Abstract
This work presents data on the application of a bacterial luciferase used to monitor gene expression of Streptococcus thermophilus in the digestive tract. The main result is that the bacterium was able to produce an active β-galactosidase in the digestive tract, although it did not multiply during its transit. This production was enhanced when lactose (the inducer) was added to the diet.
Lactase deficiency is a widespread problem occurring in approximately 70% of the world's population (17). Consumption of milk by lactase (β-galactosidase)-deficient individuals results in the underabsorption of lactose. This in turn often results in abdominal pain, diarrhea, and flatulence (1). Studies have shown that lactose digestion improves with the ingestion of yogurt compared to the ingestion of heated yogurt or milk (9, 10, 13, 14, 16, 18). These studies suggest that the beneficial effect could have occurred in the digestive tract (DT) after the consumption of the yogurt. The live bacteria present in yogurt may continue to produce β-galactosidase in the intestinal tract.
The aim of the present study was to determine if Streptococcus thermophilus is able to produce an active β-galactosidase during its transit in the DT. The S. thermophilus lactose (lac) operon contains the genes that encode a lactose permease (lacS) (12) and a β-galactosidase (lacZ) (15) for the transport and the hydrolysis of lactose, respectively. To study the expression of the bacterial β-galactosidase during the transit of S. thermophilus in the DT, the luciferase genes of Xenorhabdus luminescens (5, 8) were fused to the lac promoter. Luciferase is a well-established reporter gene system which has already been used to investigate Lactococcus lactis gene expression in the DTs of mice and rats (2, 3).
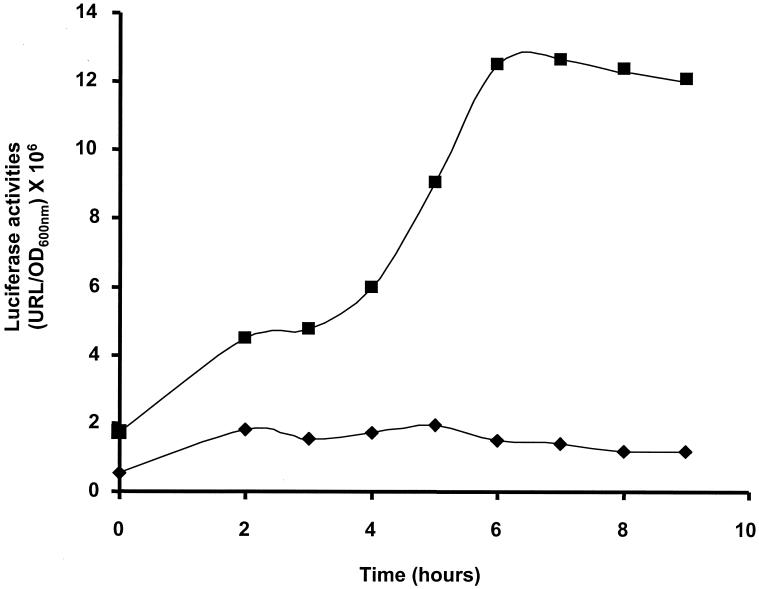
S. thermophilus strains were grown at 42°C in M17 with 0.5% lactose or with 0.5% glucose supplemented with erythromycin (5 μg/ml). Thermoresistant Bacillus subtilis spores were used as a transit marker and germinated at 60°C in G-spore medium (4). Three PCR fragments belonging to the S. thermophilus S85 lac operon were cloned into the integrative plasmid pil4267 (J. Anba, unpublished data) in front of the luciferase genes to give pFBI3 (carrying the 0.8-kb lac promoter region), pFBI4 (carrying a 0.74-kb fragment internal to the lacZ gene), and pFBI6 (carrying a 0.8-kb fragment internal to the lacS gene). These plasmids were integrated by homologous recombination into the chromosome of the S. thermophilus S85 strain grown at 42°C to produce FBI3, FBI4, and FBI6, respectively (Fig. 1). In FBI3, the integration of the plasmid duplicates the lac promoter so that the strain had a wild-type phenotype (same β-galactosidase activity and same growth rate in various media). In this strain, the luciferase genes became a functional part of the lac operon and were similarly regulated. The luciferase activity of FBI3 (estimated as described by Corthier et al. [2]) was enhanced nine times in M17-lactose compared to M17-glucose (Fig. 2). Moreover, in M17-lactose, the FBI3 strain produced light at the end of the exponential phase. These results are consistent with those described by Poolman et al. (11) and Gunnewijk and Poolman (6, 7). In FBI4 and FBI6, the integration of the pFBI4 and pFBI6 plasmids by simple crossing over interrupted the lacZ and lacS genes, respectively, leading to erythromycin-resistant recombinants devoid of β-galactosidase activity. Neither the FBI4 strain (devoid of functional LacZ) nor the FBI6 strain (devoid of functional LacS) was able to grow in milk and metabolize lactose, as expected for such mutants.
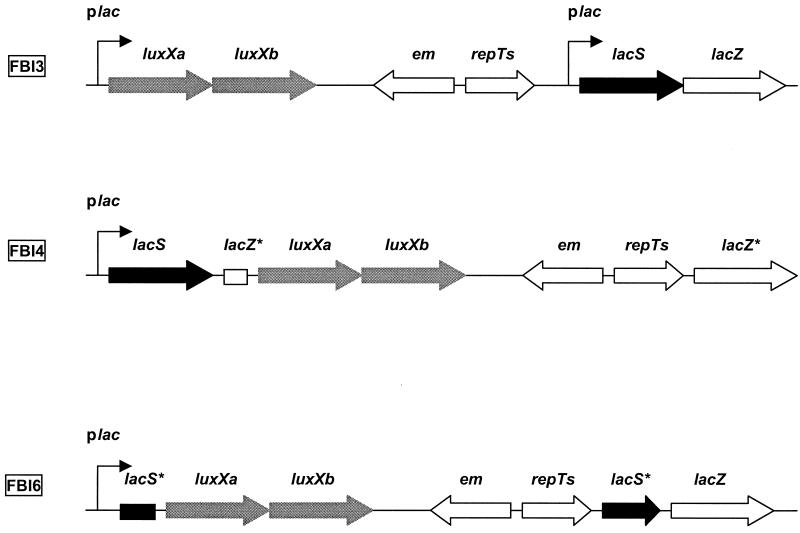
FIG. 1.
Chromosomal constructions obtained. FBI3, lac operon intact; FBI4, inactivation of lacZ; FBI6, inactivation of lacS. Plac, promoter of the lac operon; lacS, gene encoding lactose permease; lacZ, gene encoding β-galactosidase; em, gene encoding resistance to erythromycin; luxXa and luxXb, genes encoding the X. luminescens luciferase; repTs, thermosensitive replicon.
FIG. 2.
Luciferase activities of FBI3 in M17-glucose (diamonds) and in M17-lactose (squares).
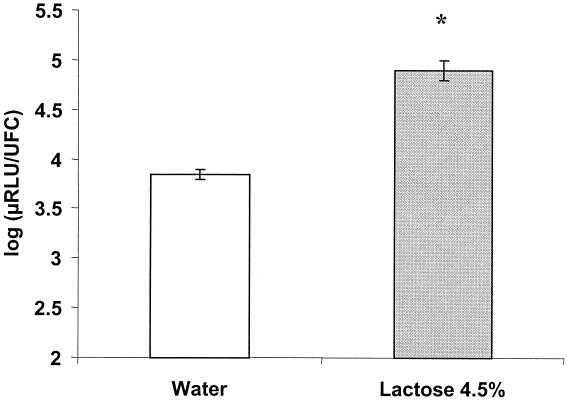
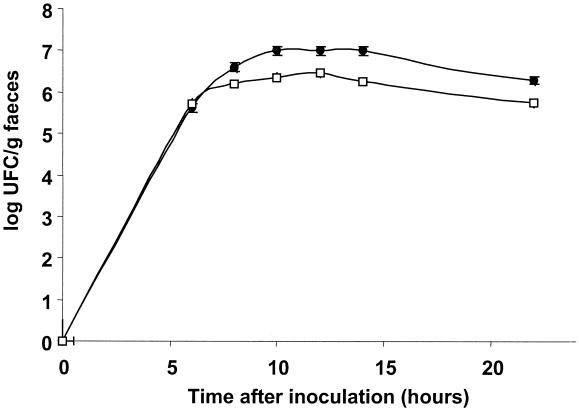
Six male adult germfree C3H/He mice, reared in sterile Trexler plastic isolators (La Calhène, Vélizy, France), received lactose (4.5% [wt/vol]; this concentration is found in milk and some yogurts) as a drinking solution or water as a control. They then received 0.5 ml of an S. thermophilus FBI3 culture (inoculum of 108 CFU/ml) mixed with B. subtilis spores (inoculum of approximately 108 CFU/ml) by oral gavage. Mice feces were collected at various times after inoculation, weighed, and diluted (1/10) in sterile water. Luciferase activities and S. thermophilus and B. subtilis counts were estimated from these dilutions. The luciferase activities per S. thermophilus organism, calculated as previously described (2), were enhanced 10 times when mice received lactose (4.5%) as a drinking solution compared to water (Fig. 3). As observed in vitro (6, 11), lactose was an activator of lac operon transcription in the DTs of germfree mice. In this experiment S. thermophilus did not multiply, did not settle in the DTs of germfree mice, and transited with the diet (Fig. 4). It was eliminated more rapidly than the spores, since 80 h after the inoculation, spores were still detectable in the feces, while the streptococci were under the threshold of detection (data not shown).
FIG. 3.
Luciferase activities in the feces of germfree mice inoculated with FBI3 and receiving either water or lactose (4.5%) as a drinking solution. The luciferase activity of the inoculum was 3.9 log (μ relative light units/unit-forming colony). ∗, standard deviation < 0.01. Error bars indicate standard deviations.
FIG. 4.
Enumeration of spores (circles) and S. thermophilus FBI3 (squares) in the feces of germfree mice.
The second experiment (with six germfree mice) was designed to determine if S. thermophilus was able to digest lactose during its transit in the DTs of mice. Metabolic cages (Tecniplast, Buguggiate, Italy) were used to measure water consumption and collect feces over a 24-h period without urine contamination. No residual lactose was detected in the feces of mice that received water as a drinking solution (Table 1, experiment I). When mice received lactose (4.5%, wt/vol) as a drinking solution, about 0.31 ± 0.01 g of lactose was recovered from feces in 24 h (Table 1, experiment II), suggesting that the mouse lactase was insufficient to digest the lactose absorbed. Oral administration of S. thermophilus (inoculum of 108 CFU/ml) to mice receiving lactose (4.5% as a drinking solution) resulted in a significant decrease (by 50%) compared to the previous group (Table 1, experiment III). No decrease was observed in mice inoculated with the FBI4 or FBI6 strain (data not shown). This experiment showed that the β-galactosidase produced by S. thermophilus is active and is able to hydrolyze lactose in vivo, resulting in an overall reduction of the lactose contents in feces.
TABLE 1.
Determination of the quantity of lactose ingested by germfree mice and excreted in the feces in 24 h
| Expta | Lactose (g)
|
|
|---|---|---|
| Ingested | Excreted in feces | |
| I | 0.00 ± 0.00 | 0.01 ± 0.00 |
| II | 0.32 ± 0.01 | 0.31 ± 0.01 |
| III | 0.32 ± 0.01 | 0.14 ± 0.01 |
Mice received either water (experiment I) or lactose (4.5%) (experiments II and III) as a drinking solution and were force fed with an S. thermophilus FBI3 culture (experiment III).
Our studies establish, with a genetic approach, that S. thermophilus produces a β-galactosidase during its transit and that this enzyme reduces the lactose content in the DT. It reinforces the observation that the bacteria in yogurt must be alive in order to help lactose digestion (10, 13, 14). Our data suggest that yogurt lactic acid bacteria are not “almost dead” in the DT but are still able to respond to diet stimuli and sustain new protein synthesis in order to adapt to environmental conditions. This approach provides new insight into host-microbe interactions and their impact on gastrointestinal health and disease.
Acknowledgments
This study was supported by the Mission Scientifique de SYNDIFRAIS, French industrial manufacturers of fresh dairy products.
We thank P. Rapine and J. Durao for technical assistances, C. Cherbut for helpful advice, and W. Holowacz for advice on English translation.
REFERENCES
- 1.Bayless, T. M., and S. S. Huang. 1971. Recurrent abdominal pain due to milk and lactose intolerance in school-aged children. Pediatrics 47:1029-1032. [PubMed] [Google Scholar]
- 2.Corthier, G., C. Delorme, S. D. Ehrlich, and P. Renault. 1998. Use of luciferase genes as biosensors to study bacterial physiology in the digestive tract. Appl. Environ. Microbiol. 64:2721-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducluzeau, R., M. Bellier, and P. Raibaud. 1970. Transit through the digestive tract of the inocula of several bacterial strains introduced “per Os” into axenic and “Holoxenic” mice. The antagonistic effect of the microflora of the gastrointestinal tract. Zentralbl. Bakteriol. Orig. 213:533-548. [PubMed] [Google Scholar]
- 5.Frackman, S., M. Anhalt, and K. H. Nealson. 1990. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J. Bacteriol. 172:5767-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunnewijk, M. G., and B. Poolman. 2000. HPr(His approximately P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34080-34085. [DOI] [PubMed] [Google Scholar]
- 7.Gunnewijk, M. G., and B. Poolman. 2000. Phosphorylation state of HPr determines the level of expression and the extent of phosphorylation of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34073-34079. [DOI] [PubMed] [Google Scholar]
- 8.Johnston, T. C., E. B. Rucker, L. Cochrum, K. S. Hruska, and V. Vandegrift. 1990. The nucleotide sequence of the luxA and luxB genes of Xenorhabdus luminescens HM and a comparison of the amino acid sequences of luciferases from four species of bioluminescent bacteria. Biochem. Biophys. Res. Commun. 170:407-415. [DOI] [PubMed] [Google Scholar]
- 9.Lerebours, E., C. N"Djitoyap Ndam, A. Lavoine, M. F. Hellot, J. M. Antoine, and R. Colin. 1989. Yogurt and fermented-then-pasteurized milk: effects of short-term and long-term ingestion on lactose absorption and mucosal lactase activity in lactase-deficient subjects. Am. J. Clin. Nutr. 49:823-827. [DOI] [PubMed] [Google Scholar]
- 10.Marteau, P., B. Flourie, P. Pochart, C. Chastang, J. F. Desjeux, and J. C. Rambaud. 1990. Effect of the microbial lactase (EC 3.2.1.23) activity in yoghurt on the intestinal absorption of lactose: an in vivo study in lactase-deficient humans. Br. J. Nutr. 64:71-79. [DOI] [PubMed] [Google Scholar]
- 11.Poolman, B., T. J. Royer, S. E. Mainzer, and B. F. Schmidt. 1990. Carbohydrate utilization in Streptococcus thermophilus: characterization of the genes for aldose 1-epimerase (mutarotase) and UDPglucose 4-epimerase. J. Bacteriol. 172:4037-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poolman, B., T. J. Royer, S. E. Mainzer, and B. F. Schmidt. 1989. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J. Bacteriol. 171:244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizkalla, S. W., J. Luo, M. Kabir, A. Chevalier, N. Pacher, and G. Slama. 2000. Chronic consumption of fresh but not heated yogurt improves breath-hydrogen status and short-chain fatty acid profiles: a controlled study in healthy men with or without lactose maldigestion. Am. J. Clin. Nutr. 72:1474-1479. [DOI] [PubMed] [Google Scholar]
- 14.Savaiano, D. A., A. AbouElAnouar, D. E. Smith, and M. D. Levitt. 1984. Lactose malabsorption from yogurt, pasteurized yogurt, sweet acidophilus milk, and cultured milk in lactase-deficient individuals. Am. J. Clin. Nutr. 40:1219-1223. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder, C. J., C. Robert, G. Lenzen, L. L. McKay, and A. Mercenier. 1991. Analysis of the lacZ sequences from two Streptococcus thermophilus strains: comparison with the Escherichia coli and Lactobacillus bulgaricus beta-galactosidase sequences. J. Gen. Microbiol. 137:369-380. [DOI] [PubMed] [Google Scholar]
- 16.Shermak, M. A., J. M. Saavedra, T. L. Jackson, S. S. Huang, T. M. Bayless, and J. A. Perman. 1995. Effect of yogurt on symptoms and kinetics of hydrogen production in lactose-malabsorbing children. Am. J. Clin. Nutr. 62:1003-1006. [DOI] [PubMed] [Google Scholar]
- 17.Simoons, F. J. 1978. The geographic hypothesis and lactose malabsorption. A weighing of the evidence. Am. J. Dig. Dis. 23:963-980. [DOI] [PubMed] [Google Scholar]
- 18.Varela-Moreiras, G., J. M. Antoine, B. Ruiz-Roso, and G. Varela. 1992. Effects of yogurt and fermented-then-pasteurized milk on lactose absorption in an institutionalized elderly group. J. Am. Coll. Nutr. 11:168-171. [PubMed] [Google Scholar]