (related to SAR307) were characteristic of halocline samples, whereas bands related to SAR11 sequences were common in all mixed-layer samples. Phylogenetic analysis of cloned sequences also showed depth-related differences in the distribution of bacterial phylotypes. Samples from the mixed layer were dominated (52%) by sequences affiliated with α-Proteobacteria, and samples from the halocline were dominated (44%) by sequences affiliated with γ-Proteobacteria.
Seasonal variation.
The γ-Proteobacteria displayed the most obvious seasonal difference of relative abundance in clone libraries. Sequences with affinities to γ-Proteobacteria constituted 35% of the sequences in libraries from samples collected on the SCICEX 95 (late winter) cruise, compared to only 19% in from the SCICEX 96 cruise (late summer) and 11% from the SCICEX 97 cruise (early fall). Members of the δ-Proteobacteria displayed a similar pattern: they accounted for 14% of the sequences from SCICEX 95 samples, 4% from SCICEX 96, and 2% from SCICEX 97. Sequences with affinities to Alteromonas and Oceanospirillum spp. were only found in samples from the SCICEX 95 cruise, suggesting they are also “winter” species. In contrast, members of the Cytophaga and CFB group were only encountered in samples from the summer and fall cruises, with most sequences coming from the fall cruise (22% of all sequences from that cruise). Sequences with affinity to Roseobacter spp. and clone NAC60 were only found during the summer and sequences with affinity to Alcanivorax borkumensis were only found in the halocline sample from the fall cruise. At the other extreme of seasonal variation, sequences with affinity to SAR407 were found everywhere, all of the time.
DISCUSSION
Phylogenetic analysis of 16S rDNA clones from the Arctic Ocean revealed significant diversity among the cloned sequences, with similarity to previously reported sequences ranging from 80 to 100% (Fig. 4, Table 2). In general, the database sequences most closely related to our Arctic sequences are from the North Atlantic, generally from boreal or temperate stations and/or deep water. This relationship may be an artifact of the global distribution of phylogenetic sampling effort, which is biased toward the North Atlantic. It is interesting that we retrieved relatively few sequences matching those from sea ice collections; however, the sea ice sequences in the database are largely derived from isolates rather than DNA collections (7, 24, 26).
The cloned sequences corresponding to the γ-Proteobacteria group had high similarity to isolate sequences, but the majority of the cloned sequences from other groups were not closely related to isolates. Based on the criterion that 93% similarity corresponds to a taxonomic grouping at the genus levels (35), 34% of our cloned 16S rDNA sequences belonged to new genera. Sequences with similarities of 94 to 97% or greater to known sequences may also represent different genera. For example, Prochlorococcus and Synechococcus isolates have 16S rRNA sequences that are 96 to 98% similar, but they have different light-harvesting components and vertical distributions (34). Beja et al. (6) reported a similar discrepancy between the similarity of SAR86 16S rDNA sequences and the sequences of genes coding for proteorhodopsin with depth and from locations as different as the Southern Ocean and a subtropical gyre.
All clones were compared by PCR-DGGE to the DGGE fingerprints of the samples from which they were recovered. We were able to assign 70% of the clones to bands in the sample based on similar DGGE mobility. In some cases we were able to obtain further verification of band identity by comparing sequences from bands with sequences of cloned inserts with the same DGGE mobility. All of the sequences from bands were identical (100% similarity) to the cloned sequences assigned to that band by PCR-DGGE. Overall, only 30 to 50% of the DGGE bands in these samples were matched with clones, suggesting that we have not completely sampled the diversity in our clone libraries. Many of the DGGE bands were faint, suggesting low relative abundance of the template (if the PCR errors are not extreme [13]) and thus a low probability of recovering a cloned insert. Most of the sequences in the clone libraries for each sample were recovered only once, further indicating that we undersampled the diversity in our clone libraries. It is thus likely that the data presented here are a minimal estimate of the richness of these assemblages.
If all of the sequences in our libraries are assigned to phylotypes based on their similarity to a database sequence as determined by BLAST, we encountered 41 different phylotypes in the 88 clones we sequenced. However, 34% of the cloned sequences were <94% similar to previously reported sequences, so the BLAST assignments are imprecise. Cloned inserts with similarities to database sequences of ≥97% fell into 18 different phylotypes. This represents a minimum estimate of the richness of the Arctic Ocean communities we sampled. At the other extreme, none of the cloned sequences were exactly the same, although the relative importance of PCR errors (55a), cloning errors (41, 51), and microdiversity (13, 32) in the 1- to 2-bp differences between some of these sequences is not known.
Our results agree with a previous analysis of a subset of these samples (17) that also showed that Arctic Ocean bacterioplankton assemblages were complex, as complex as assemblages from California coastal waters (36). Ferrari and Hollibaugh (17) were able to group 100 samples from the SCICEX 95 cruise into five major clusters with similarities greater than ∼80% based on their PCR-DGGE fingerprints. They found that four bands were almost ubiquitous in this collection of samples. We evaluated four mixed-layer samples (from a depth of 55 m) from SCICEX 95 stations together with two and three samples from SCICEX 96 and 97, respectively, on the same DGGE gel (Fig. 2) to compare the composition of the bacterial assemblages in three different seasons. DGGE fingerprints of the selected samples showed that most of the bands present in the SCICEX 95 samples were also found in samples from SCICEX 96 and 97. However, we found additional bands in some of the samples from SCICEX 96 and 97, suggesting that the late winter conditions sampled during SCICEX 95 represent a minimum in richness of Bacteria assemblages in the Arctic Ocean.
Three bands (bands H, L, and I) containing SAR11 sequences were common to all of the mixed-layer samples. Another band (band K) containing sequences similar to clone NAC60-12 (δ-proteobacterium, SAR324) was present in all of the mixed-layer and halocline samples, suggesting a cosmopolitan or ubiquitous distribution of the corresponding organism in the environment. Gonzalez et al. (23) found that clone NAC60-12 (SAR324) was a characteristic of terminal restriction fragment length polymorphism chromatograms from deepwater samples. Wright et al. (62) also showed that the SAR324 group is more common in samples from the bottom of the mixed layer of both the Atlantic and Pacific Oceans, with a maximum relative abundance occurring between depths of 160 and 500 m. We found that a band corresponding to the NAC60-12 sequence was present in all samples from the mixed layer and halocline; however, the band was more intense in the halocline samples. Wright et al. (62) suggest that SAR324 is specialized for an ecological niche found at the base of the thermocline; our data extending the range of this group into Arctic Ocean surface waters suggest that thermal tolerance may be a significant factor in controlling the distribution of this group.
Although most bands appeared to be common to all samples, some of the DGGE fingerprints and phylogenetic analysis of cloned sequences from these samples indicated spatial variation in the composition of microbial assemblages. For example, two samples from the SCICEX 95 cruise were dominated by different genera of bacteria. We found that 50% of the clones from sample 95A contained inserts that were related to Alteromonas macleodii, whereas 50% of clones from sample 95B contained inserts related to Oceanospirillum sp. Unless PCR or cloning bias can be invoked to explain the dominance of these sequences in these samples, the low diversity of these samples suggests that these bacteria were “blooming” at the time they were taken. This finding is similar to reports by Rehnstam et al. (47) and Pinhassi and Hagström (44) of occasional dominance of assemblages by one species. Survey track data contained in the study by Ferrari (16) indicate that the “patches” were only encountered once and that the along-track extent of the patches was ca. 750 km. Bacterial abundance was ca. twofold the background concentration for samples displaying band N (Alteromonas), whereas bacterioplankton abundance was not elevated for samples displaying band O (Oceanospirillum) in DGGE analysis (16).
Alteromonas macleodii is the only described species of the genus Alteromonas. Acinas et al. (3) found that clones related to Alteromonas macleodii were abundant in samples of attached bacteria and suggested that it could be an important genus, one specialized for particle-associated niches. Another study of bacterioplankton succession during an enclosure experiment in the Mediterranean showed that the bacterial community changed from a mixed assemblage to one dominated by Alteromonas-like bacteria (49). The authors of that study found that these changes were partly influenced by differential mortality of bacterial populations during incubation.
Phylogenetic analysis of cloned sequences also indicates seasonal variability in Arctic microbial assemblages. For example, sequences related to Cytophaga and Polaribacter spp. were only found in samples from the SCICEX 96 and 97 cruises, with the greatest frequency in samples from the SCICEX 97 cruise. The SCICEX 96 and 97 cruises took place during the late summer and fall, at the end of productive season, whereas the SCICEX 95 cruise sampled during late winter and early spring, prior to the onset of the spring phytoplankton and ice algal blooms. The temporal difference, in relation to the annual cycle of primary production in the Arctic Ocean, may explain our detection of Cytophagales and Polaribacter sequences in SCICEX 96 and 97 samples but not in SCICEX 95 samples. As a consequence of spring and summer phytoplankton production, dissolved organic carbon concentrations were likely to have been higher and composed of a more complex mixture of relatively labile organic compounds during the late-summer and fall SCICEX 96 and 97 cruises (60, 63). The SCICEX 95 cruise sampled waters that had likely been depleted of labile compounds over the winter. Members of the CFB phylum occur ubiquitously in marine environments and are generally associated with the degradation of complex organic substrates and more nutrient rich environments (43). Yager et al. (63) also found that Cytophaga spp. dominated during the peak algal bloom.
Our clone libraries and DGGE fingerprints showed depth-dependent differences in the distribution of bacterial ribotypes. Several previous studies have shown similar depth-dependent variation in the composition of bacterial assemblages (18, 21, 22, 29, 62). Band B represents the sequence of an uncultured organism, SAR307 (GNSB), and was characteristic of halocline samples. This is consistent with a previous study (22) reporting that the SAR307 is more common in deep water. Members of this group have also been recovered in samples from depths of 3,000 m in the Northeastern Pacific and 1,000 m in the subtropical Atlantic (19), indicating that these organisms are adapted to life in the deep ocean. Another band characteristic of halocline samples (band A) was 96% similar to a high-G+C, gram-positive bacterium, Sva0996, obtained from Arctic sediments (46). Gram-positive bacteria are normally thought to be associated with marine sediment and soil, but they have also been reported in the marine water column (20).
Sequences related to the α-Proteobacteria group, 80% of which were members of the SAR11 group, were dominant in samples from the mixed layer. The predominance of sequences with affinity to α-Proteobacteria and SAR11 in surface water samples has been reported by several authors and seems to be a widespread occurrence (18, 45).
Thirty-six percent of all of the clones in our study were related to SAR11 sequences. Giovannoni and Rappe (21) found that SAR11 sequences accounted for 13% of all retrieved ribotypes. Although SAR11 sequences appear to be widely distributed in the world ocean, they are evolutionarily distant from all known isolates. Given the widespread distribution of this group in oceanic habitats ranging from Sargasso Sea surface waters to Arctic and Antarctic surface waters (Bano and Hollibaugh, unpublished data), it seems likely that 16S rDNA sequences do not accurately reflect the true phylogenetic richness of this group. Our Arctic Ocean sequences were 96 to 99% similar over 680 bp to Antarctic and Mediterranean Sea clones reported (32). The SAR11 sequences that grouped with band H in Fig. 3 are 99% similar to the Antarctic sequences of Martinez and Valera (32). These authors only succeeded in discriminating between SAR11 populations by using the hypervariable 16S-23S intergenic spacer region.
Several studies have demonstrated variation in the relative abundance of SAR11 during phytoplankton blooms. Yager et al. (63) found that SAR11 sequences were present in the DGGE profiles of samples taken before or after the peak of an algal bloom in the Chukchi Sea but were not detected during the peak of the bloom. DGGE profiles from a study of a diatom bloom in a mesocosm showed that bands corresponding to SAR11 sequences disappeared after the peak of the bloom (48). Gonzalez et al. (23) reported that SAR11 phylotypes accounted for 11% of the sequences obtained from North Atlantic samples collected during the spring algal bloom and 26.1% in previous non-algal bloom studies of surface ocean water.
Our observations are in agreement with those of other reports (35), and we can therefore conclude that Arctic Ocean bacteria are diverse and largely represent novel groups of organisms, at least compared to the tropical and temperate waters from which most of the sequences in the database have been retrieved. Proof of this awaits broader analyses such as DNA-DNA hybridization studies (52, 58) and the availablilty of sequences from other diagnostic genes (for example, rpoA or gyrB [12, 55]) and comparison with libraries generated from the Southern Ocean. Our analysis of a limited number of samples from the Ross Sea and Palmer Peninsula region indicates that they contain the same SAR11 and β-Proteobacteria ammonia-oxidizing bacterial sequences that we have found in our Arctic Ocean samples (the present study and Hollibaugh et al., unpublished).
Acknowledgments
We appreciate the assistance of the U.S. Navy, particularly the officers and crew of the USSN Cavalla, Pogy, and Archerfish; Arctic Submarine Laboratory personnel; and the scientists who collected samples for us on the SCICEX 95, 96, and 97 cruises, especially T. DeLaca, D. Stockwell, T. Whitledge, R. Sambrotto, G. Steward, J. Gossett, M. Mosher, and B. Campbell. These studies would not have been possible without their willing collaboration. F. Lucas and S. B. Humayoun provided useful comments and other support during the preparation of the manuscript. Patrick Goodman, Ryan Hollibaugh, Briana Ransom, and Erin Biers helped with sample analysis.
This work was supported by NSF awards OPP 95-00237, OPP 96-25131 (reissued as OPP 97-96261), and OPP 98-09971 to J.T.H.
REFERENCES
- 1.Aagaard, K., L. K. Coachman, and E. C. Carmack. 1981. On the halocline of the Arctic Ocean. Deep-Sea Res. 28:529-545. [Google Scholar]
- 2.Aagaard, K., J. H. Swift, and E. C. Carmack. 1995. Thermohaline circulation in the Arctic Mediterranean Sea. J. Geophys. Res. 90:4833-4846. [Google Scholar]
- 3.Acinas, S. G., J. Antón, and F. Rodr|$$|Aa|figuez-Valera. 1999. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 65:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 5.Bano, N., and J. T. Hollibaugh. 2000. Diversity and distribution of DNA sequences with affinity to ammonia-oxidizing bacteria of the β-subdivision of the class Proteobacteria in the Arctic Ocean. Appl. Environ. Microbiol. 66:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, J. P., M. V. Brown, and D. S. Nichols. 1997. Biodiversity and ecophysiology of bacteria associated with Antarctic sea ice. Antarct. Sci. 9:134-142. [Google Scholar]
- 8.Brinkhoff, T., S. M. Sievert, J. Kuever, and G. Muyzer. 1999. Distribution and diversity of sulfur-oxidizing Thiomicrospira spp. at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Appl. Environ. Microbiol. 65:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britschgi, T. B., and S. J. Giovannoni. 1991. Phylogenetic analysis of a natural marine bacterioplankton population by rRNA cloninig and sequencing. Appl. Environ. Microbiol. 57:1707-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosius, J., M. L. Palmer, J. K. Poindexter, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruns, A., and L. Berthe-Corti. 1999. Fundibacter jadensis gen. nov., sp. nov., a new slightly halophilic bacterium, isolated from intertidal sediment. Int. J. Syst. Bacteriol. 49:441-448. [DOI] [PubMed] [Google Scholar]
- 12.Dahllof, I., H. Baillie, and S. Kjelleberg. 2000. rpoB-Based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl. Environ. Microbiol. 66:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLong, E. E., and N. R. Pace. 2001. Environmental diversity of Bacteria and Archaea. Syst. Biol. 50:470-478. [PubMed] [Google Scholar]
- 14.Eilers, H., J. Pernthaler, F. O. Glöckner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), v. 3.5. University of Washington, Seattle.
- 16.Ferrari, V. C. 1997. Analysis of the phylogenetic composition of Arctic microbial assemblages by PCR/DGGE. M.A. thesis. San Francisco State University, San Francisco, Calif.
- 17.Ferrari, V. C., and J. T. Hollibaugh. 1999. Distribution of microbial assemblages in the central Arctic Ocean basin studied by PCR/DGGE: analysis of a large data set. Hydrobiologia 401:55-68. [Google Scholar]
- 18.Field, K. G., G. Gordan, M. Wright, E. Urback, K. Vergin, and S. J. Giovannoni. 1997. Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine microbial communities from the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 63:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhrman, J. A., and A. A. Davis. 1997. Widespread Archaea and novel Bacteria from the deep sea as shown by 16S rRNA gene sequences. Mar. Ecol. Prog. Ser. 150:275-285. [Google Scholar]
- 20.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1993. Phylogenetic diversity of subsurface microbial communities from the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 59:1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannoni, S. J., and M. Rappe. 2000. Evolution, diversity and molecular ecology of marine prokaryotes, p. 47-84. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 22.Giovannoni, S. J., M. S. Rappe, K. L. Vergin, and N. L. Adair. 1996. 16S rRNA genes reveal stratified open ocean bacterioplankton populations related to the green non-sulfur bacteria. Proc. Natl. Acad. Sci. USA 93:7979-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González, J. M., R. Simó, R. Massana, J. S. Covert, E. O. Casamayor, C. Pedrós-Alió, and M. A. Moran. 2000. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 66:4237-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosink, J. J., R. P. Herwig, and J. T. Staley. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., non-pigmented, psychrophilic gas vacuolate Bacteria from polar sea ice and water. Syst. Appl. Microbiol. 20:356-365. [Google Scholar]
- 25.Gosink, J. J., R. L. Irgens, and J. T. Staley. 1993. Vertical distribution of bacteria in sea ice. FEMS Microbiol. Ecol. 102:85-90. [Google Scholar]
- 26.Gosink, J. J., and J. T. Staley. 1995. Biodiversity of gas vacuolate bacteria from Antarctic sea ice and water. Appl. Environ. Microbiol. 61:3486-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosink, J. J., C. R. Woese, and J. T. Staley. 1998. Polaribacter gen. nov., with three new species, P. irgensii sp. nov., P. franzmannii sp. nov., and P. filamentus sp. nov., gas vacuolate polar marine bacteria of the Cytophaga-Flavobacterium-Bacteroides group and reclassification of “Flectobacillus glomeratus” as Polaribacter glomeratus comb. nov. Int. J. Syst. Bacteriol. 48:223-235. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, N., R. F. Anderson, R. A. Mortlock, P. N. Froelich, P. Kubik, B. Dittrichhannen, and M. Suter. 1995. Increased biological productivity and export production in the glacial Southern Ocean. Nature 378:675-680. [Google Scholar]
- 29.Lee, S. H., and J. A. Fuhrman. 1991. Spatial and temporal variation of natural bacterioplankton assemblages studied by total genomic DNA cross-hybridization. Limnol. Oceanogr. 36:1277-1287. [Google Scholar]
- 30.Li, L. N., C. Kato, and K. Horikoshi. 1999. Bacterial diversity in deep-sea sediments from different depths. Biodivers. Conserv. 8:659-677. [Google Scholar]
- 31.Martin, J. H. 1990. Global-interglacial CO2 change: the iron hypothesis. Paleooceanography 5:1-13. [Google Scholar]
- 32.Martinez, J. G., and F. R. Valera. 2000. Microdiversity of uncultured marine prokaryotes: the SAR11 cluster and the marine Archaea of group I. Mol. Ecol. 9:935-948. [DOI] [PubMed] [Google Scholar]
- 33.Massana, R., L. T. Taylor, A. E. Murray, K. Y. Wu, W. H. Jeffrey, and E. F. DeLong. 1998. Vertical distribution and temporal variation of marine planktonic archaea in the Gerlache Strait, Antarctica, during early spring. Limnol. Oceanogr. 43:607-617. [Google Scholar]
- 34.Moore, L. R., G. Rocap, and S. W. Chisholm. 1998. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393:464-467. [DOI] [PubMed] [Google Scholar]
- 35.Mullins, T. D., T. B. Britschgi, R. L. Krest, and S. J. Giovannoni. 1995. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol. Oceanogr. 40:148-158. [Google Scholar]
- 36.Murray, A. E., J. T. Hollibaugh, and C. Orrego. 1996. Phylogenetic composition of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl. Environ. Microbiol. 62:2676-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray, A. E., C. M. Preston, R. Massana, L. T. Taylor, A. Blakis, K. Y. Wu, and E. F. DeLong. 1998. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl. Environ. Microbiol. 64:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray, A. E., K. Y. Wu, C. L. Moyer, D. M. Karl, and E. F. DeLong. 1999. Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat. Microb. Ecol. 18:263-273. [Google Scholar]
- 39.Myers, R. M., S. G. Fischer, L. S. Lerman, and T. Maniatis. 1985. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 13:3131-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opsahl, S., R. Benner, and R. M. W. Amon. 1999. Major flux of terrigenous dissolved organic matter through the Arctic Ocean. Limnol. Oceanogr. 44:2017-2023. [Google Scholar]
- 41.Paabo, S., and A. C. Wilson. 1988. Polymerase chain reaction revealing cloning artifacts. Nature 334:387-388. [DOI] [PubMed] [Google Scholar]
- 42.Paul, J. H., and B. Myers. 1982. Fluorometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl. Environ. Microbiol. 43:1393-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinhassi, J., F. Azam, J. Hempälä, R. A. Long, J. Martinez, U. L. Zweifel, and Å. Hagström. 1999. Coupling between bacterioplankton species composition, population dynamics, and organic matter degradation. Aquat. Microb. Ecol. 17:13-26. [Google Scholar]
- 44.Pinhassi, J., and A. Hagström. 2000. Seasonal succession in marine bacterioplankton. Aquat. Microb. Ecol. 21:245-256. [Google Scholar]
- 45.Rappe, M. S., P. F. Kemp, and S. J. Giovannoni. 1997. Phylogenetic diversity of marine coastal picoplankton 16S rRNA gene clones from the continental shelf off Cape Hatteras, North Carolina. Limnol. Oceanogr. 42:811-826. [Google Scholar]
- 46.Ravenschlag, K., K. Sahm, J. Pernthaler, and R. Amann. 1999. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 65:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehnstam, A.-S., S. Backman, D. C. Smith, F. Azam, and A. Hagstrom. 1993. Blooms of sequence-specific culturable bacteria in the sea. FEMS Microb. Ecol. 102:161-166. [Google Scholar]
- 48.Riemann, L., G. F. Steward, and F. Azam. 2000. Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl. Environ. Microbiol. 66:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schäfer, H., P. Servais, and G. Muyzer. 2000. Successional changes in the genetic diversity of a marine assemblage during confinement. Arch. Microbiol. 173:138-145. [DOI] [PubMed] [Google Scholar]
- 50.Schopf, T. J. M. 1980. Paleooceanography. Harvard University Press, Cambridge, Mass.
- 51.Speksnijder, A. G. C. L., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staley, J. T. 1999. Bacterial biodiversity: a time for place. ASM News 65:681-687. [Google Scholar]
- 53.Staley, J. T., and J. J. Goslink. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53:189-215. [DOI] [PubMed] [Google Scholar]
- 54.Teske, A., E. Alm, J. Regan, S. Toze, B. Rittmann, and D. Stahl. 1994. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J. Bacteriol. 176:6623-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stakebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewenella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 55a.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 56.Walsby, A. E. 1994. Gas vesicles. Microbiol. Rev. 58:94-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson, A. J., D. C. E. Bakker, A. J. Ridgwell, P. W. Boyd, and C. S. Law. 2000. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature 407:730-733. [DOI] [PubMed] [Google Scholar]
- 58.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Trüper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 35:463-464. [Google Scholar]
- 59.Wheeler, P. A., M. Gosselin, E. B. Sherr, D. Thibault, D. L. Kirchman, R. Benner, and T. E. Whitledge. 1996. Active cycling of organic carbon in the central Arctic Ocean. Nature 380:697-699. [Google Scholar]
- 60.Wheeler, P. A., J. M. Watkins, and R. L. Hansing. 1997. Nutrients, organic carbon and organic nitrogen in the upper water column of the Arctic Ocean: implications for the sources of dissolved organic carbon. Deep-Sea Res. II 44:1571-1592. [Google Scholar]
- 61.Worthington, L. V. 1968. Genesis and evolution of water masses. Meteorol. Monogr. 8:63-67. [Google Scholar]
- 62.Wright, T. D., K. L. Vergin, P. W. Boyd, and S. J. Giovannoni. 1997. A novel delta-subdivision proteobacterial lineage from the lower ocean surface layer. Appl. Environ. Microbiol. 63:1441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yager, P. L., T. L. Connelly, B. B. Mortazavi, K. E. Wommack, N. Bano, J. Bauer, S. Opsahl, and J. T. Hollibaugh. 2001. Dynamic microbial response to an Arctic algal bloom at sub-zero temperatures. Limnol. Oceanogr. 44:1882-1893.
- 64.Yakimov, M. M., G. P. N., S. Lang, E. R. Moore, W. R. Abraham, H. Lunsdorf, and N. Timmis. 1998. Alcanivorax borkumensis gen. nov., sp. nov., a new hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 48:339-348. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler, P. A. 1988. Evolution of the Arctic-North Atlantic and the Western Tethys, vol. 43. American Association of Petroleum Geologists, Tulsa, Okla.